Strategies to Prevent Early and Late-Onset Group B Streptococcal Infection via Interventions in Pregnancy
Abstract
1. Introduction
2. Incidence
3. Early vs. Late-Onset Disease
4. Transmission of GBS from Mother to Infant
5. Risk Factors
6. Biology of Streptococcus agalactiae
7. Serotype Distribution
8. Clinical Manifestations and Outcomes
| Characteristic | EOD | LOD | |
|---|---|---|---|
| Time of onset after birth | 0–6 days | 7–89 days | |
| Incidence rate in 1000 live births [7] | 0.32–0.71 | 0.04–0.65 | |
| Route of transmission [1,2,11] | vertical via direct exposure to maternal flora | horizontal from colonized mucous membranes, infected breast milk, and equipment | |
| Identified risk factors [2,12] | maternal GBS colonization | maternal GBS colonization | |
| prematurity | prematurity | ||
| young maternal age | young maternal age | ||
| black race | black race | ||
| maternal fever during labor (>100.4 F /36 C) | HIV exposure | ||
| prolonged rupture of membranes (>18 h) | |||
| Common serotypes [7,19] | III | 42.9–47% | 73–80% |
| Ia | 22.8–28.6% | 11.1–14.2% | |
| Ib | 7.1–8.0% | 2.2–5.3% | |
| V | 7.1–10.6% | 4.0–4.4% | |
| Clinical syndrome [7] | Meningitis | 16% | 43% |
| Bacteremia | 78% | 53% | |
| Adverse outcomes | Case fatality rate [6] | 7–12% | 4–9% |
| Neurodevelopmental impairment [23] | 2.2–4.3% | 2.6–3.4% |
9. Current Strategies to Prevent GBS Disease in Infants
10. Potential Future Preventive Strategies
10.1. Probiotics
10.2. Vaccines
10.2.1. Polysaccharide-Based Vaccines
10.2.2. Polysaccharide Conjugate Vaccines
10.2.3. Monovalent Conjugate Vaccines
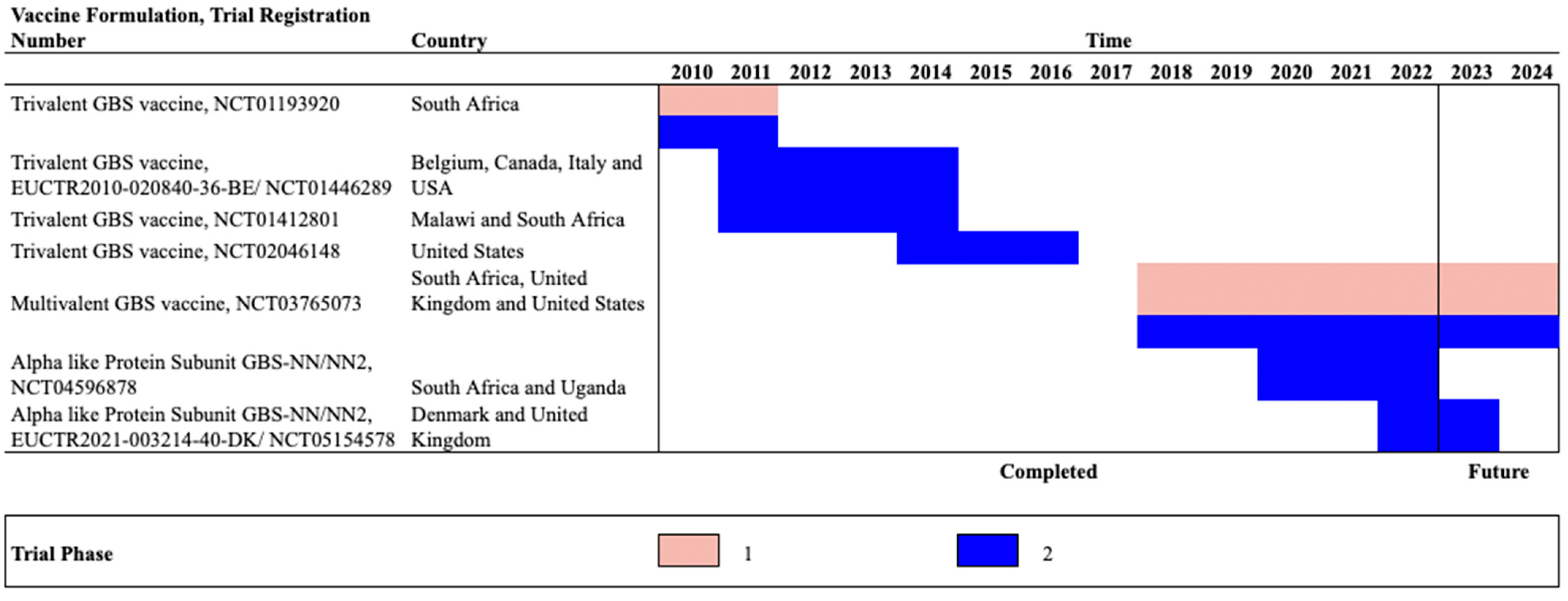
10.2.4. Multivalent Conjugate Vaccines
10.2.5. Protein-Based Vaccines
| Vaccine Formulation | Registry Number | Country/ Region | Clinical Phase (Status) | Study Duration | Age Group, Years | Gestation Weeks | Total Study Population | Population, N (Groups) | Time Points for Immunogenicity Assessment | Immunogenicity Results: Mean GMC (95% CI) | Systemic Events | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monovalent GBS III-TT conjugate vaccine | N.R. | Canada | NR | NR | 18–40 | Non-pregnant | 100 | Vaccine group: III-TT58 mg: 30 III-TT 14.5 mg: 15 III-TT 3.6 mg: 15 III CPS, 50 mg: 30 Placebo group: Saline, NA: 10 | 8 weeks | Vaccine group: III-TT 58μg: 4.53 (1.92–10.70) III-TT 14.5μg: 2.72 (0.95–7.76) III-TT 3.6μg: 1.10 (0.40–3.02) III CPS 50μg: 1.41 (0.59–3.37) Placebo group: Saline: 0.16 (0.06–0.45) | N.R. | Kasper et al., 1996 [52] |
| Monovalent GBS III-TT conjugate vaccine | N.R. | USA | Phase 1 (Completed) | NR | 18–45 | >37 | 30 | Vaccine group: 20 Placebo group: 10 | 4 weeks | Vaccine group: III-TT: >1.0 μg/mL Placebo group: Saline: 0.06 μg/mL | N.R. | Baker et al., 2003a [53] |
| Bivalent GBS conjugate | N.R. | USA | Phase 2 (Completed) | NR | 18–45 | Non-pregnant | 75 | Vaccine group: Monovalent GBS II–TT (3.6 mg of CPS) n = 25; Monovalent GBS III–TT (12.5 mg of CPS) n = 25; Bivalent GBS II–TT/III–TT n = 25 No placebo group | 8 weeks | II-TT (3.6 mg) group: IgG: 5.7 (0.7–259; 2.9–11.3) IgM: 10.9 (1.1–61.9; 7.4–16.1) IgA: 2.1 (0.2–28.1; 1.2–3.5) III-TT (12.5 mg) group: IgG: 2.0 (0.05–402; 0.7–5.8) II-TT/III-TT (3.6/12.5 mg) group: IgG: 13.1 (0.4–571; 5.6–30.6) IgM: 10.4 (0.3–81.5; 6.8–16.1) IgA: 2.1 (0.1–153; 1.1–4.1) | No serious adverse effects in any group. Mild systemic symptoms associated with low-grade fever in 2.6% of Bivalent group | Baker et al., 2003b [55] |
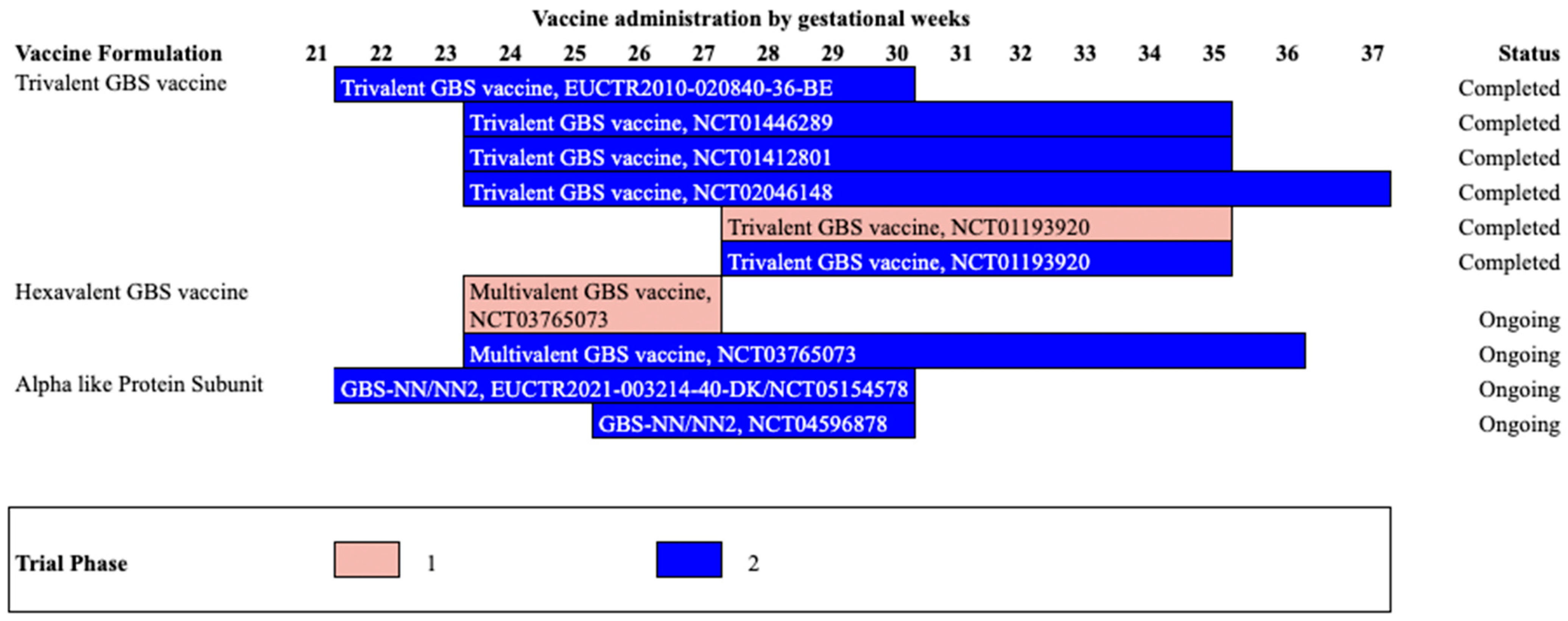
