Molecular Identification and Fungal Diversity Associated with Diseases in Hass Avocado Fruit Grown in Cauca, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Morphological Identification
2.3. DNA Extraction, Amplification and Sequencing of the Internal Transcribed Spacer Region (ITS) of the rDNA
2.4. Identity and Taxonomic Positioning of Isolates
2.5. Estimation of Diversity in Communities
3. Results
3.1. Isolation of Microorganisms
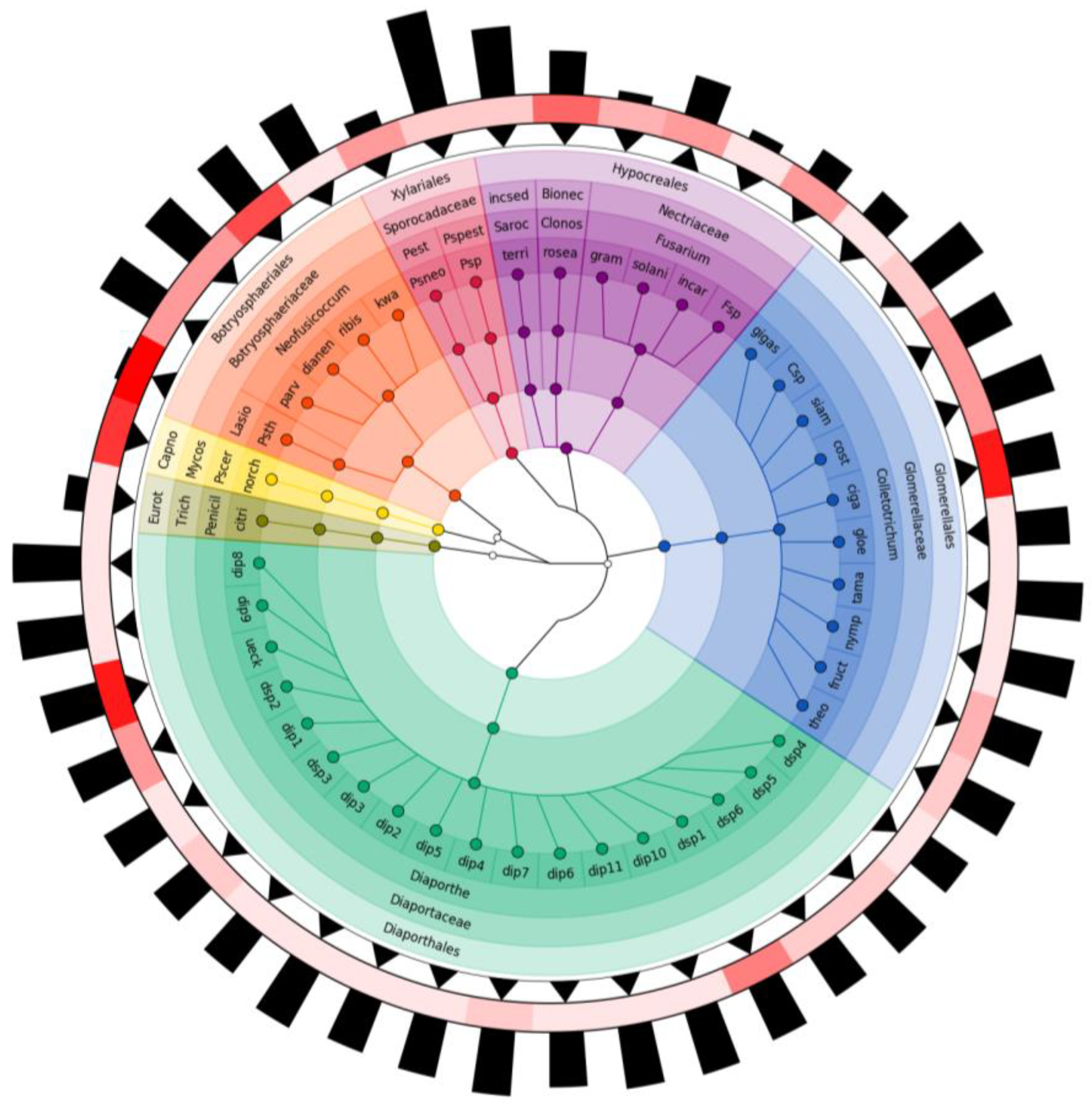
3.2. Molecular Identification and Taxonomic Positioning
3.3. Estimation of Diversity in Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AGRONET. Evaluaciones agropecuarias del Ministerio de Agricultura y Desarrollo Rural. In Evaluaciones agropecuarias del Ministerio de Agricultura y Desarrollo Rural; Information and Communication Network of the Colombian Agricultural: Bogotá, Colombia, 2020. [Google Scholar]
- Bernal, J.; Díaz, C. Actualización Tecnológica y Buenas Prácticas Agrícolas (BPA) en el Cultivo de Aguacate, 2nd ed.; Agrosavia Editorial: Mosquera, Colombia, 2020; pp. 77–644. [Google Scholar]
- Ramírez, G.J.G.; López, J.H.; Henao, R.J.C. Causes of Hass Avocado Fruit Rejection in Preharvest, Harvest, and Packinghouse: Economic Losses and Associated Variables. Agronomy 2020, 10, 8–21. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Reina-Noreña, J.; Mayorga-Cobos, M.J.; Caldas-Herrera, S.J.; Rodríguez-Valenzuela, J.; Varón-Devia, E.H. El problema de la peca en cultivos de aguacate (Persea americana Mill.) del norte del Tolima, Colombia. Corpoica Cienc. Tecnol. Agropecu. 2016, 16, 265–278. [Google Scholar] [CrossRef]
- Everett, K.R.; Hallett, I.C.; Rees-George, J.; Chynoweth, R.W.; Pak, H.A. Avocado lenticel damage: The cause and the effect on fruit quality. Postharvest Biol. Technol. 2008, 48, 383–390. [Google Scholar] [CrossRef]
- Iyanyi, N.G.; Ataga, A.E.; Rotimi, I.S.; Blessing, I. Molecular identification of fungi associated with avocado (Persea americana Mill.) fruits. Agro-Science 2021, 20, 1. [Google Scholar] [CrossRef]
