Invasive and Non-Invasive Human Salmonellosis Cases Admitted between 2015 and 2021 in Four Suburban Hospitals in the Metropolitan Area of Milan (Italy): A Multi-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Procedures
- Anagraphic data (name, age, sex, nationality, place of residence).
- Date and hospital of diagnosis.
- Body fluid on which Salmonella grew (defined as blood, feces, urine, or other).
- Species, serogroup, and serotype (defined as the O-antigen group and serotype’s name, when available) of the isolate.
- AMR pattern of the isolate.
- Clinical history (including a history of immunodepression).
- Area of hospitalization (defined as OBGYN/Pediatrics, Internal Medicine Division, Surgery Division, Intensive Care, Emergency Department).
- Symptoms at presentation.
- Date of symptoms’ onset.
- Blood tests at presentation.
- Date and length of hospital admission.
- Antimicrobial treatment (including empirical and antibiogram-aimed treatment).
- Infectious Diseases Specialist consultation during the hospital stay.
- Outcome (defined as dead or discharged).
2.3. Definitions
2.4. Microbiology
2.5. Statistical Analyses
3. Results
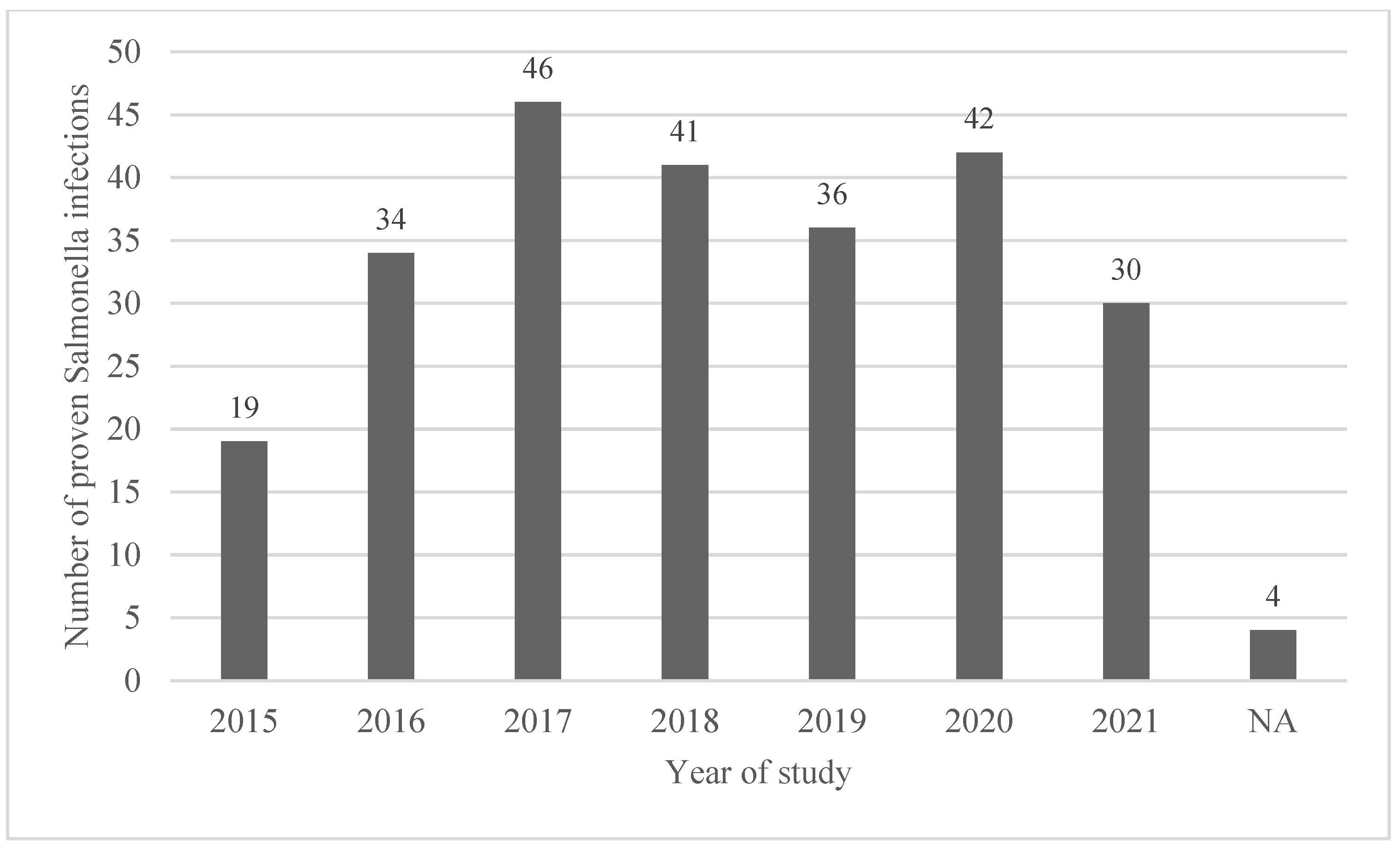
3.1. Demographics and Hospital Admission
3.2. Site of Salmonella spp. Isolation
3.3. Mortality
3.4. Serogroups and Serotypes Prevalence
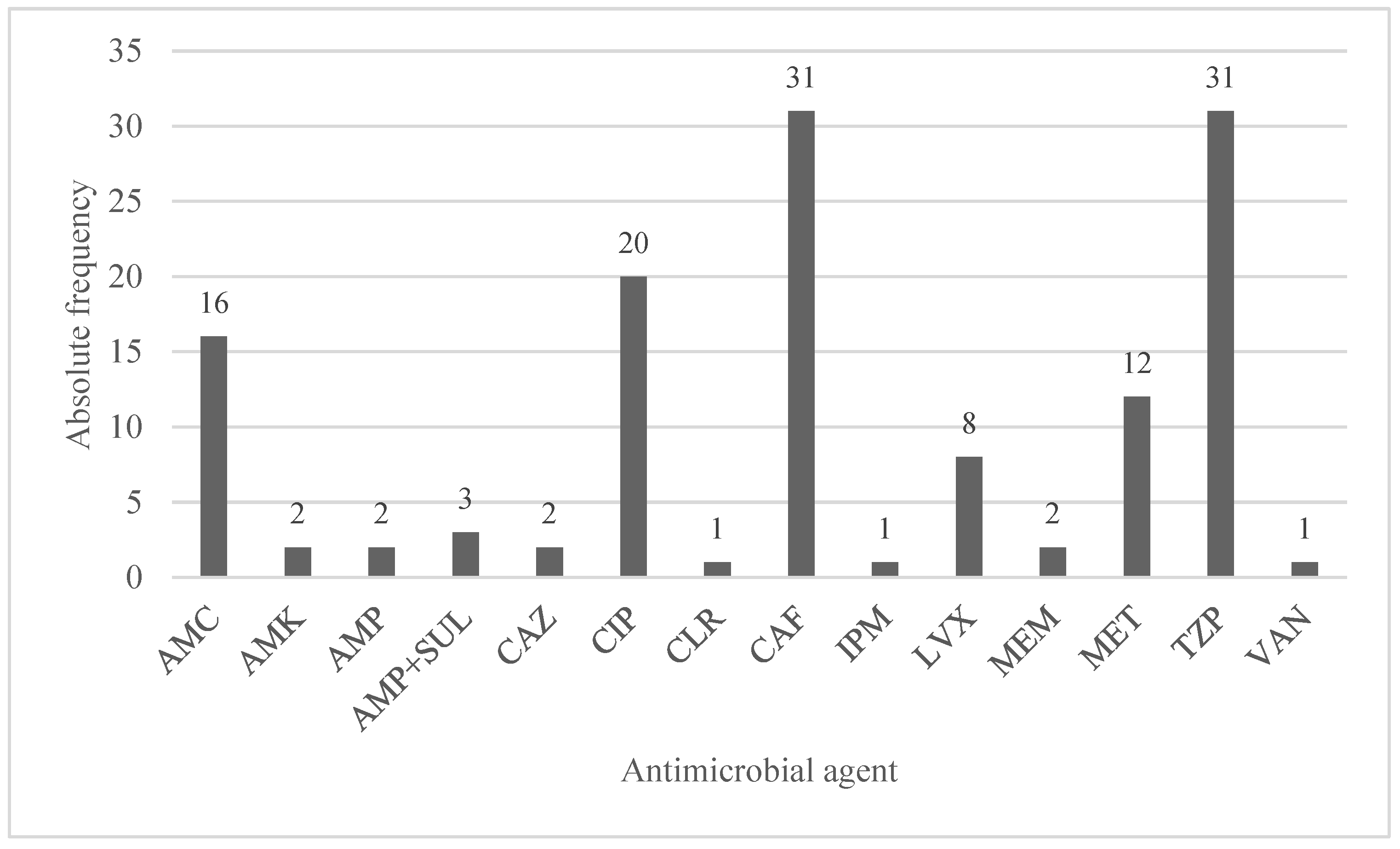
3.5. Antimicrobial Treatments and Antimicrobial Resistance
3.6. Signs and Symptoms
3.7. Laboratory Values
3.8. Factors Associated with Bacteremia
3.9. Factors Associated with Antibiotic-Resistant Salmonella Infection
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannella, R.A. Salmonella. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8435/ (accessed on 15 March 2023).
- GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar]
- Authority, E.F.S.; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Lan, N.P.H.; Le Thi Phuong, T.; Huu, H.N.; Thuy, L.; Mather, A.E.; Park, S.E.; Marks, F.; Thwaites, G.E.; Van Vinh Chau, N.; Thompson, C.N.; et al. Invasive Non-typhoidal Salmonella Infections in Asia: Clinical Observations, Disease Outcome, and Dominant Serovars from an Infectious Disease Hospital in Vietnam. PLoS Negl. Trop. Dis. 2016, 10, e0004857. [Google Scholar]
- Mughini-Gras, L.; Pijnacker, R.; Duijster, J.; Heck, M.; Wit, B.; Veldman, K.; Franz, E. Changing epidemiology of invasive non-typhoid Salmonella infection: A nationwide population-based registry study. Clin. Microbiol. Infect. 2020, 26, 941.e9–941.e14. [Google Scholar] [CrossRef] [PubMed]
- Butler, T. Treatment of typhoid fever in the 21st century: Promises and shortcomings. Clin. Microbiol. Infect. 2011, 17, 959–963. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Zhao, S.; Tate, H. Antimicrobial Resistance in Nontyphoidal Salmonella. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30314348/ (accessed on 15 March 2023).
- Varma, J.K.; Mølbak, K.; Barrett, T.J.; Beebe, J.L.; Jones, T.F.; Rabatsky-Ehr, T.; Smith, K.E.; Vugia, D.J.; Chang, H.H.; Angulo, F.J. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 2005, 191, 554–561. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union One Health 2018 Zoonoses Report. EFSA 2019, 17, e05926. [Google Scholar] [CrossRef]
- Alessiani, A.; Goffredo, E.; Mancini, M.; Occhiochiuso, G.; Faleo, S.; Didonna, A.; Fischetto, R.; Suglia, F.; De Vito, D.; Stallone, A.; et al. Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy. Microorganisms 2022, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Lauteri, C.; Festino, A.R.; Conter, M.; Vergara, A. Prevalence and antimicrobial resistance profile in Salmonella spp. isolates from the swine food chain. Ital. J. Food Saf. 2022, 11, 9980. [Google Scholar] [CrossRef] [PubMed]
- Huedo, P.; Gori, M.; Amato, E.; Bianchi, R.; Valerio, E.; Magnoli, L.; Pontello, M. A Multischool Outbreak Due to Salmonella enterica serovar Napoli Associated with Elevated Rates of Hospitalizations and Bacteremia, Milan, Italy, 2014. Foodborne Pathog. Dis. 2016, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- EpiCentro. Salmonella. Available online: https://www.epicentro.iss.it/salmonella/ (accessed on 15 March 2023).
- Login—ENTERNET. Available online: https://w3.iss.it/site/RMI/enternet/Default.aspx?ReturnUrl=%2fsite%2frmi%2fenternet (accessed on 15 March 2023).
