The Diverse Pathogenicity of Various Babesia Parasite Species That Infect Dogs
Abstract
1. Introduction
2. Comparing the Clinical Presentations Babesia Infections in Dogs Have in Common
3. Contrasting Features of the Disease Caused by Babesia Species
3.1. Mortality
3.2. Systemic Inflammatory Response Syndrome (SIRS) and Multiple Organ Dysfunction Syndrome (MODS)
3.3. Organ Pathology
3.3.1. Brain Pathology
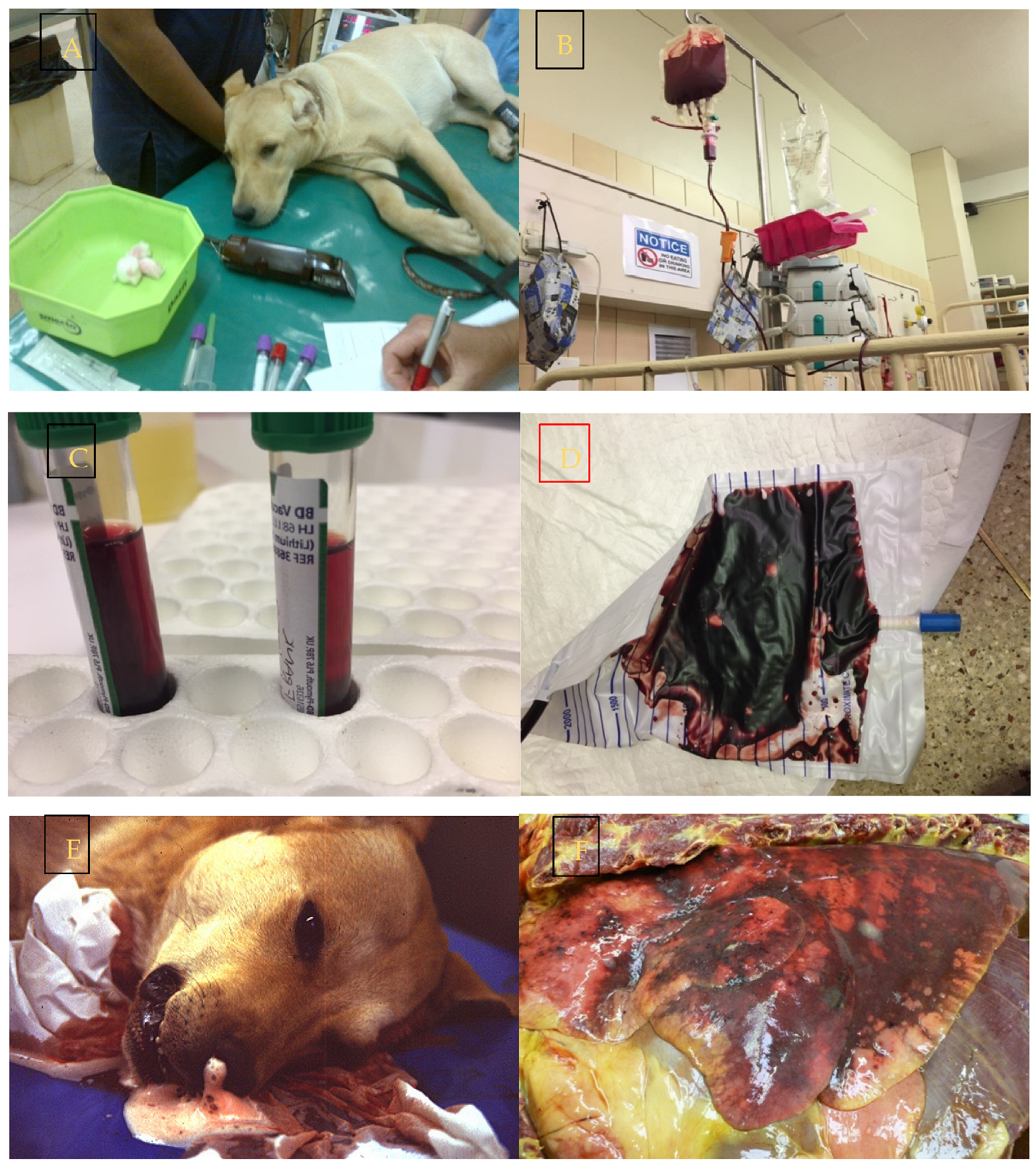

3.3.2. Renal Pathology
3.3.3. Liver Pathology
3.3.4. Lung Pathology
3.3.5. Pancreatic Pathology
3.3.6. Coagulopathy
3.3.7. Systemic Inflammation
3.3.8. Macropathology, Histopathology, and Immunohistochemistry
3.4. Serum Biochemistry Markers of Disease Severity
3.5. Endocrine Markers of Disease Severity
3.6. Future Directions
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collett, M.G. Survey of canine babesiosis in South Africa. J. S. Afr. Vet. Assoc. 2000, 71, 180–186. [Google Scholar] [CrossRef][Green Version]
- Nevils, M.A.; Figueroa, J.V.; Turk, J.R.; Canto, G.J.; Le, V.; Ellersieck, M.R.; Carson, C.A. Cloned lines of Babesia bovis differ in their ability to induce cerebral babesiosis in cattle. Parasitol. Res. 2000, 86, 437–443. [Google Scholar] [CrossRef]
- Rapsang, A.G.; Shyam, D.C. Scoring systems in the intensive care unit: A compendium. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2014, 18, 220. [Google Scholar] [CrossRef]
- Ruys, L.J.; Gunning, M.; Teske, E.; Robben, J.H.; Sigrist, N.E. Evaluation of a veterinary triage list modified from a human five-point triage system in 485 dogs and cats. J. Vet. Emerg. Crit. Care 2012, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Mathews, K.; Kruth, S.; Doig, G.; Dewey, C. Illness severity scores in veterinary medicine: What can we learn? J. Vet. Intern. Med. 2010, 24, 457–466. [Google Scholar] [CrossRef]
- Goggs, R.; Dennis, S.; Di Bella, A.; Humm, K.R.; McLauchlan, G.; Mooney, C.; Ridyard, A.; Tappin, S.; Walker, D.; Warman, S. Predicting outcome in dogs with primary immune-mediated hemolytic anemia: Results of a multicenter case registry. J. Vet. Intern. Med. 2015, 29, 1603–1610. [Google Scholar] [CrossRef]
- Leisewitz, A.L.; Goddard, A.; Clift, S.; Thompson, P.N.; de Gier, J.; Van Engelshoven, J.; Schoeman, J.P. A clinical and pathological description of 320 cases of naturally acquired Babesia rossi infection in dogs. Vet. Parasitol. 2019, 271, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mathe, A.; Voros, K.; Papp, L.; Reiczigel, J. Clinical manifestations of canine babesiosis in Hungary (63 cases). Acta Vet. Hung. 2006, 54, 367–385. [Google Scholar] [CrossRef]
- Conrad, P.; Thomford, J.; Yamane, I.; Whiting, J.; Bosma, L.; Uno, T.; Holshuh, H.J.; Shelly, S. Hemolytic anemia caused by Babesia gibsoni infection in dogs. J. Am. Vet. Med. Assoc. 1991, 199, 601–605. [Google Scholar] [PubMed]
- Kjemtrup, A.M.; Conrad, P.A. A review of the small canine piroplasms from California: Babesia conradae in the literature. Vet. Parasitol. 2006, 138, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, W.D.; Immelman, A.; Haupt, W.H.; Walzl, H.J. The diagnosis and treatment of acid-base deranged dogs infected with Babesia canis. J. S. Afr. Vet. Assoc. 1976, 47, 29–33. [Google Scholar]
- Malherbe, W.D. A Clinico-Pathological Study of Babesia canis Infection in Dogs; University of Pretoria: Pretoria, South Africa, 1968. [Google Scholar]
- Malherbe, W.D.; Parkin, B.S. Atypical symptomatology in Babesia canis infection. J. S. Afr. Vet. Assoc. 1951, 22, 25–36. [Google Scholar]
- Uilenberg, G.; Franssen, F.F.; Perie, N.M.; Spanjer, A.A. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet. Q. 1989, 11, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Carret, C.; Walas, F.; Carcy, B.; Grande, N.; Precigout, E.; Moubri, K.; Schetters, T.P.; Gorenflot, A. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: Differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J. Eukaryot Microbiol. 1999, 46, 298–303. [Google Scholar] [CrossRef]
- Maegraith, B.; Gilles, H.M.; Devakul, K. Pathological processes in Babesia canis infections. Z. Fur Tropenmedizin Und Parasitol. 1957, 8, 485–514. [Google Scholar]
- Baneth, G.; Cardoso, L.; Brilhante-Simões, P.; Schnittger, L. Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasites Vectors 2019, 12, 129. [Google Scholar] [CrossRef]
- Barash, N.R.; Thomas, B.; Birkenheuer, A.J.; Breitschwerdt, E.B.; Lemler, E.; Qurollo, B.A. Prevalence of Babesia spp. and clinical characteristics of Babesia vulpes infections in North American dogs. J. Vet. Intern. Med. 2019, 33, 2075–2081. [Google Scholar] [CrossRef]
- Camacho, A.T.; Guitian, F.J.; Pallas, E.; Gestal, J.J.; Olmeda, A.S.; Goethert, H.K.; III, S.R.T.; Spielman, A. Azotemia and mortality among Babesia microti-like infected dogs. J. Vet. Intern. Med. 2004, 18, 141–146. [Google Scholar]
- Miró, G.; Checa, R.; Paparini, A.; Ortega, N.; González-Fraga, J.L.; Gofton, A.; Bartolomé, A.; Montoya, A.; Gálvez, R.; Mayo, P.P. Theileria annae (syn. Babesia microti-like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: Clinical report of 75 cases. Parasites Vectors 2015, 8, 217. [Google Scholar] [CrossRef]