- Wanjiku, E.K.; Waceke, J.W.; Wanjala, B.W.; Mbaka, J.N. Identification and Pathogenicity of Fungal Pathogens Associated with Stem End Rots of Avocado Fruits in Kenya. Int. J. Microbiol. 2020, 2020, 4063697. [Google Scholar] [CrossRef]
- Hernández, D.; García, O.; Perera, S.; Gonzáles, M.; Rodríguiez, A.; Siverio, F. Fungal Pathogens Associated with Aerial Symptoms of Avocado (Persea americana Mill.) in Tenerife (Canary Islands, Spain) Focused on Species of the Family Botryosphaeriaceae. Microorganisms 2023, 11, 585–607. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Ijaz, S.; Haq, I.U.; Mukhtar, S.; Habib, Z. Molecular Phytopathometry. In Trends in Plant Disease Assessment; Ul Haq, I., Ijaz, S., Eds.; Springer: Singapore, 2012; pp. 167–202. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14–46. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B. Illustrated Genera of Imperfect Fungi, 4th ed.; Burgess Publishing Company: Saint Paul, MN, USA; APS Press: Saint Paul, MN, USA, 1998; p. 241. [Google Scholar]
- Jaramillo-Laverde, A.; Martinez, M.F.; Murcia-Riaño, N.; Martinez-Caballero, L.N.; Ceballos-Aguirre, G.; Angel-García, C.; Rodríguez, K.A. Microorganismos asociados a lenticelosis y pudrición de pedúnculo del aguacate Hass del departamento del Cauca. v1.0. Corporación Colombiana de Investigación Agropecuaria-AGROSAVIA. Dataset/Occur 2023. [Google Scholar] [CrossRef]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Application; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, Y.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1967, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.; Darling; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Weingart, G.; Tickle, T.L.; Huttenhower, C.; Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 2015, 3, e1029. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 8 April 2023).