- Backhaus, E.; Berg, S.; Andersson, R.; Ockborn, G.; Malmström, P.; Dahl, M.; Nasic, S.; Trollfors, B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence, and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Graziani, C.; Busani, L.; Dionisi, A.M.; Caprioli, A.; Ivarsson, S.; Hedenström, I.; Luzzi, I.; Volpe, G.; Delibato, E.; Fabiani, L.; et al. Virulotyping of Salmonella enterica serovar Napoli strains isolated in Italy from human and nonhuman sources. Foodborne Pathog. Dis. 2011, 8, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Graziani, C.; Luzzi, I.; Owczarek, S.; Dionisi, A.M.; Busani, L. Salmonella enterica Serovar Napoli Infection in Italy from 2000 to 2013: Spatial and Spatio-Temporal Analysis of Cases Distribution and the Effect of Human and Animal Density on the Risk of Infection. PLoS ONE 2015, 10, e0142419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huedo, P.; Gori, M.; Zolin, A.; Amato, E.; Ciceri, G.; Bossi, A.; Pontello, M. Salmonella enterica Serotype Napoli is the First Cause of Invasive Nontyphoidal Salmonellosis in Lombardy, Italy (2010–2014), and Belongs to Typhi Subclade. Foodborne Pathog. Dis. 2017, 14, 148–151. [Google Scholar] [CrossRef] [PubMed]
- The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2022.7209 (accessed on 15 March 2023).

| Bacteriemic Infections | Non-Bacteriemic Infections | Overall | p-Value | |
|---|---|---|---|---|
| Proven cases, N (%) | 41 (16.3%) | 211 (83.7%) | 252 | |
| Age (median, IQR) | 68 (19–81) | 10 (3–67) | 15 (4–71) | <0.001 |
| Sex, N (%) | 0.260 | |||
| Male | 21 (51.2) | 128 (60.7) | 149 (59.1) | |
| Female | 20 (48.8) | 83 (39.3) | 103 (40.9) | |
| Male/Female | 1.05:1 | 1.51:1 | 1.45:1 | |
| Hospital unit, N (%) | 0.001 | |||
| OB/GYN and Pediatrics | 10 (24.5) | 122 (57.8) | 132 (52.4) | |
| Internal Medicine | 17 (41.6) | 59 (28) | 76 (30.2) | |
| Emergency Room | 12 (29.4) | 22 (10.5) | 34 (13.4) | |
| Surgery | 2 (4.5) | 6 (2.8) | 8 (3.2) | |
| Intensive Care | 0 | 2 (0.9) | 2 (0.8) | |
| Municipality of origin, N (%) | 0.172 | |||
| Abbiategrasso | 5 (12.2) | 28 (13.4) | 33 (13.1) | |
| Magenta | 5 (12.2) | 64 (30.3) | 69 (27.4) | |
| Cuggiono | 7 (17.1) | 30 (14.3) | 37 (14.4) | |
| Legnano | 14 (34.1) | 52 (24.6) | 66 (26.2) | |
| Other | 10 (24.4) | 37 (17.5) | 47 (18.6) | |
| Immune-suppression, N (%) | 0.078 | |||
| Iatrogenic | 4 (9.8) | 2 (0.9) | 6 (2.3) | |
| Oncohaematological | 1 (2.4) | 5 (2.4) | 6 (2.3) | |
| HIV-related | 0 | 2 (0.9) | 2 (0.8) | |
| Length of hospital stay, days (median, IQR) * | 10 (8–13) | 4 (3–7) | 5 (3–9) | <0.001 |
| Symptoms-to-diagnosis interval (median, IQR) | 4 (1–6) | 3 (2–5) | 3 (2–5) | 0.055 |
| ID consultation N (%) | 6 | 21 | 27 | 0.263 |
| Outcome, N (%) | 0.013 | |||
