Stability of the Virucidal Activity of Commercial Disinfectants against Avian Influenza Viruses under Different Environmental Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Disinfectants
2.4. Cytotoxicity Assay
2.5. Virucidal Activity Assay
- Two-fold serial dilutions (30 μL each) of each disinfectant were made in 96-well U-bottom plates with either MEM or 10% fetal calf serum (FCS), which is recommended as a source of organic contamination by the guideline of the German Association for the Control of Viral Disease and Robert Kock Institute [33], starting from the 102-fold dilution (based on the results of cytotoxicity assays) in distilled water, corresponding to the absence or presence of organic matter, respectively.
- The diluted disinfectants were mixed with 6000 TCID50 of each virus tested (30 μL).
- The virus–disinfectant mixtures were incubated at room temperature (RT) or 4 °C for 1 h.
- The virus-disinfectant mixtures were 100-fold diluted with MEM (in duplicates), so that each 50 μL of the mixtures contained 100 TCID50 of the tested virus and a 104-fold dilution of the disinfectant in the final volume (the non-toxic dilution of all disinfectants confirmed from the cytotoxicity assay).
- The AX4 cells in 96-well cell culture plates were inoculated with the diluted virus–disinfectant mixtures and incubated at 37 °C for 1 h.
- The inoculated AX4 cells were washed with PBS twice and cultured in the infection medium at 37 °C for 3 days.
- The CPE in the inoculated AX4 cells was observed under a light microscope.
3. Results
3.1. Selection of Disinfectants for Testing
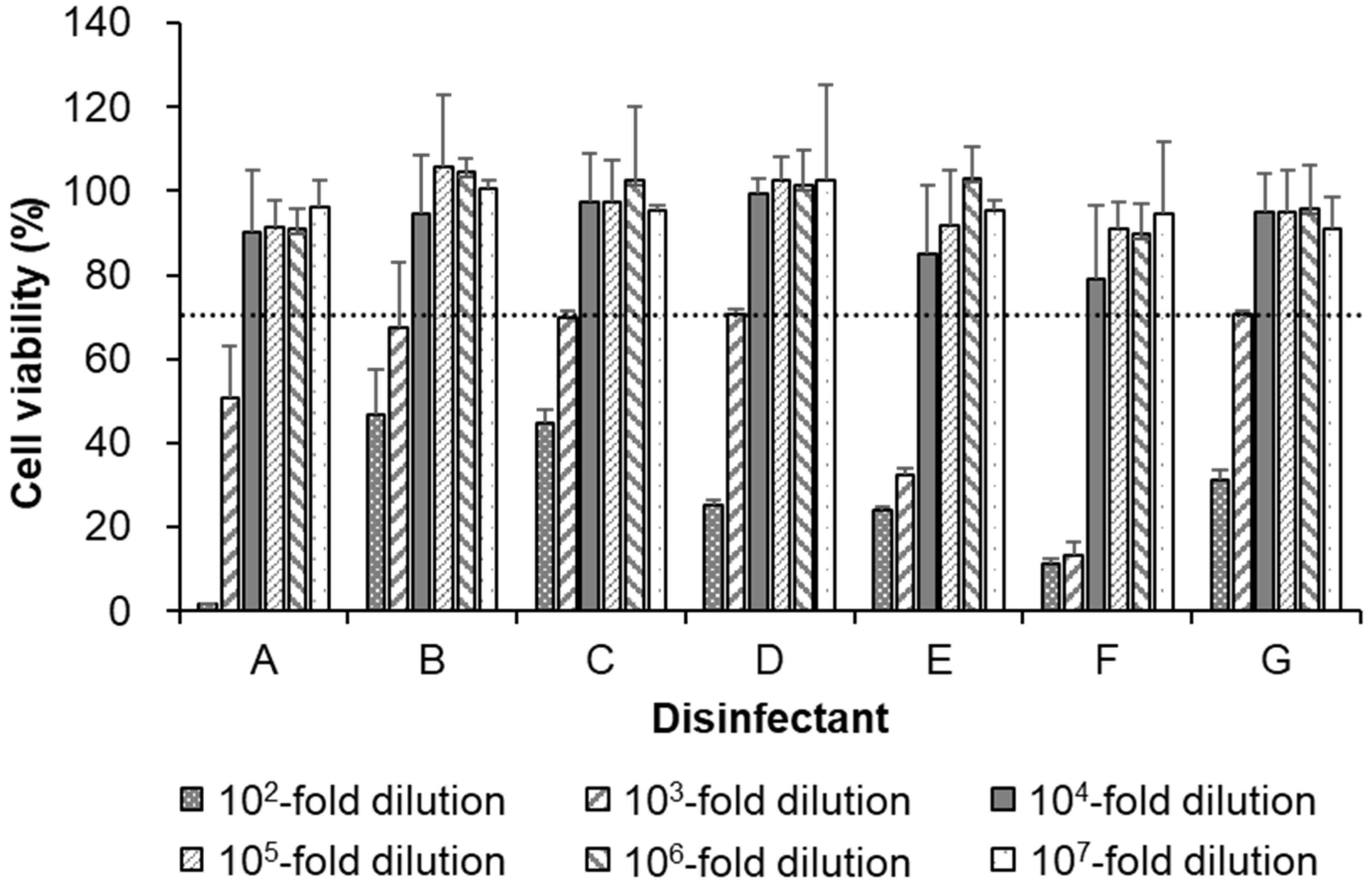
3.2. Cytotoxicity of Seven Disinfectants in AX4 Cells
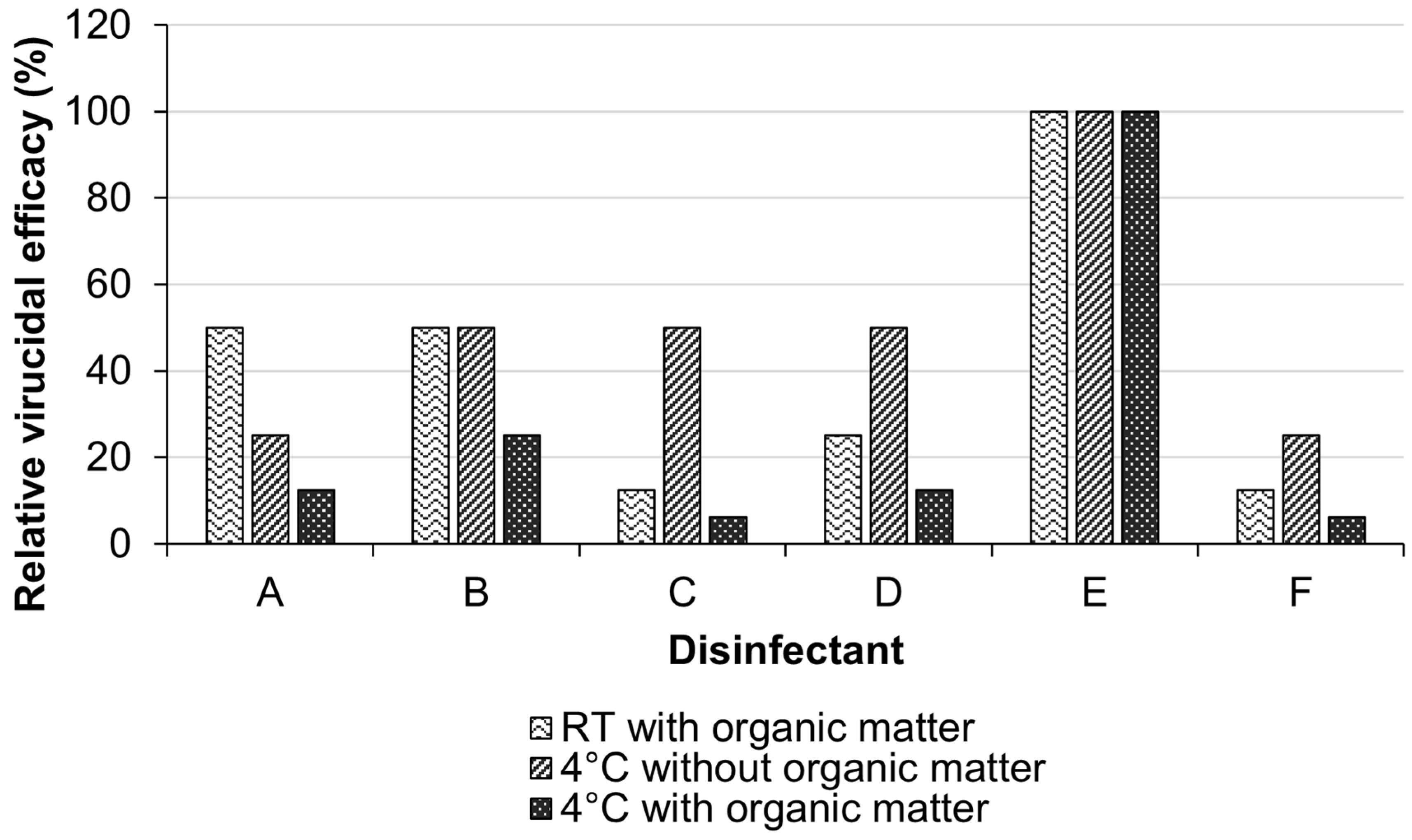
3.3. Virucidal Activity of the Disinfectants
3.4. Virucidal Activity of Disinfectant E against AIVs of Various Subtypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Kawaoka, Y. Transmission of influenza A viruses. Virology 2015, 479–480, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Rooney, J.A.; Blanton, L.; Rolfes, M.A.; Nelson, D.I.; Gomez, T.M.; Karli, S.A.; Trock, S.C.; Fry, A.M. Estimating Risk to Responders Exposed to Avian Influenza A H5 and H7 Viruses in Poultry, United States, 2014–2017. Emerg. Infect. Dis. 2019, 25, 1011–1014. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L. Highly pathogenic avian influenza. Rev. Sci. Tech. 2000, 19, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yao, Z.; Tang, Y.; Yang, M.; Li, Y.; Yang, G.; Chen, J.; Chen, G.; Feng, W.; Zheng, H.; et al. Highly Pathogenic Avian Influenza A (H5N1) Virus in Swans, Central China, 2021. Microbiol. Spectr. 2022, 10, e0231522. [Google Scholar] [CrossRef]
- Nagy, A.; Cernikova, L.; Stara, M.; Hofmannova, L.; Sedlak, K. Genotype Uniformity, Wild Bird-to-Poultry Transmissions, and Farm-to-Farm Carryover during the Spread of the Highly Pathogenic Avian Influenza H5N8 in the Czech Republic in 2021. Viruses 2022, 14, 1411. [Google Scholar] [CrossRef]
- King, J.; Schulze, C.; Engelhardt, A.; Hlinak, A.; Lennermann, S.L.; Rigbers, K.; Skuballa, J.; Staubach, C.; Mettenleiter, T.C.; Harder, T.; et al. Novel HPAIV H5N8 Reassortant (Clade 2.3.4.4b) Detected in Germany. Viruses 2020, 12, 281. [Google Scholar] [CrossRef]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef]
- Abolnik, C.; Phiri, T.P.; van der Zel, G.; Anthony, J.; Daniell, N.; de Boni, L. Wild Bird Surveillance in the Gauteng Province of South Africa during the High-Risk Period for Highly Pathogenic Avian Influenza Virus Introduction. Viruses 2022, 14, 2027. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Brojer, C.; Zohari, S.; Noremark, M.; Uhlhorn, H.; Jansson, D.S. Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020–2021 Season. Vet Sci 2022, 9, 344. [Google Scholar] [CrossRef]
- Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Rahman, M.M.; Chowdhury, M.N.U.; Nine, H.; Rahman, M.K.; Jarhult, J.D.; Hoque, M.A. Serological Evidence of Avian Influenza in Captive Wild Birds in a Zoo and Two Safari Parks in Bangladesh. Vet. Sci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Soda, K.; Sumi, K.; Ozaki, H.; Tomioka, Y.; Ito, H.; Murase, T.; Kawamoto, T.; Miura, M.; Komatsu, M.; et al. Outbreaks of highly pathogenic avian influenza in zoo birds caused by HA clade 2.3.4.4 H5N6 subtype viruses in Japan in winter 2016. Transbound. Emerg. Dis. 2020, 67, 686–697. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, P.; Beato, M.S.; Capua, I. Inactivation of avian influenza viruses by chemical agents and physical conditions: A review. Zoonoses Public Health 2007, 54, 51–68. [Google Scholar] [CrossRef]