- Simões, P.B.; Cardoso, L.; Araújo, M.; Yisaschar-Mekuzas, Y.; Baneth, G. Babesiosis due to the canine Babesia microti-like small piroplasm in dogs-first report from Portugal and possible vertical transmission. Parasites Vectors 2011, 4, 50. [Google Scholar] [CrossRef]
- Zahler, M.; Rinder, H.; Schein, E.; Gothe, R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 2000, 89, 241–248. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Neel, J.; Ruslander, D.; Levy, M.; Breitschwerdt, E. Detection and molecular characterization of a novel large Babesia species in a dog. Vet. Parasitol. 2004, 124, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Penzhorn, B.L. Don’t let sleeping dogs lie: Unravelling the identity and taxonomy of Babesia canis, Babesia rossi and Babesia vogeli. Parasites Vectors 2020, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J.; Buch, J.; Beall, M.J.; Braff, J.; Chandrashekar, R. Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100471. [Google Scholar] [CrossRef] [PubMed]
- Dear, J.D.; Birkenheuer, A. Babesia in North America: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1193–1209. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, A.C.; Foley, P.M.; Clancey, N.P. Babesia vulpes in a dog from Prince Edward Island, Canada. Can. Vet. J. 2022, 63, 589. [Google Scholar]
- Radyuk, E.; Karan, L. A case of Babesia vulpes infection in a dog in Russia. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100467. [Google Scholar] [CrossRef]
- Morters, M.K.; Archer, J.; Ma, D.; Matthee, O.; Goddard, A.; Leisewitz, A.L.; Matjila, P.T.; Wood, J.L.N.; Schoeman, J.P. Long-term follow-up of owned, free-roaming dogs in South Africa naturally exposed to Babesia rossi. Int. J. Parasitol. 2020, 50, 103–110. [Google Scholar] [CrossRef]
- Weingart, C.; Helm, C.S.; Müller, E.; Schäfer, I.; Skrodzki, M.; von Samson-Himmelstjerna, G.; Krücken, J.; Kohn, B. Autochthonous Babesia canis infections in 49 dogs in Germany. J. Vet. Intern. Med. 2023, 37, 140–149. [Google Scholar] [CrossRef]
- Liu, P.-C.; Lin, C.-N.; Su, B.-L. Clinical characteristics of naturally Babesia gibsoni infected dogs: A study of 60 dogs. Vet. Parasitol. Reg. Stud. Rep. 2022, 28, 100675. [Google Scholar] [CrossRef]
- Botros, B.A.; Moch, R.W.; Barsoum, I.S. Some observations on experimentally induced infection of dogs with Babesia gibsoni. Am. J. Vet. Res. 1975, 36, 293–296. [Google Scholar] [PubMed]
- Birkenheuer, A.J.; Levy, M.G.; Savary, K.C.; Gager, R.B.; Breitschwerdt, E.B. Babesia gibsoni infections in dogs from North Carolina. J. Am. Anim. Hosp. Assoc. 1999, 35, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Macintire, D.K.; Boudreaux, M.K.; West, G.D.; Bourne, C.; Wright, J.C.; Conrad, P.A. Babesia gibsoni infection among dogs in the southeastern United States. J. Am. Vet. Med. Assoc. 2002, 220, 325–329. [Google Scholar] [CrossRef]
- Dear, J.D.; Owens, S.D.; Lindsay, L.L.; Biondo, A.W.; Chomel, B.B.; Marcondes, M.; Sykes, J.E. Babesia conradae infection in coyote hunting dogs infected with multiple blood-borne pathogens. J. Vet. Intern. Med. 2018, 32, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Rojas, D.; Montenegro, V.; Gutiérrez, R.; Yasur-Landau, D.; Baneth, G. Vector-borne pathogens in dogs from Costa Rica: First molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet. Parasitol. 2014, 199, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Di Cataldo, S.; Ulloa-Contreras, C.; Cevidanes, A.; Hernandez, C.; Millan, J. Babesia vogeli in dogs in Chile. Transbound. Emerg. Dis. 2020, 67, 2296–2299. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Trotta, M.; Carli, E.; Carcy, B.; Caldin, M.; Furlanello, T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Parasitol. 2008, 157, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.S. The South African form of severe and complicated canine babesiosis: Clinical advances 1994–2004. Vet. Parasitol. 2006, 138, 126–139. [Google Scholar] [CrossRef]
- Matijatko, V.; Mrljak, V.; Kis, I.; Kucer, N.; Forsek, J.; Zivicnjak, T.; Romic, Z.; Simec, Z.; Ceron, J.J. Evidence of an acute phase response in dogs naturally infected with Babesia canis. Vet. Parasitol. 2007, 144, 242–250. [Google Scholar] [CrossRef]
- Groves, M.G.; Dennis, G.L. Babesia gibsoni: Field and laboratory studies of canine infections. Exp. Parasitol. 1972, 31, 153–159. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Kelly, P.; Zheng, X.; Li, M.; You, J.; Huang, K.; Qiu, H.; Wang, Y.; Zhang, R. First description of the pathogenicity of Babesia vogeli in experimentally infected dogs. Vet. Parasitol. 2018, 253, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Shiel, R.E.; Irwin, P.J. Clinical, haematological, cytokine and acute phase protein changes during experimental Babesia gibsoni infection of beagle puppies. Exp. Parasitol. 2015, 157, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Bilwal, A.K.; Mandali, G.C.; Tandel, F.B. Clinicopathological alterations in naturally occurring Babesia gibsoni infection in dogs of Middle-South Gujarat, India. Vet. World 2017, 10, 1227–1232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birkenheuer, A.J.; Correa, M.T.; Levy, M.G.; Breitschwerdt, E.B. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J. Am. Vet. Med. Assoc. 2005, 227, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Matijatko, V.; Torti, M.; Kiš, I.; Šmit, I.; Štoković, I.; Vranješ-Đurić, S.; Milanović, S.; Mrljak, V.; Brkljačić, M. Serum cor tisol and insulin concentrations in dogs naturally infected Serum cor tisol and insulin concentrations in dogs naturally infected with Babesia canis. Vet. Arh. 2014, 84, 551–562. [Google Scholar]