| Death | 4 (9.8%) | 3 (1.4%) | 7 (2.8%) | |
| Discharge | 37 (90.2%) | 208 (98.6%) | 245 (97.2%) | |
| Antibiotic regimen, N (%) | 38 (92.7%) | 82 (38.9%) | 120 (47.6%) | <0.001 |
| Inappropriate first-line regimen, N (%) | 2 (5.3%) | 14 (17.5%) | 16 (13.3%) | 0.135 |
| Serogroups/Serovars | Absolute Frequency | Frequency % |
|---|---|---|
| O:4 | 133 | 52.8 |
| Typhimurium | 98 | 38.1 |
| Brandenburg | 9 | 3.5 |
| Derby | 9 | 3.5 |
| Var. Monofasica | 8 | 3.1 |
| Agbeni | 1 | 0.4 |
| Bredeney | 1 | 0.4 |
| Chester | 1 | 0.4 |
| Kapemba | 1 | 0.4 |
| Saintpaul | 1 | 0.4 |
| ND | 10 | 4.0 |
| O:9 | 56 | 22.2 |
| Napoli | 33 | 15.5 |
| Enteritidis | 18 | 7.1 |
| Typhi | 2 | 0.6 |
| Panama | 1 | 0.4 |
| ND | 4 | 1.6 |
| O:7 | 25 | 9.9 |
| Infantis | 13 | 5.1 |
| Braenderup | 1 | 0.4 |
| Isangi | 1 | 0.4 |
| Livingstone | 1 | 0.4 |
| Rissen | 1 | 0.4 |
| Strathcona | 1 | 0.4 |
| Thompson | 1 | 0.4 |
| Virchow | 1 | 0.4 |
| ND | 4 | 1.6 |
| O:8 | 9 | 3.6 |
| Bovismorbificans | 4 | 1.6 |
| Goldcoast | 2 | 0.8 |
| Blockley | 1 | 0.4 |
| ND | 2 | 0.8 |
| O:3, 10 | 2 | 0.8 |
| Muenster | 1 | 0.4 |
| London | 1 | 0.4 |
| O:11 | 2 | 0.8 |
| Veneziana | 2 | 0.8 |
| O:13 | 2 | 0.8 |
| Kedougou | 1 | 0.4 |
| Poona | 1 | 0.4 |
| O:2 | 1 | 0.4 |
| Paratyphi A | 1 | 0.4 |
| ND | 14 | 5.6 |
| Overall | 252 | 100 |
| Serogroups, N (%) | Bacteriemic Infections (n = 41) | Non-Bacteriemic Infections (n = 211) | Overall (n = 252) | p-Value |
|---|---|---|---|---|
| O:4 | 11 (26.8) | 122 (57.7) | 133 (52.8) | 0.001 |
| O:9 | 18 (43.9) | 38 (18) | 56 (22.2) | <0.001 |
| O:7 | 5 (12.3) | 20 (9.5) | 25 (9.9) | 0.594 |
| ND | 6 (14.6) | 16 (7.5) | 22 (8.7) | / |
| O:8 | 0 | 9 (4.3) | 9 (3.6) | / |
| O:3, 10 | 0 | 2 (1.0) | 2(0.8) | / |
| O:11 | 0 | 2 (1.0) | 2 (0.8) | / |
| O:13 | 0 | 2 (1.0) | 2 (0.8) | / |
| O:2 | 1 (2.4) | 0 | 1 (0.4) | 0.163 |
| Overall | 41 (100) | 211 (100) | 252 (100) |
| Serovars, N (%) | Bacteriemic Infections (n = 41) | Non-bacteriemic Infections (n = 211) | Overall (n = 252) | p-Value |
|---|---|---|---|---|
| Typhimurium | 7 (17) | 91 (43.1) | 98 (38.9) | 0.002 |
| Napoli | 13 (31.7) | 20 (9.5) | 33 (13.1) | <0.001 |
| Enteritidis | 5 (12.2) | 13 (6.2) | 18 (7.1) | 0.332 |
| Infantis | 2 (4.9) | 11 (5.2) | 13 (5.6) | 0.929 |
| Derby | 0 | 9 (4.3) | 9 (3.6) | 0.362 |
| Brandenburg | 2 (4.9) | 7 (3.3) | 9 (3.6) | 0.361 |
| Other | 12 (29.3) | 60 (28.4) | 72 (28.6) |
| Frequency of Antimicrobial Resistance, N (%) * | |
|---|---|
| Isolates showing resistance to at least 1 antibiotic | 122 (60.4) |
| Amikacin | 22 (10.9) |
| Gentamicin | 29 (14.3) |
| Amoxicillin/clavulanate | 54 (26.7) |
| Ampicillin | 44 (21.8) |
| Cefepime | 1 (0.5) |
| Cefotaxime | 4 (2.0) |
| Ceftazidime | 1 (0.5) |