| Trivalent GBS conjugate vaccine | NCT01193920 | South Africa | Phase1b/2 (Completed) | 2010–2011 | 18–40 | 28–35 | 380 | Vaccine group (non-pregnant): GBS vaccine 20 μg n = 40 Placebo group: (non-pregnant): Placebo n = 20 Vaccine group (Pregnant) GBS vaccine 0.5 mg n= 80; GBS vaccine 2.5 μg n = 80; GBS vaccine 5.0 μg n= 80; Placebo Group (Pregnant) n= 80 | At delivery | Vaccine group: At delivery GBS vaccine 2.5 μg Serotype Ia: 5.57 (2.25–14) Serotype Ib: 2.10 (0.65–6.78) Serotype III: 0.50 (0.10–2.46) Placebo group: At delivery GBS vaccine 2.5 μg Serotype Ia: 0.21 (0.15–0.31) Serotype Ib: 0.16 (0.07–0.37) Serotype III: 0.05 (0.03–0.07) | In non-pregnant vaccine vs. placebo groups: Chills: (37.50% vs. 30%) Fatigue: (72.50% vs. 60%) Pyrexia: (15.00% vs. 0%) In pregnant participants: vaccine 0.5mg, 2.5mg,5mg vs. placebo Chills: 20.00% vs. 8.75% vs. 10% vs. 11.25%) Fatigue: (48.75% vs. 42.5% vs. 53.75% vs. 46.25%); Pyrexia: 6.25% vs. 1.25% vs. 5% vs. 2.5%) 1/80 (1.25%) death in GBS vaccine 2·5 μg group in pregnant women | Madhi et al., 2016 [57] |
| Trivalent GBS conjugate vaccine | NCT01412801 | Malawi, South Africa | Phase 2 (Completed) | 2011–2012 | 18–40 | 24–35 | 270 | GBS vaccine in: HIV-infected, low CD4 cell count >50 to ≤350 group: n = 91 HIV-infected, high CD4 cell count >350 cells per μ group: n = 89 HIV-uninfected group: 90 | 4 Weeks | Serotype Ia HIV-infected, low CD4 cell count group: 2.68 (1.74–4.10) HIV-infected, high CD4 cell count group: 3.26 (2.14–4.98) HIV-uninfected group: 6.63 (4.37–10) Serotype Ib HIV-infected, low CD4 cell count group: 2.62 (1.62–4.24 HIV-infected, high CD4 cell count group: 3.68 (2.38–5.70) HIV-uninfected group: 5.35 (3.63–7.87) Serotype III HIV-infected, low CD4 cell count group: 1.51 (0.97–2.35) HIV-infected, high CD4 cell count group: 1.31 (0.85–2.02) HIV-uninfected group: 5.35 (3.66–7.83) | any systemic reactions (chills/ nausea/ malaise/ myalgia/ arthralgia/ headache/ fatigue/ rash/ fever) in low CD4 vaccinated vs. high CD4 vaccinated vs. uninfected group: (40% vs. 55% vs. 59%) | Heyderman et al., 2016 [58] |
| Trivalent GBS conjugate vaccine | NCT01446289/ EUCTR2010-020840-36-BE | Belgium, Canada, Italy | Phase 2 (Completed) | 2011–2013 | 18–40 | 24–35 | 86 | Vaccine group: n = 49 Placebo group: n = 34 | At delivery | Vaccine group: Serotype Ia: 5.22 (3.37–8.10) Serotype Ib: 2.41 (1.48–3.94) Serotype III: 1.90 (1.15–3.12) Placebo group: Serotype Ia: 0.37 (0.22–0.63) Serotype Ib: 0.13 (0.07–0.23) Serotype III: 0.11 (0.06–0.19) | Vaccine vs. placebo group: Chills: 4/49 (8% vs. 8%) Malaise:14/49 (28% vs. 26%) Myalgia:27/49 (55% vs. 8%) Arthralgia: 4/49 (8% vs. 26%) Headache: 16/49 (32% vs. 61%) Fatigue: 31/49 (63% vs. 76%) | Donders et al., 2016 [59] |
| Trivalent GBS conjugate vaccine | NCT02046148 | USA | Phase 2 (Completed) | 2014–2015 | 18–40 | 24–35 | 75 | Vaccine group: 49 Placebo group: 26 | At delivery | Vaccine group: In mothers: sIgA- Serotype Ia: 283 (104–770) Serotype Ib: 418 (151–1155) Serotype III: 112 (44–286) IgG- Serotype Ia: 0.64 (0.29–1.42) Serotype Ib: 0.10 (0.04–0.24) Serotype III: 0.41 (0.21–0.79) Placebo group: In mothers: sIgA- Serotype Ia: 2.94 (0.89–9.68) Serotype Ib: 4.17 (1.37–13) Serotype III: 0.45 (0.13–1.58) IgG- Serotype Ia: 0.12 (0.05–0.28) Serotype Ib: 0.04 (0.02–0.11) Serotype III: 0.15 (0.07–0.30) | Vaccine vs. placebo group: Fatigue: (38% vs. 23%) Nausea: (15% vs. 12%)) Headache: (13% vs. 12%)) Loss of appetite: (13% vs. 8%) Myalgia: (4% vs. 8%) Arthralgia: (4% vs. 8%) Chills: (2% vs. 4%) Rash: (2% vs. 0%) Urticaria: (2% vs. 0%) | Swamy et al., 2020 [61] |