- Lewis, D.L.; Arens, M. Resistance of microorganisms to disinfection in dental and medical devices. Nat. Med. 1995, 1, 956–958. [Google Scholar] [CrossRef]
- Muscarella, L.F. Sterilizing dental equipment. Nat. Med. 1995, 1, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- WHO. Review of Latest Available Evidence on Potential Transmission of Avian Influenza (H5N1) through Water and Sewage and Ways to Reduce the Risks to Human Health; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Schmitz, A.; Pertusa, M.; Le Bouquin, S.; Rousset, N.; Ogor, K.; LeBras, M.-O.; Martenot, C.; Daniel, P.; Belen Cepeda Hontecillas, A.; Scoizec, A.; et al. Natural and Experimental Persistence of Highly Pathogenic H5 Influenza Viruses in Slurry of Domestic Ducks, with or without Lime Treatment. Appl. Environ. Microbiol. 2020, 86, e02288-20. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Q.; Ma, M.; Shi, H.; He, G. Persistence of avian influenza virus (H9N2) on plastic surface. Sci. Total Environ. 2022, 834, 155355. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Khalil, A.M.; Fujimoto, Y.; Kojima, I.; Esaki, M.; Ri, K.; Masatani, T.; Matusi, T.; Ozawa, M. Genetic Characterization of H5N8 highly pathogenic avian influenza virus isolated from falcated ducks and environmental water in Japan in November 2020. Pathogens 2021, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Hatai, H.; Fujimoto, Y.; Kojima, I.; Okajima, M.; Esaki, M.; Kinoshita, K.; Ozawa, M. A lethal case of natural infection with the H5N8 highly pathogenic avian influenza virus of clade 2.3.4.4. in a Mandarin duck. Zoonotic Dis. 2022, 2, 32–36. [Google Scholar] [CrossRef]
- Okuya, K.; Khalil, A.M.; Esaki, M.; Nishi, N.; Koyamada, D.; Saito, R.; Tokorozaki, K.; Hasegawa, T.; Ozawa, M. Newly emerged genotypes of highly pathogenic H5N8 avian influenza viruses in Kagoshima Prefecture, Japan during winter 2020/21. JGV 2023, 104, 001870. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Sakai-Tagawa, Y.; Kiso, M.; Goto, H.; Kawakami, C.; Mitamura, K.; Sugaya, N.; Suzuki, Y.; Kawaoka, Y. Enhanced expression of an alpha2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J. Clin. Microbiol. 2005, 43, 4139–4146. [Google Scholar] [CrossRef]