| Ceftriaxone | 10 (5.0) |
| Ciprofloxacin | 19 (9.4) |
| Fosfomycin | 0 |
| Meropenem | 0 |
| Piperacillin | 26 (12.9) |
| Tigecyclin | 12 (5.9) |
| Piperacillin/tazobactam | 4 (2.0) |
| Trimethoprim/sulfamethoxazole | 18 (8.9) |
| Ertapenem | 0 |
| Signs and Symptoms, N (%) | Bacteriemic Infections (n = 41) | Non Bacteriemic Infections (n = 211) | Overall (n = 252) | p-Value |
|---|---|---|---|---|
| Diarrhea | 32 (78.0) | 196 (92.9) | 228 (90.5) | 0.007 |
| Fever | 35 (85.4) | 171 (81.1) | 206 (81.7) | 0.292 |
| Vomit | 16 (39.0) | 84 (39.8) | 100 (39.7) | 0.535 |
| Abdominal pain | 14 (34.1) | 64 (30.3) | 78 (31) | 0.377 |
| Malaise | 10 (24.4) | 19 (9.0) | 29 (11.5) | 0.008 |
| Nausea | 3 (7.3) | 12 (5.7) | 15 (6) | 0.435 |
| Sensory alterations | 4 (9.8) | 11 (5.2) | 15 (6) | 0.213 |
| Dyspnea | 5 (12.2) | 8 (3.8) | 13 (5.2) | 0.042 |
| Neurological symptoms | 8 (19.5) | 2 (0.9) | 9 (3.6) | 0.505 |
| Pneumonia | 1 (2.4) | 1 (0.5) | 2 (0.8) | 0.299 |
| Septic arthritis | 0 | 1 (0.5) | 1 (0.4) | 0.842 |
| Bacteriemic Infections (n = 41) Median (IQR) | Non Bacteriemic Infections (n = 211) Median (IQR) | Overall (n = 252) Median (IQR) | p-Value | |
|---|---|---|---|---|
| Hb (g/dL) | 13.7 (12.1–14.2) | 13.2 (12.1–14.1) | 13.2 (12.1–14.1) | 0.354 |
| Hct (%) | 39.2 (35.0–43.8) | 39.2 (36.5–42.3) | 39.4% (36.4–42.9) | 0.863 |
| PLT (/mcL) | 179,500 (123,750–240,500) | 236,000 (182,500–299,000) | 239,000 (175,000–287,000) | <0.001 |
| WBC (/mcL) | 6600 (4375–9428) | 9300 (7500–13,200) | 9100 (6700–12,450) | <0.001 |
| N (/mcL) | 5150 (3019–7591) | 6836 (4357–9728) | 6396 (4230–9379) | 0.635 |
| L (/mcL) | 943 (496–1441) | 1124 (770–2165) | 1189 (746–2081) | 0.970 |
| M (/mcL) | 617 (284–891) | 831 (607–1109) | 807 (555–1091) | 0.395 |
| E (/mcL) | 6 (0–15) | 9 (0–28) | 8 (0–27) | 0.629 |
| B (/mcL) | 20 (10–36) | 28 (17–45) | 27 (16–45) | 0.912 |
| LDH (U/L) | 258 (178–405) | 242 (202–310) | 244 (200–331) | 0.270 |
| AST (U/L) | 36 (23–53) | 30 (21–40) | 30 (21–41) | 0.093 |
| ALT (U/L) | 27 (21–43) | 20 (16–29) | 22 (17–31) | 0.002 |
| BR (mg/dL) | 0.63 (0.4–1.01) | 0.56 (0.37–0.77) | 0.58 (0.37–0.79) | 0.185 |
| Glu (mg/dL) | 119 (98–143) | 106 (89–122) | 107 (90–126) | 0.008 |
| BUN (mg/dL) | 35 (23–74) | 26 (19–50) | 27 (19–57) | 0.036 |
| sCr (mg/dL) | 0.96 (0.79–1.78) | 0.56 (0.38–1.15) | 0.71 (0.39–1.22) | 0.001 |
| CRP (mg/L) | 6.90 (3.20–14.6) | 6.12 (2.68–10.83) | 6.20 (2.79–11.40) | 0.314 |
| PT (INR) | 1.17 (1.09–1.34) | 1.23 (1.09–1.45) | 1.22 (1.09–1.42) | 0.421 |
| aPTT | 0.97 (0.91–1.07) | 0.98 (0.90–1.08) | 0.9 (0.9–1.07) | 0.681 |
| Na+ (mEq/L) | 137 (134–140) | 136 (133–138) | 136 (133–139) | 0.359 |