| Quadrivalent GBS | N.R. | USA | Phase 1 (Completed) | NR | 18–40 | Non-pregnant | 40 | Vaccine group: n = 40 Absence of placebo group | 6 weeks | Vaccine group: GMC of type-specific antibody (range) Ia: 5.2 (1–260) IIb: Not done II: 3.6 (0–72) III: 43.4 (2–496) Placebo group: No placebo group | Vaccine group: nausea, malaise, myalgia, or headache: 15% No placebo group | Kotloff et al,1996 [63] |
| Hexavalent GBS conjugate vaccine | NCT03170609 | USA | Phase 1/2 (Completed) | 2017–2018 | 18–49 | Non-pregnant | 365 | Vaccine group: GBS6 5 μg with AlPO4: n = 52; GBS6 5 μg without AlPO4: n = 52; GBS6 10 μg with AlPO4: n = 52; GBS6 10 μg without AlPO4: n = 52; GBS6 20 μg with AlPO4: n = 52; GBS6 20 μg without AlPO4: n = 52; Placebo group: n = 52 | 2 Weeks | Vaccine group: Ia: 17.829 (7.542–42.149) Ib: 1.948 (0.785–4.836) II: 31.786 (17.490–57.770) III: 3.766 (1.887–7.517) IV: 7.018 (4.518–10.903) V: 6.760 (3.230–14.146) Placebo group: Ia: 0.755 (0.372–1.536) Ib: 0.034 (0.019–0.059) II: 0.187 (0.126–0.279) III: 0.081 (0.054–0.122) IV: 0.046 (0.027–0.078) V: 0.143 (0.084–0.244) | Vaccine vs. placebo groups: fatigue or tiredness and headache: 2% vs. 2% | Absalon et al., 2021 [64] |
| Hexavalent conjugate vaccine | NCT03765073 | USA, UK, South Africa | Phase 2 (Ongoing) | 2019–2024 | 18–40 | 24–36 | 586 | N.R | N.R. | N.R. | N.R. | Pfizer [65] |
| Alpha like Protein subunit | NCT05154578/ EUCTR2021-003214-40-DK | Denmark, South Africa | Phase 2 (Ongoing) | 2022–2023 | 18–64 | 22–30 | 270 | N.R | N.R. | N.R. | N.R. | Minervax (a) [67]; Minervax (c) [66] |
| Alpha like Protein subunit | NCT04596878 | South Africa, Uganda | Phase 2 (Ongoing) | 2020–2022 | 18–40 | 26–30 | 205 | N.R | N.R. | N.R. | N.R. | Minervax (b) [68] |
11. Challenges
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanna, M.; Noor, A. Streptococcus Group B; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Morgan, J.A.; Zafar, N.; Cooper, D.B. Group B Streptococcus and Pregnancy; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Vornhagen, J.; Waldorf, K.M.A.; Rajagopal, L. Perinatal group B streptococcal infections: Virulence factors, immunity, and prevention strategies. Trends Microbiol. 2017, 25, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, A.; Furuta, A.; Dacanay, M.; Rajagopal, L.; Adams Waldorf, K.M. Bacterial and host determinants of group B streptococcal vaginal colonization and ascending infection in pregnancy. Front. Cell. Infect. Microbiol. 2021, 11, 720789. [Google Scholar] [CrossRef] [PubMed]
- Berner, R. Group B streptococcus vaccines: One step further. Lancet Infect. Dis. 2020, 21, 158–160. [Google Scholar] [CrossRef]
- Davies, H.G.; Carreras-Abad, C.; Le Doare, K.; Heath, P.T. Group B Streptococcus: Trials and Tribulations. Pediatr. Infect. Dis. J. 2019, 38, S72–S76. [Google Scholar] [CrossRef]
- Madrid, L.; Seale, A.C.; Kohli-Lynch, M.; Edmond, K.M.; Lawn, J.E.; Heath, P.T.; Madhi, S.A.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Infant group B streptococcal disease incidence and serotypes worldwide: Systematic review and meta-analyses. Clin. Infect. Dis. 2017, 65, S160–S172. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Smith, B.; George, C.; McMullan, B.; Kesson, A.; Lahra, M.M.; Palasanthiran, P. Epidemiology of Late and Very Late Onset Group B Streptococcal Disease. Pediatr. Infect. Dis. J. 2017, 36, 20–24. [Google Scholar] [CrossRef]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-onset neonatal sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef]