- Crnogaj, M.; Cerón, J.J.; Šmit, I.; Kiš, I.; Gotić, J.; Brkljačić, M.; Matijatko, V.; Rubio, C.P.; Kučer, N.; Mrljak, V. Relation of antioxidant status at admission and disease severity and outcome in dogs naturally infected with Babesia canis canis. BMC Vet. Res. 2017, 13, 114. [Google Scholar] [CrossRef]
- Zygner, W.; Gójska, O.; Rapacka, G.; Jaros, D.; Wędrychowicz, H. Hematological changes during the course of canine babesiosis caused by large Babesia in domestic dogs in Warsaw (Poland). Vet. Parasitol. 2007, 145, 146–151. [Google Scholar] [CrossRef]
- Lee, M.J.; Yu, D.H.; Yoon, J.S.; Li, Y.H.; Lee, J.H.; Chae, J.S.; Park, J. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Dis. 2009, 9, 681–686. [Google Scholar] [CrossRef]
- Meinkoth, J.H.; Kocan, A.A.; Loud, S.D.; Lorenz, M.D. Clinical and hematologic effects of experimental infection of dogs with recently identified Babesia gibsoni-like isolates from Oklahoma. J. Am. Vet. Med. Assoc. 2002, 220, 185–189. [Google Scholar] [CrossRef]
- Di Cicco, M.F.; Downey, M.E.; Beeler, E.; Marr, H.; Cyrog, P.; Kidd, L.; Diniz, P.P.V.; Cohn, L.A.; Birkenheuer, A.J. Re-emergence of Babesia conradae and effective treatment of infected dogs with atovaquone and azithromycin. Vet. Parasitol. 2012, 187, 23–27. [Google Scholar] [CrossRef]
- Zaki, A.A.; Attia, M.M.; Ismael, E.; Mahdy, O.A. Prevalence, genetic, and biochemical evaluation of immune response of police dogs infected with Babesia vogeli. Vet. World 2021, 14, 903. [Google Scholar] [CrossRef]
- Gülanber, A.; Gorenflot, A.; Schetters, T.P.; Carcy, B. First molecular diagnosis of Babesia vogeli in domestic dogs from Turkey. Vet. Parasitol. 2006, 139, 224–230. [Google Scholar] [CrossRef]
- Henning, A.; Clift, S.J.; Leisewitz, A.L. The pathology of the spleen in lethal canine babesiosis caused by Babesia rossi. Parasite Immunol. 2020, 42, e12706. [Google Scholar] [CrossRef] [PubMed]
- Welzl, C.; Leisewitz, A.L.; Jacobson, L.S.; Vaughan-Scott, T.; Myburgh, E. Systemic inflammatory response syndrome and multiple-organ damage/dysfunction in complicated canine babesiosis. J. S. Afr. Vet. Assoc. 2001, 72, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Matijatko, V.; Kiš, I.; Torti, M.; Brkljačić, M.; Barić Rafaj, R.; Žvorc, Z.; Mrljak, V. Systemic inflammatory response syndrome and multiple organ dysfunction syndrome in canine babesiosis. Vet. Arh. 2010, 80, 611–626. [Google Scholar]
- Kandasamy, R.; Venkatasubramanian, L.; Loganathasamy, K.; Latha, B.R.; Mani, B. Prognostic markers and their discriminant score in predicting the outcome of Babesia gibsoni infection. Vet. Rec. 2021, 188, e29. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculić, A.; Beck, A.; Živičnjak, T.; Cacciò, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Oakley, M.S.; Gerald, N.; McCutchan, T.F.; Aravind, L.; Kumar, S. Clinical and molecular aspects of malaria fever. Trends Parasitol. 2011, 27, 442–449. [Google Scholar] [CrossRef]
- van Zyl, E.; Leisewitz, A.L.; Atkinson, B.K.; Goddard, A.; Rautenbach, Y.; Thompson, P.N.; Schoeman, J.P. Serial changes in the concentrations of cortisol and thyroid hormones in Beagle dogs infected with Babesia rossi. Ticks Tick Borne Dis 2022, 14, 102107. [Google Scholar] [CrossRef]
- Bodi, J.; Nsibu, C.; Hirayama, K. Immunogenetic mechanisms of black water fever: Article review. Gene Technol. 2021, 10, 1–8. [Google Scholar]
- Smith, R.L.; Goddard, A.; Boddapati, A.; Brooks, S.; Schoeman, J.P.; Lack, J.; Leisewitz, A.; Ackerman, H. Experimental Babesia rossi infection induces hemolytic, metabolic, and viral response pathways in the canine host. BMC Genom. 2021, 22, 619. [Google Scholar] [CrossRef] [PubMed]
- Defauw, P.; Schoeman, J.P.; Leisewitz, A.L.; Goddard, A.; Duchateau, L.; Aresu, L.; Meyer, E.; Daminet, S. Evaluation of acute kidney injury in dogs with complicated or uncomplicated Babesia rossi infection. Ticks Tick Borne Dis 2020, 11, 101406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Applefeld, W.N.; Sun, J.; Solomon, S.B.; Feng, J.; Couse, Z.G.; Risoleo, T.F.; Danner, R.L.; Tejero, J.; Lertora, J. Mechanistic Insights into Cell-free Hemoglobin-induced Injury During Septic Shock. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H2385–H2400. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, E.J.; Barr, B.C.; Thomford, J.W.; Yamane, I.; McDonough, S.P.; Moore, P.F.; Naydan, D.; Robinson, T.W.; Conrad, P.A. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris). J. Parasitol. 1997, 83, 692–699. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Winiarczyk, S.; Skrzypczak, M. The clinical course of babesiosis in 76 dogs infected with protozoan parasites Babesia canis canis. Pol. J. Vet. Sci. 2009, 12, 81–87. [Google Scholar] [PubMed]
- Penzhorn, B.L. Why is Southern African canine babesiosis so virulent? An evolutionary perspective. Parasites Vectors 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Sibbald, W.J.; Sprung, C.L. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992, 101, 1481–1483. [Google Scholar] [CrossRef]