- Khalil, A.M.; Nishi, N.; Kojima, I.; Fukunaga, W.; Kuwahara, M.; Masatani, T.; Matsui, T.; Ozawa, M. Transition in genetic constellations of H3N8 and H4N6 low-pathogenic avian influenza viruses isolated from an overwintering site in Japan throughout different winter seasons. Arch. Virol. 2020, 165, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Okuya, K.; Kawabata, T.; Matsuu, A.; Takase, K.; Kuwahara, M.; Toda, S.; Ozawa, M. Genetic characterization of low-pathogenic avian influenza viruses isolated on the Izumi plain in Japan: Possible association of dynamic movements of wild birds with AIV evolution. Arch. Virol. 2018, 163, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Kojima, I.; Fukunaga, W.; Okajima, M.; Mitarai, S.; Fujimoto, Y.; Matsui, T.; Kuwahara, M.; Masatani, T.; Okuya, K.; et al. Improved method for avian influenza virus isolation from environmental water samples. Transbound. Emerg. Dis. 2022, 69, e2889–e2897. [Google Scholar] [CrossRef]
- Cannella, V.; Altomare, R.; Chiaramonte, G.; Di Bella, S.; Mira, F.; Russotto, L.; Pisano, P.; Guercio, A. Cytotoxicity Evaluation of Endodontic Pins on L929 Cell Line. BioMed Res. Int. 2019, 2019, 3469525. [Google Scholar] [CrossRef]
- Romano, M.R.; Gatto, C.; Giurgola, L.; Ragazzi, E.; D’Amato Tóthová, J. Toxicity Threshold of Perfluorocarbon Liquids for Intraocular Use: Dose–Response Assessment of In Vitro Cytotoxicity of Possible Contaminants. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Romano, M.R.; Ferrara, M.; Gatto, C.; Ferrari, B.; Giurgola, L.; D’Amato Tóthová, J. Evaluation of Cytotoxicity of Perfluorocarbons for Intraocular Use by Cytotoxicity Test In Vitro in Cell Lines and Human Donor Retina Ex Vivo. Transl. Vision. Sci. Technol. 2019, 8, 24. [Google Scholar] [CrossRef]
- Querido, M.M.; Rosário, F.; Bessa, M.J.; Mendes, F.; Teixeira, J.C.; Teixeira, J.P.; Pereira, C.C. In Vitro Cyto- and Genotoxicity Assessment of Antibacterial Paints with Triclosan and Isoborneol. Toxics 2022, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, H.F.; Schwebke, I.; Blumel, J.; Eggers, M.; Glebe, D.; Rapp, I.; Sauerbrei, A.; Steinmann, E.; Steinmann, J.; Willkommen, H.; et al. Guideline of the German Association for the Control of Viral Diseases (DVV) eV and the Robert Koch Institute (RKI) for testing chemical disinfectants for effectiveness against viruses in human medicine. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2015, 58, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, T.O.; Takakuwa, K.; Komatsu, H.; Anti-Avian Influenza, H. Virus Properties of Ortho dichlorobenzene Cresol Complex Formulation with Strong Antimicrobial Activity. J. Jpn. Vet. Med. Assoc. 2019, 72, 205–209. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Lambert, P.A.; Hammond, S.M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 1973, 54, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Badanthadka, M.; Mehendale, H.M. Cresols. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 1061–1065. [Google Scholar]
- Oxford, J.S.; Lambkin, R.; Gibb, I.; Balasingam, S.; Chan, C.; Catchpole, A. A throat lozenge containing amyl meta cresol and dicholorobenzyl alchol has a direct virucidal effect on respiratory syncytial virus, influenza A and SARS-CoV. Antivir. Chem. Chemother. 2005, 16, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Olitsky, P.K.; Boez, L. Studies on the physical and chemical properties of the virus of foot-and-mouth disease: III. Resistance to chemicals. J. Exp. Med. 1927, 45, 815–831. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Smith-Burchnell, C.A.; Dalgleish, A.G. Evaluation of hypochlorite-releasing disinfectants against the human immunodeficiency virus (HIV). J. Hosp. Infect. 1990, 15, 237–238. [Google Scholar] [CrossRef]
- Dee, S.; Deen, J.; Burns, D.; Douthit, G.; Pijoan, C. An evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperature. Can. J. Vet. Res. 2005, 69, 64–70. [Google Scholar]
- Davison, S.; Benson, C.E.; Ziegler, A.F.; Eckroade, R.J. Evaluation of disinfectants with the addition of antifreezing compounds against non-pathogenic H7N2 avian influenza virus. Avian Dis. 1999, 43, 533–537. [Google Scholar] [CrossRef]
- Guan, J.; Chan, M.; Brooks, B.W.; Rohonczy, E. Enhanced inactivation of avian influenza virus at −20 °C by disinfectants supplemented with calcium chloride or other antifreeze agents. Can. J. Vet. Res. 2015, 79, 347–350. [Google Scholar] [PubMed]
- Jang, Y.; Lee, J.; So, B.; Lee, K.; Yun, S.; Lee, M.; Choe, N. Evaluation of changes induced by temperature, contact time, and surface in the efficacies of disinfectants against avian influenza virus. Poult. Sci. 2014, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rohaim, M.A.; El-Naggar, R.F.; Gamal, A.M.; Ismael, E.; Hamoud, M.M.; Moubarak, S.T.; Metwally, A.M.; Zaki, M.M.; Nasr, S.A.E.; Elsaid, S.; et al. Efficacy of disinfectants against Egyptian H5N1 avian influenza virus. Br. J. Virol. 2015, 2, 80–87. [Google Scholar] [CrossRef]