- Costa, N.S.; Oliveira, L.M.A.; Meštrović, T.; Obiero, C.W.; Lee, S.S.; Pinto, T.C.A. The urgent need to recognize and properly address prenatal-onset group B Streptococcus disease. Int. J. Infect. Dis. 2022, 124, 168–170. [Google Scholar] [CrossRef]
- Morinis, J.; Shah, J.; Murthy, P.; Fulford, M. Horizontal transmission of group B streptococcus in a neonatal intensive care unit. Paediatr. Child Health 2011, 16, e48–e50. [Google Scholar] [CrossRef]
- Berardi, A.; Trevisani, V.; Di Caprio, A.; Bua, J.; China, M.; Perrone, B.; Pagano, R.; Lucaccioni, L.; Fanaro, S.; Iughetti, L.; et al. Understanding Factors in Group B Streptococcus Late-Onset Disease. Infect. Drug Resist. 2021, 14, 3207–3218. [Google Scholar] [CrossRef]
- Cools, P.; van de Wijgert, J.H.; Jespers, V.; Crucitti, T.; Sanders, E.J.; Verstraelen, H.; Vaneechoutte, M. Role of HIV exposure and infection in relation to neonatal GBS disease and rectovaginal GBS carriage: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 13820. [Google Scholar] [CrossRef]
- Maisey, H.C.; Doran, K.S.; Nizet, V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 2008, 10, E27. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, A.; Rinaudo, C.D.; Maione, D. Group B Streptococcus vaccine: State of the art. Ther. Adv. Vaccines 2015, 3, 76–90. [Google Scholar] [CrossRef]
- Teatero, S.; Neemuchwala, A.; Yang, K.; Gomes, J.; Athey, T.B.T.; Martin, I.; Demczuk, W.; McGeer, A.; Fittipaldi, N. Genetic evidence for a novel variant of the pilus island 1 backbone protein in group B Streptococcus. J. Med. Microbiol. 2017, 66, 1409–1415. [Google Scholar] [CrossRef]
- Cozzi, R.; Malito, E.; Lazzarin, M.; Nuccitelli, A.; Castagnetti, A.; Bottomley, M.J.; Margarit, I.; Maione, D.; Rinaudo, C.D. Structure and Assembly of Group B Streptococcus Pilus 2b Backbone Protein. PLoS ONE 2015, 10, e0125875. [Google Scholar] [CrossRef] [PubMed]
- Shabayek, S.; Spellerberg, B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.-Q.; Thoon, K.C.; Tee, W.S.N.; Ang, M.L.T.; Tan, N.W.H.; Yeo, K.T.; Li, J.; Chong, C.Y. Serotype distribution and incidence of invasive early onset and late onset group B streptococcal disease amongst infants in Singapore. BMC Infect. Dis. 2021, 21, 1221. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.; Barkham, T.; Kyaw, W.M.; Ho, H.J.; Chan, M. Characterisation of bone and joint infections due to Group B Streptococcus serotype III sequence type 283. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Group B Streptococcus Vaccine: Full Value of Vaccine Assessment; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kohli-Lynch, M.; Russell, N.J.; Seale, A.C.; Dangor, Z.; Tann, C.J.; Baker, C.J.; Bartlett, L.; Cutland, C.; Gravett, M.G.; Heath, P.T.; et al. Neurodevelopmental Impairment in Children After Group B Streptococcal Disease Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S190–S199. [Google Scholar] [CrossRef] [PubMed]
- Horváth-Puhó, E.; van Kassel, M.N.; Gonçalves, B.P.; de Gier, B.; Procter, S.R.; Paul, P.; van der Ende, A.; Søgaard, K.K.; Hahné, S.J.; Chandna, J.; et al. Mortality, neurodevelopmental impairments, and economic outcomes after invasive group B streptococcal disease in early infancy in Denmark and the Netherlands: A national matched cohort study. Lancet Child Adolesc. Health 2021, 5, 398–407. [Google Scholar] [CrossRef]