- Ganley, A.R.; Kobayashi, T. Highly efficient concerted evolution in the ribosomal DNA repeats: Total rDNA repeat variation revealed by whole-genome shotgun sequence data. Genome Res. 2007, 17, 184–191. [Google Scholar] [CrossRef]
- Pérez-Jiménez, R.M. Significant avocado diseases caused by fungi and oomycetes. Eur. J. Plant Sci. Biotechnol. 2008, 2, 1–24. [Google Scholar]
- Mosquera, S.; Cataño Useche, C.; Saavedra, S.; Villegas-Escobar, V. Characterization of fungal communities associated with the lenticel-like damage of avocado cv. Hass in two geographical locations in Colombia. Phytobiomes J. 2023. [Google Scholar] [CrossRef]
- Crous, P.W.; Braun, U.; Hunter, G.C.; Wingfield, M.J.; Verkley, G.J.M.; Shin, H.D.; Nakashima, C.; Groenewald, J.Z. Phylogenetic lineages in Pseudocercospora. Stud. Mycol. 2013, 75, 37–114. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.I.; Osorio-Navarro, C.; Fernández, Y.; Bourret, T.B.; Zamorano, A.; Henríquez-Sáez, J.L. First Record of Colletotrichum anthrisci Causing Anthracnose on Avocado Fruits in Chile. Pathogens 2022, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Gañán, L.; Álvarez, E.; Castaño-Zapata, J. Identificación genética de aislamientos de Colletotrichum spp. causantes de antracnosis en frutos de aguacate, banano, mango y tomate de árbol. Rev. De La Acad. Colomb. De Cienc. Exactas Físicas Y Nat. 2015, 39, 339–347. [Google Scholar] [CrossRef]
- Hunupolagama, D.M.; Wijesundera, R.L.C.; Chandrasekharan, N.V.; Wijesundera, W.S.S.; Kathriarachchi, H.; Fernando, T.H.P.S. Characterization of Colletotrichum isolates causing avocado anthracnose and first report of C. gigasporum infecting avocado in Sri Lanka. Plant Pathol. Quar. 2015, 5, 132–143. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia-Mol. Phylogeny Evol. Fungi 2014, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Valencia, A.L.; Gil, P.; Latorre, B.A.; Rosales, I.M. Characterization and Pathogenicity of Botryosphaeriaceae Species Obtained from Avocado Trees with Branch Canker and Dieback and from Avocado Fruit with Stem End Rot in Chile. Plant Dis. 2019, 103, 996–1005. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; Henao-Rojas, J.C.; Morales-Osorio, J.G. Postharvest diseases and disorders in avocado cv. Hass and their relationship to preharvest management practices. Heliyon 2021, 7, e05905. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chao, A. Distance-based functional diversity measures and their decomposition: A framework based on Hill numbers. PLoS ONE 2014, 9, e100014. [Google Scholar] [CrossRef]
- Hakizimana, J.D.; Gryzenhout, M.; Coutinho, T.A.; Van den Berg, N. Endophytic diversity in Persea americana (avocado) trees and their ability to display biocontrol activity against Phytophthora cinnamomi. In Proceedings of the VII World Avocado Congress, Cairns, Australia, 5–9 September 2011. [Google Scholar]
- Shetty, K.G.; Rivadeneira, D.V.; Jayachandran, K.; Walker, D.M. Isolation and molecular characterization of the fungal endophytic microbiome from conventionally and organically grown avocado trees in South Florida. Mycol. Prog. 2016, 15, 977–986. [Google Scholar] [CrossRef]
- Gazis, R.; Rehner, S.; Chaverri, P. Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Mol. Ecol. 2011, 20, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Henao, R.J.; Lopez, J.H.; Osorio, N.W.; Ramírez, G.J. Fruit quality in Hass avocado and its relationships with different growing areas under tropical zones. Rev. Ceres 2019, 66, 341–350. [Google Scholar] [CrossRef]