- Okano, S.; Yoshida, M.; Fukushima, U.; Higuchi, S.; Takase, K.; Hagio, M. Usefulness of systemic inflammatory response syndrome criteria as an index for prognosis judgement. Vet. Rec. 2002, 150, 245. [Google Scholar] [CrossRef]
- Vincent, J.-L. Dear SIRS, I’m sorry to say that I don’t like you. Crit. Care Med. 1997, 25, 372–374. [Google Scholar] [CrossRef]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Beletić, A.; Janjić, F.; Radaković, M.; Spariosu, K.; Andrić, J.F.; Chandrashekar, R.; Tyrrell, P.; Radonjić, V.; Balint, B.; Ajtić, J. Systemic inflammatory response syndrome in dogs naturally infected with Babesia canis: Association with the parasite load and host factors. Vet. Parasitol. 2021, 291, 109366. [Google Scholar] [CrossRef]
- Schetters, T.P.M.; Kleuskens, J.A.G.M.; Van De Crommert, J.; De Leeuw, P.W.J.; Finizio, A.L.; Gorenflot, A. Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. Vet. Parasitol. 2009, 162, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gourd, N.M.; Nikitas, N. Multiple organ dysfunction syndrome. J. Intensive Care Med. 2020, 35, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Osterbur, K.; Mann, F.; Kuroki, K.; DeClue, A. Multiple organ dysfunction syndrome in humans and animals. J. Vet. Intern. Med. 2014, 28, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Schetters, T.P.; Moubri, K.; Cooke, B.M. Comparison of Babesia rossi and Babesia canis isolates with emphasis on effects of vaccination with soluble parasite antigens: A review. J. S. Afr. Vet. Assoc. 2009, 80, 75–78. [Google Scholar] [CrossRef]
- Lewis, M. Histopathology of Cerebral Babesiosis in Dogs with Naturally Acquired Babesia rossi Infection; University of Pretoria: Pretoria, South Africa, 2022. [Google Scholar]
- Adaszek, L.; Górna, M.; Klimiuk, P.; Kalinowski, M.; Winiarczyk, S. A presumptive case of cerebral babesiosis in a dog in Poland caused by a virulent Babesia canis strain. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2012, 40, 367–371. [Google Scholar]
- Daste, T.; Lucas, M.N.; Aumann, M. Cerebral babesiosis and acute respiratory distress syndrome in a dog. J. Vet. Emerg. Crit. Care 2013, 23, 615–623. [Google Scholar] [CrossRef]
- Zygner, W.; Gojska-Zygner, O.; Baska, P.; Dlugosz, E. Increased concentration of serum TNF alpha and its correlations with arterial blood pressure and indices of renal damage in dogs infected with Babesia canis. Parasitol. Res. 2014, 113, 1499–1503. [Google Scholar] [CrossRef]
- Ullal, T.; Birkenheuer, A.; Vaden, S. Azotemia and Proteinuria in Dogs Infected with Babesia gibsoni. J. Am. Anim. Hosp. Assoc. 2018, 54, 156–160. [Google Scholar] [CrossRef]
- Martin, C.; Clift, S.; Leisewitz, A. Lung pathology of natural Babesia rossi infection in dogs. J. S. Afr. Vet. Assoc. 2023, 94, 59–69. [Google Scholar] [CrossRef]
- Leisewitz, A.L.; Jacobson, L.S.; de Morais, H.S.; Reyers, F. The mixed acid-base disturbances of severe canine babesiosis. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2001, 15, 445–452. [Google Scholar]
- Koster, L.S.; Steiner, J.M.; Suchodolski, J.S.; Schoeman, J.P. Serum canine pancreatic-specific lipase concentrations in dogs with naturally occurring Babesia rossi infection. J. S. Afr. Vet. Assoc. 2015, 86, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.J.; Lobetti, R.G.; van der Lugt, J.J. Acute pancreatitis: A newly recognised potential complication of canine babesiosis. J. S. Afr. Vet. Assoc. 2000, 71, 232–239. [Google Scholar] [CrossRef]
- Masuda, M.; Otsuka-Yamasaki, Y.; Shiranaga, N.; Iguchi, A.; Uchida, N.; Sato, R.; Yamasaki, M. Retrospective study on intercurrent pancreatitis with Babesia gibsoni infection in dogs. J. Vet. Med. Sci. 2019, 81, 1558–1563. [Google Scholar] [CrossRef]
- Goddard, A.; Wiinberg, B.; Schoeman, J.P.; Kristensen, A.T.; Kjelgaard-Hansen, M. Mortality in virulent canine babesiosis is associated with a consumptive coagulopathy. Vet. J. 2012, 196, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Dubova, O.; Feshchenko, D.; Bakhur, T.; Zghozinska, O.; Antipov, A.; Rublenko, S.; Goncharenko, V.; Shahanenko, R.; Shahanenko, V. Disseminated intravascular coagulation syndrome as a complication in acute spontaneous canine babesiosis. Mac. Vet. Rev. 2020, 43, 141–149. [Google Scholar] [CrossRef]
- de Gopegui, R.R.; Peñalba, B.; Goicoa, A.; Espada, Y.; Fidalgo, L.E.; Espino, L. Clinico-pathological findings and coagulation disorders in 45 cases of canine babesiosis in Spain. Vet. J. 2007, 174, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Weltan, S.M.; Leisewitz, A.L.; Goddard, A. A case-controlled retrospective study of the causes and implications of moderate to severe leukocytosis in dogs in South Africa. Vet. Clin. Pathol./Am. Soc. Vet. Clin. Pathol. 2008, 37, 164–172. [Google Scholar] [CrossRef]
- Lobetti, R. Leukaemoid response in two dogs with Babesia canis infection. J. S. Afr. Vet. Assoc. 1995, 66, 182–184. [Google Scholar]
- Atkinson, B.K.; Thompson, P.; Van Zyl, E.; Goddard, A.; Rautenbach, Y.; Schoeman, J.P.; Mukorera, V.; Leisewitz, A. Kinetics of the inflammatory response during experimental Babesia rossi infection of beagle dogs. Vet. Parasitol. 2022, 306, 109717. [Google Scholar] [CrossRef]