- Baltimore, R.S. Consequences of Prophylaxis for Group B Streptococcal Infections of the Neonate. Semin. Perinatol. 2007, 31, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Money, D.; Allen, V.M.; Yudin, M.H.; Bouchard, C.; Boucher, M.; Caddy, S.; Castillo, E.; Murphy, K.E.; Ogilvie, G.; Paquet, C.; et al. The Prevention of Early-Onset Neonatal Group B Streptococcal Disease. J. Obstet. Gynaecol. Can. 2013, 35, 939–948. [Google Scholar] [CrossRef] [PubMed]
- ACOG. ACOG Prevention of Group B Streptococcal Early-Onset Disease in Newborns. 2020. Available online: https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns (accessed on 22 December 2022).
- Patras, K.A.; Nizet, V. Group B streptococcal maternal colonization and neonatal disease: Molecular mechanisms and preventative approaches. Front. Pediatr. 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Souza da Cunha, S.; Santorelli, G.; Pearce, N.; Wright, J.; Oddie, S.; Petherick, E.; Pembrey, L. Evidence for causal associations between prenatal and postnatal antibiotic exposure and asthma in children, England. Clin. Exp. Allergy 2021, 51, 1438–1448. [Google Scholar] [CrossRef]
- Delara, M.; McMillan, D.E.; Nickel, N.C.; Jong, G.W.; Seitz, D.P.; Mignone, J. Early life exposure to antibiotics and the risk of mood and anxiety disorders in children and adolescents: A population-based cohort study. J. Psychiatr. Res. 2021, 137, 621–633. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhou, Y.-Y.; Pan, L.-Y.; Zhang, X.; Jiang, H.-Y. Early Life Antibiotic Exposure and the Subsequent Risk of Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. J. Autism Dev. Disord. 2021, 52, 2236–2246. [Google Scholar] [CrossRef]
- Hanson, L.; Vusse, L.V.; Duster, M.; Warrack, S.; Safdar, N. Feasibility of Oral Prenatal Probiotics against Maternal Group B Streptococcus Vaginal and Rectal Colonization. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 294–304. [Google Scholar] [CrossRef]
- Farr, A.; Sustr, V.; Kiss, H.; Rosicky, I.; Graf, A.; Makristathis, A.; Foessleitner, P.; Petricevic, L. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci. Rep. 2020, 10, 19745. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.-Y.; Chang, W.-C.; Lin, H.-C.; Wang, M.-H.; Lin, W.-C.; Chiu, T.-H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef]
- Olsen, P.; Williamson, M.; Traynor, V.; Georgiou, C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 2018, 31, 31–37. [Google Scholar] [CrossRef]
- Sharpe, M.; Shah, V.; Freire-Lizama, T.; Cates, E.C.; McGrath, K.; David, I.; Cowan, S.; Letkeman, J.; Stewart-Wilson, E. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: A midwifery-led double-blind randomized controlled pilot trial. J. Matern. Neonatal Med. 2021, 34, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N. Effects of Oral Probiotic Supplementation on Group B Strep (GBS) Rectovaginal Colonization in Pregnancy. 2018. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01479478 (accessed on 18 July 2022).
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Pham, M.; Lemberg, D.A.; Day, A.S. Probiotics: Sorting the evidence from the myths. Med. J. Aust. 2008, 188, 304–308. [Google Scholar] [CrossRef]
- Butler, M. Oral Probiotic Supplementation in Pregnancy to Reduce Group B Streptococcus Colonization (OPSiP). Available online: https://clinicaltrials.gov/ct2/show/NCT03407157 (accessed on 18 July 2022).
- Nardini, K.A. Group B Streptococcus Response after Probiotic Exposure (GRAPE). Available online: https://clinicaltrials.gov/ct2/show/NCT04721912 (accessed on 18 July 2022).
- Sharpe, M. Effect of Probiotics on GBS Colonization Status During Pregnancy: A Pilot Randomized Controlled Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02528981 (accessed on 18 July 2022).