| Class | Disinfectant ID | Active Ingredient | Proportion |
|---|---|---|---|
| Chlorine | A | Sodium dichloro isocyanurate | 60% (w/w) |
| B | Potassium peroxomonosulphate | 50% (w/w) | |
| Sodium chloride | 1.5% (w/w) | ||
| Glutaraldehyde | C | Glutaraldehyde | 25% (w/v) |
| Phenol | D | Ortho-dichlorobenzene | 88.5% (w/w) |
| Quinomethionate | 1.5% (w/w) | ||
| E | Ortho-dichlorobenzene | 75% (w/w) | |
| Cresol | 7% (w/w) | ||
| F | Ortho-dichlorobenzene | 72% (w/w) | |
| Didecyldimethylammonium chloride | 12% (w/w) | ||
| Chlorocresol | 5% (w/w) | ||
| G | Ortho-dichlorobenzene | 67% (w/w) | |
| Chlororthophenylphenol | 2% (w/w) | ||
| Chlorocresol | 10% (w/w) |
| Temperature | Organic Matter | Highest Dilution of Disinfectant E with Virucidal Activity against AIV of this Subtype * | ||||
|---|---|---|---|---|---|---|
| H4N6 | H6N2 | H7N9 | H11N9 | H5N8 | ||
| RT | Absent | 100 | 200 | 200 | 200 | 100 |
| Present | 100 | 200 | 200 | 200 | 100 | |
| 4 °C | Absent | 100 | 200 | 200 | 200 | 100 |
| Present | 100 | 200 | 200 | 200 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.M.; Esaki, M.; Okuya, K.; Ozawa, M. Stability of the Virucidal Activity of Commercial Disinfectants against Avian Influenza Viruses under Different Environmental Conditions. Pathogens 2023, 12, 1382. https://doi.org/10.3390/pathogens12121382
Khalil AM, Esaki M, Okuya K, Ozawa M. Stability of the Virucidal Activity of Commercial Disinfectants against Avian Influenza Viruses under Different Environmental Conditions. Pathogens. 2023; 12(12):1382. https://doi.org/10.3390/pathogens12121382
Chicago/Turabian StyleKhalil, Ahmed Magdy, Mana Esaki, Kosuke Okuya, and Makoto Ozawa. 2023. "Stability of the Virucidal Activity of Commercial Disinfectants against Avian Influenza Viruses under Different Environmental Conditions" Pathogens 12, no. 12: 1382. https://doi.org/10.3390/pathogens12121382
APA StyleKhalil, A. M., Esaki, M., Okuya, K., & Ozawa, M. (2023). Stability of the Virucidal Activity of Commercial Disinfectants against Avian Influenza Viruses under Different Environmental Conditions. Pathogens, 12(12), 1382. https://doi.org/10.3390/pathogens12121382