- Rautenbach, Y.A.G.P.N.T.R.J.M.; Leisewitz, A.L. A flow cytometric assessment of the lymphocyte immunophenotypes in dogs naturally infected with Babesia Ross. Vet. Parasitol. 2017, 241, 26–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koster, L.S.; Van Schoor, M.; Goddard, A.; Thompson, P.N.; Matjila, P.T.; Kjelgaard-Hansen, M. C-reactive protein in canine babesiosis caused by Babesia rossi and its association with outcome. J. S. Afr. Vet. Assoc. 2009, 80, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leisewitz, A.; Goddard, A.; De Gier, J.; Van Engelshoven, J.; Clift, S.; Thompson, P.; Schoeman, J.P. Disease severity and blood cytokine concentrations in dogs with natural Babesia rossi infection. Parasite Immunol. 2019, 41, e12630. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.; Leisewitz, A.L.; Kjelgaard-Hansen, M.; Kristensen, A.T.; Schoeman, J.P. Excessive Pro-Inflammatory Serum Cytokine Concentrations in Virulent Canine Babesiosis. PLoS ONE 2016, 11, e0150113. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Mayer, I.; Rafaj, R.B.; Bendelja, K.; Susic, V.; Ceron, J.J.; Mrljak, V. MCP-1, KC-like and IL-8 as critical mediators of pathogenesis caused by Babesia canis. PLoS ONE 2018, 13, e0190474. [Google Scholar] [CrossRef] [PubMed]
- Bumby, M.M. Cytological and Histopathological Bone Marrow Findings in Dogs with Natural Babesia rossi Infection; University of Pretoria: Pretoria, South Africa, 2021. [Google Scholar]
- Rees, P.; Schoeman, J.P. Plasma insulin concentrations in hypoglycaemic dogs with Babesia canis rossi infection. Vet. Parasitol. 2008, 152, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Jacobson, L.S.; Nel, M.; de Clerq, M.; Thompson, P.N.; Schoeman, J.P. Prevalence and risk factors of hypoglycemia in virulent canine babesiosis. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2004, 18, 265–270. [Google Scholar] [CrossRef]
- Nel, M.; Lobetti, R.G.; Keller, N.; Thompson, P.N. Prognostic value of blood lactate, blood glucose, and hematocrit in canine babesiosis. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2004, 18, 471–476. [Google Scholar] [CrossRef]
- Zygner, W.; Rapacka, G.; Gojska-Zygner, O.; Dlugosz, E.; Wedrychowicz, H. Biochemical abnormalities observed in serum of dogs infected with large Babesia in Warsaw (Poland). Pol. J. Vet. Sci. 2007, 10, 245–253. [Google Scholar]
- Torti, M.; Kuleš, J.; Matijatko, V.; Brkljačić, M.; Kiš, I.; Gotić, J.; Mrljak, V.; Šmit, I. Acid-base status in canine babesiosis caused by Babesia canis. Vet. Arh. 2020, 90, 603–610. [Google Scholar] [CrossRef]
- Schoeman, J.P.; Herrtage, M.E. Adrenal response to the low dose ACTH stimulation test and the cortisol-to-adrenocorticotrophic hormone ratio in canine babesiosis. Vet. Parasitol. 2008, 154, 205–213. [Google Scholar] [CrossRef]
- Schoeman, J.P.; Herrtage, M.E. Serum thyrotropin, thyroxine and free thyroxine concentrations as predictors of mortality in critically ill puppies with parvovirus infection: A model for human paediatric critical illness? Microbes Infect./Inst. Pasteur 2008, 10, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Defauw, P.; Schoeman, J.P.; Smets, P.; Goddard, A.; Meyer, E.; Liebenberg, C.; Daminet, S. Assessment of renal dysfunction using urinary markers in canine babesiosis caused by Babesia rossi. Vet. Parasitol. 2012, 190, 326–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lobetti, R.G.; Reyers, F.; Nesbit, J.W. The comparative role of haemoglobinaemia and hypoxia in the development of canine babesial nephropathy. J. S. Afr. Vet. Assoc. 1996, 67, 188–198. [Google Scholar] [PubMed]
- de Scally, M.P.; Leisewitz, A.L.; Lobetti, R.G.; Thompson, P.N. The elevated serum urea:creatinine ratio in canine babesiosis in South Africa is not of renal origin. J. S. Afr. Vet. Assoc. 2006, 77, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Horrell, H.; Clift, S.; Leisewitz, A. Liver Histopathology in Dogs with Naturally Acquired Babesia rossi Infection. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2023. [Google Scholar]
- DeStefano, I.; Wayne, A.; Cudney, S.; Rozanski, E. Successful treatment of suspect Babesia-induced ARDS in a dog using lung-protective positive-pressure ventilation and neuromuscular blockade. Authorea Prepr. 2022, 10, e6234. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, C.; Goddard, A.; Wiinberg, B.; Kjelgaard-Hansen, M.; van der Merwe, L.L.; Thompson, P.N.; Matjila, P.T.; Schoeman, J.P. Hemostatic abnormalities in uncomplicated babesiosis (Babesia rossi) in dogs. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2013, 27, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Cartin-Ceba, R. Balance between Hyperinflammation and Immunosuppression in Sepsis. Semin. Respir. Crit. Care Med. 2016, 37, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Shinohara, M.L. Hyperinflammation, T cells, and endotoxemia. Oncotarget 2015, 6, 23040–23041. [Google Scholar] [CrossRef]
- Christensen, M.B.; Langhorn, R.; Goddard, A.; Andreasen, E.B.; Moldal, E.; Tvarijonaviciute, A.; Kirpensteijn, J.; Jakobsen, S.; Persson, F.; Kjelgaard-Hansen, M. Comparison of serum amyloid A and C-reactive protein as diagnostic markers of systemic inflammation in dogs. Can. Vet. J. 2014, 55, 161. [Google Scholar]