- Baker, C.J.; Kasper, D.L. Correlation of Maternal Antibody Deficiency with Susceptibility to Neonatal Group B Streptococcal Infection. N. Engl. J. Med. 1976, 294, 753–756. [Google Scholar] [CrossRef]
- Lin, F.C.; Philips, J.B., III; Azimi, P.H.; Weisman, L.E.; Clark, P.; Rhoads, G.G.; Regan, J.; Concepcion, N.F.; Frasch, C.E.; Troendle, J.; et al. Level of maternal antibody required to protect neonates against early-onset disease caused by group B Streptococcus type Ia: A multicenter, seroepidemiology study. J. Infect. Dis. 2001, 184, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.C.; Weisman, L.E.; Azimi, P.H.; Iii, J.B.P.; Clark, P.; Regan, J.; Rhoads, G.G.; Frasch, C.E.; Gray, B.M.; Troendle, J.; et al. Level of Maternal IgG Anti–Group B Streptococcus Type III Antibody Correlated with Protection of Neonates against Early-Onset Disease Caused by This Pathogen. J. Infect. Dis. 2004, 190, 928–934. [Google Scholar] [CrossRef]
- Baker, C.J.; Carey, V.J.; Rench, M.A.; Edwards, M.S.; Hillier, S.L.; Kasper, D.L.; Platt, R. Maternal Antibody at Delivery Protects Neonates from Early Onset Group B Streptococcal Disease. J. Infect. Dis. 2014, 209, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Kasper, D.L. Group B streptococcal vaccines. Rev. Infect. Dis. 1985, 7, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Rench, M.A.; Edwards, M.S.; Carpenter, R.J.; Hays, B.M.; Kasper, D.L. Immunization of Pregnant Women with a Polysaccharide Vaccine of Group B Streptococcus. N. Engl. J. Med. 1988, 319, 1180–1185. [Google Scholar] [CrossRef]
- Gaultier, G.N.; McCready, W.; Ulanova, M. The effect of pneumococcal immunization on total and antigen-specific B cells in patients with severe chronic kidney disease. BMC Immunol. 2019, 20, 41. [Google Scholar] [CrossRef]
- Cruz, S.C.; Souza, S.L.; Cruz, A.C.; Silva, G.P.; Milagres, L.G. Human antibody and memory B and T-cell responses after primary and booster immunisation against Neisseria meningitidis B. Vaccine 2011, 29, 7387–7394. [Google Scholar] [CrossRef]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef]
- Avci, F.Y.; Kasper, D.L. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.L.; Paoletti, L.C.; Wessels, M.R.; Guttormsen, H.K.; Carey, V.J.; Jennings, H.J.; Baker, C.J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J. Clin. Investig. 1996, 98, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Rench, M.A.; McInnes, P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine 2003, 21, 3468–3472. [Google Scholar] [CrossRef]
- Baker, C.J.; Paoletti, L.C.; Rench, M.A.; Guttormsen, H.; Edwards, M.S.; Kasper, D.L. Immune Response of Healthy Women to 2 Different Group B Streptococcal Type V Capsular Polysaccharide–Protein Conjugate Vaccines. J. Infect. Dis. 2004, 189, 1103–1112. [Google Scholar] [CrossRef]
- Baker, C.J.; Rench, M.A.; Fernandez, M.; Paoletti, L.C.; Kasper, D.L.; Edwards, M.S. Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J. Infect. Dis. 2003, 188, 66–73. [Google Scholar] [CrossRef]
- Madhi, S.A.; Koen, A.; Cutland, C.L.; Jose, L.; Govender, N.; Wittke, F.; Olugbosi, M.; Sobanjo-ter Meulen, A.; Baker, S.; Dull, P.M.; et al. Antibody kinetics and response to routine vaccinations in infants born to women who received an investigational trivalent group B Streptococcus polysaccharide CRM197-conjugate vaccine during pregnancy. Clin. Infect. Dis. 2017, 65, 1897–1904. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Jose, L.; Koen, A.; Govender, N.; Wittke, F.; Olugbosi, M.; Meulen, A.S.-T.; Baker, S.; Dull, P.M.; et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: A randomised phase 1b/2 trial. Lancet Infect. Dis. 2016, 16, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Heyderman, R.S.; Madhi, S.A.; French, N.; Cutland, C.; Ngwira, B.; Kayambo, D.; Mboizi, R.; Koen, A.; Jose, L.; Olugbosi, M.; et al. Group B streptococcus vaccination in pregnant women with or without HIV in Africa: A non-randomised phase 2, open-label, multicentre trial. Lancet Infect. Dis. 2016, 16, 546–555. [Google Scholar] [CrossRef]
- Donders, G.G.; Halperin, S.A.; Devlieger, R.; Baker, S.; Forte, P.; Wittke, F.; Slobod, K.S.; Dull, P.M. Maternal immunization with an investigational trivalent group B streptococcal vaccine: A randomized controlled trial. Obstet. Gynecol. 2016, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.; Rigat, F.; Tuscano, G.; Chiarot, E.; Donders, G.; Devlieger, R.; Filippini, S.; Frigimelica, E.; Forte, P.; Wittke, F.; et al. Functional activity of maternal and cord antibodies elicited by an investigational group B Streptococcus trivalent glycoconjugate vaccine in pregnant women. J. Infect. 2018, 76, 449–456. [Google Scholar] [CrossRef]
- Swamy, G.K.; Metz, T.D.; Edwards, K.M.; Soper, D.E.; Beigi, R.H.; Campbell, J.D.; Grassano, L.; Buffi, G.; Dreisbach, A.; Margarit, I.; et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in pregnant women and their infants: Results from a randomized placebo-controlled phase II trial. Vaccine 2020, 38, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Novartis Vaccines and Diagnostics GmbH. A Phase II Randomized, Observer-Blind, Multi-Center, Controlled Study of a Trivalent group B Streptococcus Vaccine in Healthy Pregnant Women. 2013. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-020840-36/BE (accessed on 9 February 2022).