| K+ (mEq/L) | 3.78 (3.4–4.18) | 3.93 (3.53–4.20) | 3.9 (3.5–4.2) | 0.253 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Age 12–65 year-old vs. age ≤ 12 year-old | 3.21 | 1.24–8.29 | 0.016 | 3.35 | 1.17–9.56 | 0.024 |
| Age > 65 year-old vs. age ≤ 12 year-old | 4.79 | 2.06–11.16 | 0.001 | 5.78 | 2.28–14.67 | 0.001 |
| Female sex | 1.47 | 0.75–2.88 | 0.262 | 0.68 | 0.32–1.44 | 0.309 |
| Days from symptoms to diagnosis | 0.99 | 0.94–1.06 | 0.971 | 0.98 | 0.93–1.05 | 0.591 |
| O:4 vs. others | 0.39 | 0.17–0.89 | 0.025 | 0.65 | 0.27–1.59 | 0.349 |
| O:9 vs. others | 1.84 | 0.79–4.23 | 0.154 | 2.96 | 1.15–7.63 | 0.025 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Age 12–65 year-old vs. age ≤ 12 year-old | 0.48 | 0.23–0.99 | 0.049 | 0.39 | 0.16–0.91 | 0.031 |
| Age > 65 year-old vs. age ≤ 12 year-old | 0.53 | 0.28–1.03 | 0.06 | 0.52 | 0.24–1.11 | 0.089 |
| Female sex | 2.12 | 1.17–3.84 | 0.014 | 2.94 | 1.47–5.87 | 0.002 |
| Year of infection (per every year more) | 1.37 | 1.13–1.67 | 0.002 | 1.50 | 1.19–1.89 | 0.001 |
| O:4 vs. others | 2.67 | 1.39–5.14 | 0.003 | 2.18 | 1.06–4.47 | 0.035 |
| O:9 vs. others | 0.41 | 0.17–0.98 | 0.045 | 0.22 | 0.08–0.63 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagani, G.; Parenti, M.; Franzetti, M.; Pezzati, L.; Bassani, F.; Osnaghi, B.; Vismara, L.; Pavia, C.; Mirri, P.; Rusconi, S. Invasive and Non-Invasive Human Salmonellosis Cases Admitted between 2015 and 2021 in Four Suburban Hospitals in the Metropolitan Area of Milan (Italy): A Multi-Center Retrospective Study. Pathogens 2023, 12, 1298. https://doi.org/10.3390/pathogens12111298
Pagani G, Parenti M, Franzetti M, Pezzati L, Bassani F, Osnaghi B, Vismara L, Pavia C, Mirri P, Rusconi S. Invasive and Non-Invasive Human Salmonellosis Cases Admitted between 2015 and 2021 in Four Suburban Hospitals in the Metropolitan Area of Milan (Italy): A Multi-Center Retrospective Study. Pathogens. 2023; 12(11):1298. https://doi.org/10.3390/pathogens12111298
Chicago/Turabian StylePagani, Gabriele, Marco Parenti, Marco Franzetti, Laura Pezzati, Francesco Bassani, Bianca Osnaghi, Laura Vismara, Claudia Pavia, Paola Mirri, and Stefano Rusconi. 2023. "Invasive and Non-Invasive Human Salmonellosis Cases Admitted between 2015 and 2021 in Four Suburban Hospitals in the Metropolitan Area of Milan (Italy): A Multi-Center Retrospective Study" Pathogens 12, no. 11: 1298. https://doi.org/10.3390/pathogens12111298
APA StylePagani, G., Parenti, M., Franzetti, M., Pezzati, L., Bassani, F., Osnaghi, B., Vismara, L., Pavia, C., Mirri, P., & Rusconi, S. (2023). Invasive and Non-Invasive Human Salmonellosis Cases Admitted between 2015 and 2021 in Four Suburban Hospitals in the Metropolitan Area of Milan (Italy): A Multi-Center Retrospective Study. Pathogens, 12(11), 1298. https://doi.org/10.3390/pathogens12111298