| Babesia rossi | Babesia canis | Babesia gibsoni | Babesia conradae | Babesia vogeli | |
|---|---|---|---|---|---|
| Tick vector | Haemophysalis elliptica | Dermacentor reticularis | Haemaphysalis longicornis Haemaphysalis hystricis | Ripicephalus sanguineus (?) Ornothodoris (?) | Ripicephalus sanguineus |
| Assymptomatic illness | Recently demonstrated [29] but probably unusual. | Has also been shown and may be quite common [30] | A significant proportion are subclinical. In a Korean study, 10% of 60 infected dogs showed no clinical signs [31]. In an experimental infection study, 2/10 dogs remained subclinical after needle infection [32]. In 9 cases described from North Carolina (USA), 1 was parasitemic but healthy [33]. In another American study, 9/10 parasitemic dogs were healthy [34] | Reported in a case series of 29 dogs [35] | Subclinical infection is common; seen in 5/12 cases in one study [36], all 4 cases described in a Chilean study [37], however an Italian study reported 11 dogs with B. vogeli infection, and all were ill [38]. |
| Lethargy, anorexia, vomiting and diarrhea | Well described in almost all infected dogs [7,39]. | Seen in 63/63 cases from Hungary, in 49/49 cases from Germany and 50/50 cases from Croatia [8,30,40]. Anorexia was described in 76% of dogs (23/30) and depression in 93% (28/30) in an Italian study [38] and in 43/50 (86%) cases from Croatia [40]. Anorexia was described in 76% of dogs (23/30) and depression in 93% (28/30) in an Italian study [38]. | In the chronic relapsing infections weight loss may be a feature [32,41]. Lethargy was observed in 48/60 (80%) and anorexia in 51/60 (85%) naturally B. gibsoni infected dogs from Taiwan [31]. A smaller proportion of these dogs were also noted to have vomiting (18/60) and diarrhea (6/60) as part of their clinical histories [31] | In a description of 11 naturally infected B. conradae dogs, vomiting and lethargy are described as common [9] | None of 4 infected dogs from Chile showed any clinical illness [37]. Only splenectomised dogs (n = 3) in an experimental B. vogeli infection became depressed and anorexic and one of these dogs self-cured [42]. Five of 11 naturally infected dogs from Italy were described as lethargic and anorexic [38] |
| Pyrexia | Well described in almost all infected dogs [7,39]. | One study documented pyrexia in 84% (27/32) [8] and another in 43% (13/30) of cases [38]. | Pyrexia is also not a consistent feature and is poorly correlated with parasitemia [9,32] and seldom rises above 40 °C [41]. Temperature is frequently described as normal despite parasitemia [32]. In an experimental infection pyrexia developed on days 13 and 14 post infection before parasites were seen on blood smear. Fever also resolved within days and never recurred despite a climbing parasitemia [43]. In another study, 8/10 dogs developed a transient fever which mostly resolved [32]. A study of 79 naturally infected dogs in India did not regard it as a cardinal clinical finding as only 31% (5/16) of dogs with positive blood smears were febrile [44]. In another study pyrexia was only detected in 2/8 naturally infected dogs that were anemic and PCR positive for B. gibsoni DNA [45]. | Pyrexia is noted to occur in B. conradae infections, it appears not to be a consistent finding [9,35] | Pyrexia was common in 11 dogs with evidence of disease [38]. All 8 dogs experimentally infected showed a mild pyrexia which self-resolved in a matter of days despite the infection persisting [42]. Five of 11 naturally infected Italian dogs developed pyrexia [38]. Five of 11 naturally infected Italian dogs developed pyrexia [38] |
| Anemia | Over a third of 320 dogs were severely anemic (hematocrit < 15%, requiring blood transfusions), a quarter were moderately so, and just under a quarter were mildly anemic. A small proportion of cases had normal hematocrits at presentation [7] | A significantly proportion of dogs reported with mild to moderate anemia and a very small proportion of these were treated with blood transfusions [8,30,38,46,47,48]. | Caused anemia in over 80% of 60 infected dogs. A quarter of these dogs had mild to moderate anemia whilst just over 10% of dogs had hematocrits < 20%. Similar findings in other studies describe a severe life-threatening anemia rarely with mild to moderate anemia being more characteristic of the infection [43,44,45,49,50]. | Severe anemia is described in 11 cases (before B. gibsoni and B. conradae were understood to be separate species) [9]. The anemia is described as more pronounced than what is observed in B. gibsoni infections [50]. One study from California demonstrated mild anemia in 13/29 infected dogs [35] while another demonstrated severe anemia in 3/12 infected dogs [51]. | Anemia is reported but appears to be a feature of the infection in puppies (where it described as hemolytic and severe) or in immunocompromised rather than immunocompetent adult dogs (where the infections is usually subclinical or reported as a co-infection) [38,42,52,53]. |