- Kotloff, K.L.; Fattom, A.; Basham, L.; Hawwari, A.; Harkonen, S.; Edelman, R. Safety and immunogenicity of a tetravalent group B streptococcal polysaccharide vaccine in healthy adults. Vaccine 1996, 14, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Absalon, J.; Segall, N.; Block, S.L.; Center, K.J.; Scully, I.L.; Giardina, P.C.; Peterson, J.; Watson, W.J.; Gruber, W.C.; Jansen, K.U.; et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: A phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect. Dis. 2021, 21, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Trial to Evaluate the Safety, Tolerability, and Immunogenicity of a Multivalent Group B Streptococcus Vaccine in Healthy Nonpregnant Women and Pregnant Women and Their Infants. Available online: https://clinicaltrials.gov/ct2/show/NCT03765073 (accessed on 9 February 2022).
- Minervax. A Multicentre, Multinational, Parallel Group, Observer-Blind, Randomised, Placebo-Controlled Study on the Group B Streptococcus Vaccine (Gbs-Nn/Nn2), Investigating the Immunogenicity and Safety of Four Vaccination Regimens in Pregnant Woman, Assessing Igg Specific to Alpn Proteins in Cord Blood and Maternal Blood, and the Safety Profile in Mother and Infant up to 6 Months Post-Delivery. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-003214-40/DK (accessed on 9 February 2022).
- Minervax. Immunogenicity and Safety of GBS-NN/NN2 in Pregnant Women. Available online: https://clinicaltrials.gov/ct2/show/NCT05154578 (accessed on 9 February 2022).
- Minervax. Study of a Group B Streptococcus Vaccine in Pregnant Women Living with HIV and in Pregnant Women Who Do Not Have HIV. Available online: https://clinicaltrials.gov/ct2/show/NCT04596878 (accessed on 9 February 2022).
- Vekemans, J.; Crofts, J.; Baker, C.J.; Goldblatt, D.; Heath, P.T.; Madhi, S.A.; Le Doare, K.; Andrews, N.; Pollard, A.J.; Saha, S.K.; et al. The role of immune correlates of protection on the pathway to licensure, policy decision and use of group B Streptococcus vaccines for maternal immunization: Considerations from World Health Organization consultations. Vaccine 2019, 37, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Isbrucker, R.; Andrews, N.; Goldblatt, D.; Heath, P.T.; Izu, A.; Madhi, S.A.; Moulton, L.; Schrag, S.J.; Shang, N.; et al. Methodology for a correlate of protection for group B Streptococcus: Report from the Bill & Melinda Gates Foundation workshop held on 10 and 11 February 2021. Vaccine 2022, 40, 4283–4291. [Google Scholar]
- Carreras-Abad, C.; Ramkhelawon, L.; Heath, P.T.; Le Doare, K. A vaccine against group B Streptococcus: Recent advances. Infect. Drug Resist. 2020, 13, 1263. [Google Scholar] [CrossRef]
- Delara, M.; Sadarangani, M. Immunization in pregnancy to protect pregnant people and their newborns against COVID-19. Expert Rev. Vaccines 2022, 21, 593–595. [Google Scholar] [CrossRef]
- Camurus, A.B. A Randomized, Multi-Center, Open-Label, Active-Controlled Phase 3 Trial to Assess the Efficacy and Safety of Octreotide Subcutaneous Depot (Cam2029) Versus Octreotide Lar or Lanreotide Atg in Patie. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2021-000849-40 (accessed on 9 February 2022).
- Janssens, E.; Flamaing, J.; Vandermeulen, C.; Peetermans, W.E.; Desmet, S.; De Munter, P. The 20-valent pneumococcal conjugate vaccine (PCV20): Expected added value. Acta Clin. Belg. 2022, 78, 78–86. [Google Scholar] [CrossRef]
- Bellais, S.; Six, A.; Fouet, A.; Longo, M.; Dmytruk, N.; Glaser, P.; Trieu-Cuot, P.; Poyart, C. Capsular Switching in Group B Streptococcus CC17 Hypervirulent Clone: A Future Challenge for Polysaccharide Vaccine Development. J. Infect. Dis. 2012, 206, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Malley, R.; Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011, 378, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Orije, M.R.P.; Van Damme, P.; Leuridan, E. Vaccination during pregnancy: Current and possible future recommendations. Eur. J. Pediatr. 2020, 179, 235–242. [Google Scholar] [CrossRef]
- Sadarangani, M.; Soe, P.; Shulha, H.P.; Valiquette, L.; Vanderkooi, O.G.; Kellner, J.D.; Muller, M.P.; Top, K.A.; Isenor, J.E.; McGeer, A.; et al. Safety of COVID-19 vaccines in pregnancy: A Canadian National Vaccine Safety (CANVAS) network cohort study. Lancet Infect. Dis. 2022, 22, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 32, 7057–7064. [Google Scholar] [CrossRef]
- Edmond, K.M.; Kortsalioudaki, C.; Scott, S.; Schrag, S.J.; Zaidi, A.K.; Cousens, S.; Heath, P.T. Group B streptococcal disease in infants aged younger than 3 months: Systematic review and meta-analysis. Lancet 2012, 379, 547–556. [Google Scholar] [CrossRef]
- Dauby, N.; Chamekh, M.; Melin, P.; Slogrove, A.L.; Goetghebuer, T. Increased risk of group B streptococcus invasive infection in HIV-exposed but uninfected infants: A review of the evidence and possible mechanisms. Front. Immunol. 2016, 7, 505. [Google Scholar] [CrossRef]