| Associated Genus | Number of Isolations | Percentage of Population | Number of OTUs |
|---|---|---|---|
| Colletotrichum spp. | 38 | 1.03 | 15 |
| Pseudocercospora sp. | 38 | 16.03 | 2 |
| Neofusicoccum spp. | 26 | 10.97 | 6 |
| Lasiodiplodia spp. | 20 | 8.44 | 3 |
| Diaporthe spp. | 37 | 15.61 | 17 |
| Pestalotiopsis sp. | 2 | 0.84 | 1 |
| Neopestalotiopsis sp. | 24 | 10.13 | 1 |
| Fusarium spp. | 32 | 13.50 | 9 |
| Clonostachys sp. | 16 | 6.75 | 1 |
| Penicillium sp. | 1 | 0.42 | 1 |
| Sarocladium sp. | 2 | 0.84 | 1 |
| Schizophyllum sp. | 1 | 0.42 | 1 |
| ∑ | 237 | 100 | 58 |
| Assemblages | * SC | m | SC.Est | Richness (q0) | Diversity (q1) | Diversity (q2) |
|---|---|---|---|---|---|---|
| Diversity analysis by municipality | ||||||
| Cajibío | 0.75 | 48 | 0.83 | 17.4 ± 7.74 | 13.45 ± 6.07 | 11.33 ± 5.07 |
| Corinto | 0.11 | 10 | 0.51 | 8.56 ± 3.63 | 9.06 ± 4.09 | 10.00 ± 5.14 |
| El Tambo | 0.89 | 74 | 0.96 | 13.48 ± 4.85 | 9.18 ± 2.82 | 7.02 ± 2.44 |
| Morales | 0.72 | 48 | 0.84 | 16.23 ± 7.18 | 9.15 ± 4.87 | 5.04 ± 3.64 |
| Piendamó | 0.83 | 70 | 0.91 | 17.31 ± 6.41 | 11.57 ± 4.00 | 8.43 ± 3.39 |
| Popayán | 0.63 | 42 | 0.78 | 18.17 ± 7.32 | 13.21 ± 6.26 | 9.30 ± 5.37 |
| Sotará | 0.94 | 56 | 1 | 9.61 ± 3.36 | 8.28 ± 2.21 | 7.01 ± 2.18 |
| Timbío | 0.88 | 64 | 0.96 | 14.47 ± 5.03 | 11.96 ± 3.06 | 10.53 ± 3.03 |
| Toribío | 0.78 | 16 | 0.92 | 5.15 ± 2.99 | 4.16 ± 2.51 | 3.37 ± 2.27 |
| Diversity by altitude intervals (masl) | ||||||
| 1613–1793 | 0.94 | 224 | 0.98 | 29.14 ± 6.65 | 18.39 ± 3.30 | 12.77 ± 3.27 |
| 1794–1973 | 0.92 | 190 | 0.98 | 29.53 ± 7.15 | 19.89 ± 3.60 | 15.08 ± 3.34 |
| 1974–2153 | 0.76 | 48 | 0.91 | 15.68 ± 5.80 | 12.41 ± 4.44 | 9.44 ± 4.20 |
| 2154–2333 | 0.33 | 6 | 0.8 | 4.41 ± 2.28 | 4.98 ± 2.74 | 6.00 ± 3.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ángel-García, C.; Rodríguez-Arevalo, K.A.; Murcia Riaño, N.; Martínez-Caballero, L.N.; Ceballos-Aguirre, G.; Jaramillo Laverde, A.; Martínez, M.F. Molecular Identification and Fungal Diversity Associated with Diseases in Hass Avocado Fruit Grown in Cauca, Colombia. Pathogens 2023, 12, 1418. https://doi.org/10.3390/pathogens12121418
Ángel-García C, Rodríguez-Arevalo KA, Murcia Riaño N, Martínez-Caballero LN, Ceballos-Aguirre G, Jaramillo Laverde A, Martínez MF. Molecular Identification and Fungal Diversity Associated with Diseases in Hass Avocado Fruit Grown in Cauca, Colombia. Pathogens. 2023; 12(12):1418. https://doi.org/10.3390/pathogens12121418
Chicago/Turabian StyleÁngel-García, Carolina, Kevin Alejandro Rodríguez-Arevalo, Nubia Murcia Riaño, Luz Natalia Martínez-Caballero, Germán Ceballos-Aguirre, Alejandro Jaramillo Laverde, and Mauricio Fernando Martínez. 2023. "Molecular Identification and Fungal Diversity Associated with Diseases in Hass Avocado Fruit Grown in Cauca, Colombia" Pathogens 12, no. 12: 1418. https://doi.org/10.3390/pathogens12121418
APA StyleÁngel-García, C., Rodríguez-Arevalo, K. A., Murcia Riaño, N., Martínez-Caballero, L. N., Ceballos-Aguirre, G., Jaramillo Laverde, A., & Martínez, M. F. (2023). Molecular Identification and Fungal Diversity Associated with Diseases in Hass Avocado Fruit Grown in Cauca, Colombia. Pathogens, 12(12), 1418. https://doi.org/10.3390/pathogens12121418