| Hemolysis | 84% (269/320) of cases had hemoglobinuria (as a result of massive intravascular hemolysis) at presentation [7] | Reported to be common with macroscopically visible hemoglobin in urine and/or blood in >2/3rds of cases [8], in 24/49 dogs [30] and in 63% of 63 cases [8] | Evidence of hemolysis is reported but in a small percentage of cases [49] | Not reported/unknown | Hemolysis is reported in 11 cases [38] |
| Splenomegaly | Well described [54]. A detailed description of splenic pathology has been reported [54] | Well described [8] | Well described [31] | Has been described [35] | Not specifically described. |
| Mortality | The majority of infected dogs that die, succumb within the first 24 h of hospitalization, despite intensive treatment [7,39]. Ranges between 5 and 35% with a rate of over 80% for cerebral or hemoconcentrating cases [1]. Others have reported mortality of 45% for complicated cases with death in 10–12% of all admitted cases [55]. Lower mortality rates have also been reported with 1–3% of cases euthanized because of a grave prognosis and about 5% of all cases dying [39]. In a series of 320 cases the overall mortality rate was 11% | In one study 10% of dogs were diagnosed with multiple organ dysfunction syndrome and 67% of these died. Five percent of dogs that did not develop MODS died [56]. The mortality across all 332 dogs included in the study was around 6%. | In a Korean study of 9/39 dogs (31%) were regarded as subclinical and none were reported to have died [49]. In a study of 60 infected dogs from Taiwan, the majority of dogs had mild to moderate disease, 10 dogs were severely anemic and relieved a blood transfusion and no deaths were reported [31]. In an American study of 150 cases, most were reported as mild or moderate disease and there were no reported deaths [45]. One of 9 naturally infected dogs from North Carolina (USA) died [33]. In an Indian study, 10% of B. gibsoni infected dogs died whilst 34% of dual B. gibsoni/B. vogeli infected dogs died. None of the dogs with B. vogeli infection alone died [57]. In an experimental infection study, 2 of 9 infected dogs died [32] | Can cause significant mortality with between 25 and 40% of naturally infected dogs dying or being euthanized because of severe illness in two separate studies [50,51]. Other studies indicate a significantly lower mortality than this [9,35] but data from large study populations involving this infection are lacking. | Most cases reported as subclinical or only mildly affected [37]. No dogs in an experimental infection died [57]. One of 11 dogs in a case series died [38]. Severe disease (and the single death) were only seen in puppies [38]. |
| Babesia rossi | Babesia canis | Babesia gibsoni | Babesia conradae | Babesia vogeli | |
|---|---|---|---|---|---|
| SIRS and MODS | SIRS has been described [7,55] MODS has been described [7,55] | SIRS has been described [56,72,76] MODS has been described [56] | Neither SIRS or MODS have been described | Neither SIRS or MODS have been described | Neither SIRS or MODS have been described |
| Brain pathology | Cerebral disease well described [77] | Cerebral disease described [78,79]. | Not described | Not described | Not described |
| Renal pathology | Recoverable renal injury is common [63]. Severe irreversible renal failure is uncommon but occurs and carries a very poor prognosis [7,16,39] | Renal injury is described [56,80]. In one study 4/9 dogs with acute renal failure alone died [8] | A reversable protein losing nephropathy has been identified in a small number of infected American pit bull terriers [81] | An IgM positive membranoproliferative glomerulonephritis (consistent with a type III hypersensitivity) has been described [65] | Not described |
| Liver pathology | The liver was the single most common organ showing biochemical evidence of injury [55]. Icterus was observed in almost two thirds of infected dogs and elevated serum bilirubin concentrations were predictive of a poor outcome. Icterus was present on post mortem in 68% (17/25) of cases and 16% (52/320) of a large cohort study [7]. Acute lung injury (ALI) is common with all dogs that died in one study demonstrating it [82] | The liver the second most common organ showing biochemical evidence of injury [56]. Icterus is also reported in 80% (39/49) of infected dogs although this does not appear to correlate with outcome [30] | Evidence of liver injury is described [31,57]. Icterus is common in with the incidence ranging from 14–25% of cases [31] | Icterus and hyperbilirubinemia has been reported however elevated liver enzyme activity appears to be rare [9,35]. | Only 1/11 dogs presenting ill was icteric [38]. |
| Lung pathology | Acute lung injury (ALI) is common with all dogs that died in one study demonstrating it [82] Acute Respiratory Distress Syndrome (ARDS) is rare but has been reported [82]. The proportion of dogs with ARDS was 18% (18/98) of cases having an arterial pO2 < 60 mmHg in one study [7] and 9% (3/34) in another [83]. | Acute Respiratory Distress Syndrome (ARDS) is rare but has been reported [82]. 16/331 (just <5%) of dogs demonstrated diagnostic criteria consistent with ARDS [56] | Not reported | Not reported | Not reported |