- Eaton, J.W.; Rehle, T.M.; Jooste, S.; Nkambule, R.; Kim, A.A.; Mahy, M.; Hallett, T.B. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: Implications for HIV estimates. AIDS Lond. Engl. 2014, 28, S507. [Google Scholar] [CrossRef]
- Kharsany, A.B.M.; Frohlich, J.A.; Yende-Zuma, N.; Mahlase, G.; Samsunder, N.; Dellar, R.C.; Zuma-Mkhonza, M.; Karim, S.S.A.; Karim, Q.A. Trends in HIV Prevalence in Pregnant Women in Rural South Africa. Am. J. Ther. 2015, 70, 289–295. [Google Scholar] [CrossRef]
- Borrow, R.; Dagan, R.; Zepp, F.; Hallander, H.; Poolman, J. Glycoconjugate vaccines and immune interactions, and implications for vaccination schedules. Expert Rev. Vaccines 2011, 10, 1621–1631. [Google Scholar] [CrossRef]
- White, A.; Madhi, S.A. Ethical considerations for designing GBS maternal vaccine efficacy trials in low-middle income countries. Vaccine 2015, 33, 6396–6400. [Google Scholar] [CrossRef] [PubMed]
- Grenham, A.; Villafana, T. Vaccine development and trials in low and lower-middle income countries: Key issues, advances and future opportunities. Hum. Vaccines Immunother. 2017, 13, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Puthavathana, P.; Nghiem, N.M.; van Doorn, H.R.; Nguyen, T.V.; Pham, H.V.; Subekti, D.; Harun, S.; Malik, S.; Robinson, J.; et al. Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN). PLoS Med. 2010, 7, e1000231. [Google Scholar] [CrossRef] [PubMed]
| Study (NCT Registration) | Country/ Region | Study Design | Study Population, N | Gestational Age (Weeks) | Mean Age in Years (SD) | Therapy | Treatment Course | Efficacy | Preterm Birth | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/total (%) | p value | n/total (%) | p value | ||||||||
| Farr et al., 2020 (NCT03008421) [32] | Austria | Randomized clinical trial | 82 | 33–37 | Probiotic:38.5 ± 1.4; Placebo: 38.6 ± 1.2 | A dietary probiotic supplement (containing L. jensenii, L. crispatus, L. rhamnosus, and L.gasseri ) or placebo | Twice daily | GBS positivity: Probiotic = 12/33 (63.6%); Placebo = 6/27 (77.8%) | 0.24 | Probiotic = 4 (9.8%); Placebo = 1 (2.4%) | 0.20 |
| Olsen et al., 2018 [34] | Australia | Randomized clinical trial | 34 | 36 | Probiotic:32 ± 4; Standard: 30 ± 4.3 | A daily oral dose of probiotics (containing L. rhamnosus and L. fermentum/reuteir); Placebo arm: Standard antenatal care | Once daily for 3 weeks | GBS negativity: Probiotic = 4/19 (21.1%); Standard of Care = 3/21 23.1%) | 0.7 | N/A | |
| Hanson et al., 2014 [31] | US | Open label, two group quasi experiment | 20 | 28, 32, 36 | Probiotic:25.8 ± 3.8; Control:25.9 ± 5.1 | oral probiotic (containing L. acidophillus, B. lactis, and B. longum) or placebo | Once daily | GBS positivity: Probiotic= 2/10 (20%); Standard of Care = 3/10 (20%) | >0.05 | N/A | |
| Sharpe et al., 2021 [35] | Canada | Double blind randomized control pilot trial | 139 | 35–37 | Probiotic:32.04; Placebo:32.47 | Two capsules of probiotics (L. rhamnosus and L. reuteri) or placebo | Twice daily | GBS positivity: Probiotic = 9/57(15.8%); Placebo = 12/56 (21.4%) | 0.48 | N/A | |
| Ho et al., 2016 (NCT03688321) [33] | Taiwan | Prospective, double-blind randomized clinical trial | 110 | 35–37 | Probiotic:32.0 ± 4.0; Placebo:32.0 ± 3.7 | Two probiotic capsules (containing L. rhamnosus and L. reuteri) before bedtime or placebo | Once daily | GBS negativity: Probiotic = 21/49 (42.9%); Placebo = 9/50 (18%) | 0.007 | N/A | |
| Aziz 2017 (NCT01479478) [36] | US | Randomized clinical trial | 251 | 35–37 | Probiotic:31.4 ± 5.7; Placebo; 31.9 ± 5.3 | Probiotic dietary supplement (containing L. rhamnosus and L. reuteri) or placebo | Once daily | GBS positivity: Probiotic= 20/108 (18.5%); Placebo= 23/117 (19.7%) | 0.87 | N\A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delara, M.; Vadlamudi, N.K.; Sadarangani, M. Strategies to Prevent Early and Late-Onset Group B Streptococcal Infection via Interventions in Pregnancy. Pathogens 2023, 12, 229. https://doi.org/10.3390/pathogens12020229
Delara M, Vadlamudi NK, Sadarangani M. Strategies to Prevent Early and Late-Onset Group B Streptococcal Infection via Interventions in Pregnancy. Pathogens. 2023; 12(2):229. https://doi.org/10.3390/pathogens12020229
Chicago/Turabian StyleDelara, Mahin, Nirma Khatri Vadlamudi, and Manish Sadarangani. 2023. "Strategies to Prevent Early and Late-Onset Group B Streptococcal Infection via Interventions in Pregnancy" Pathogens 12, no. 2: 229. https://doi.org/10.3390/pathogens12020229
APA StyleDelara, M., Vadlamudi, N. K., & Sadarangani, M. (2023). Strategies to Prevent Early and Late-Onset Group B Streptococcal Infection via Interventions in Pregnancy. Pathogens, 12(2), 229. https://doi.org/10.3390/pathogens12020229