| Pancreatic pathology | Diagnosed in 28% of admitted dogs based on pancreatic lipase immunoreactivity level (cPLI) [84]. In another study, pancreatitis was histologically confirmed [85] | Suspected in an old study (based on amylase and lipase concentrations) in 33% of 31 infected dogs although it was never the only organ with signs of damage [8]. In 13/46 (28%) an increased lipase DGGR was found and 8 of these were clinical for pancreatitis (including ultrasound findings) [30]. | Described in 2% of 20 dogs by means of canine specific pancreatic lipase activity [86] | Not reported | Not reported |
| Coagulopthy | Hemorrhage was common internally during post mortem examination of dogs that died (seen in 22/25 post mortems [7]. Disseminated intravascular coagulation (DIC) and its association with mortality has been described [87]. | DIC has been described [88,89]. | No description | No description | No description |
| White cell count | Total counts are higher in the more severely affected dogs but often still within the normal range. Severe leukocytosis is occasionally seen [90,91]. The band cell count was significantly higher in complicated cases and cases that died [7,92]. Complicated disease had significantly depressed CD3+, CD3+/CD4+ and CD3+/CD8+ lymphocytes in circulation [93]. | WCC described as normal or low with only an occasional finding of an increased band cell count. A lymphopenia has been described but its association with disease severity or immunophenotyping have not been reported [8,30]. | The WCC is generally unremarkable [43,65] or mildly elevated due to neutrophilia with the dogs more severely affected having moderately elevated counts without remarkable changes in the band cell numbers [31]. | The WCC in infected dogs appears to be varied though 41% of infected dogs in one study were leukopenic [9,35]. | An increase in the band cell count was described in 4/11 cases presented with illness. An increased WCC was common in the 11 sick dogs [38]. |
| C-reactive protein | Elevates significantly during infection in concert with disease progression and decreases with resolution, but does not predict outcome [92,94] | Elevates significantly with infection and decreases with resolution but does not predict outcome [30,38,40]. | Infection induces a sudden rise which coincides with the appearance of the peripheral parasitemia in experimental infections (which is very delayed compared to similar experimental infections with other parasite species) [43]. | Unknown | Elevated in 4/5 cases that presented ill [38] |
| Cytokines | Induces a cytokine storm, hyperinflammation and cytokine mediated immune dysregulation with proinflammatory cytokine levels correlating with disease severity [92,95,96] | Induces a cytokine storm in which complicated disease and poor outcome are associated with higher concentrations of proinflammatory cytokines [97] | Cytokine profiles were described in 2 experimentally B. gibsoni infected dogs but, as with CRP, the onset of increases coincided with the very delayed onset of parasitemia [43]. | Not described | Not described |
| Macropathology, histopathology and immunohistochemistry | Organ damage has been described in all the organs studied thus far (spleen, bone marrow, brain, liver and lung [54,77,82,98]). | No descriptions | A unique an immune complex mediated glomerulonephritis with proteinuria and azotemia has also been described in 34% of 35 dogs with B. gibsoni infection [81] | A single report describing some pathology caused by B. conradae infection [65] | No descriptions |
| Blood glucose and lactate | Hypoglycemia (present in around 23% of complicated cases) is associated with a poor outcome and hyperglycemia (which is common) is less strongly correlated with a poor outcome [7,99,100]. Hyperlactatemia that is treatment refractory is a good indicator of a poor outcome [101]. | Hypoglycemia is present in around 20% of complicated cases but its association with outcome is unknown [102]. Hyperlactatemic metabolic acidosis is described but there is no report on its association with outcome [103]. | There is one report of a small number dogs in which hyperlactatemia was associated with a poor outcome [57]. | Nor reported | Not reported |
| Endocrine markers of disease severity | Hypercortisolemia and a low thyroid hormone are well correlated with disease severity and have been reported in several studies [7,104,105] | Hypercortisolemia and a low thyroid hormone are correlated with disease severity and have been reported [46]. | Not reported | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leisewitz, A.L.; Mrljak, V.; Dear, J.D.; Birkenheuer, A. The Diverse Pathogenicity of Various Babesia Parasite Species That Infect Dogs. Pathogens 2023, 12, 1437. https://doi.org/10.3390/pathogens12121437
Leisewitz AL, Mrljak V, Dear JD, Birkenheuer A. The Diverse Pathogenicity of Various Babesia Parasite Species That Infect Dogs. Pathogens. 2023; 12(12):1437. https://doi.org/10.3390/pathogens12121437
Chicago/Turabian StyleLeisewitz, Andrew L., Vladimir Mrljak, Jonathan D. Dear, and Adam Birkenheuer. 2023. "The Diverse Pathogenicity of Various Babesia Parasite Species That Infect Dogs" Pathogens 12, no. 12: 1437. https://doi.org/10.3390/pathogens12121437
APA StyleLeisewitz, A. L., Mrljak, V., Dear, J. D., & Birkenheuer, A. (2023). The Diverse Pathogenicity of Various Babesia Parasite Species That Infect Dogs. Pathogens, 12(12), 1437. https://doi.org/10.3390/pathogens12121437






