Evidence for Dicot Plants as Alternative Hosts of Banana Bunchy Top Virus and Its Alphasatellites in South-East Asia
Abstract
:1. Introduction
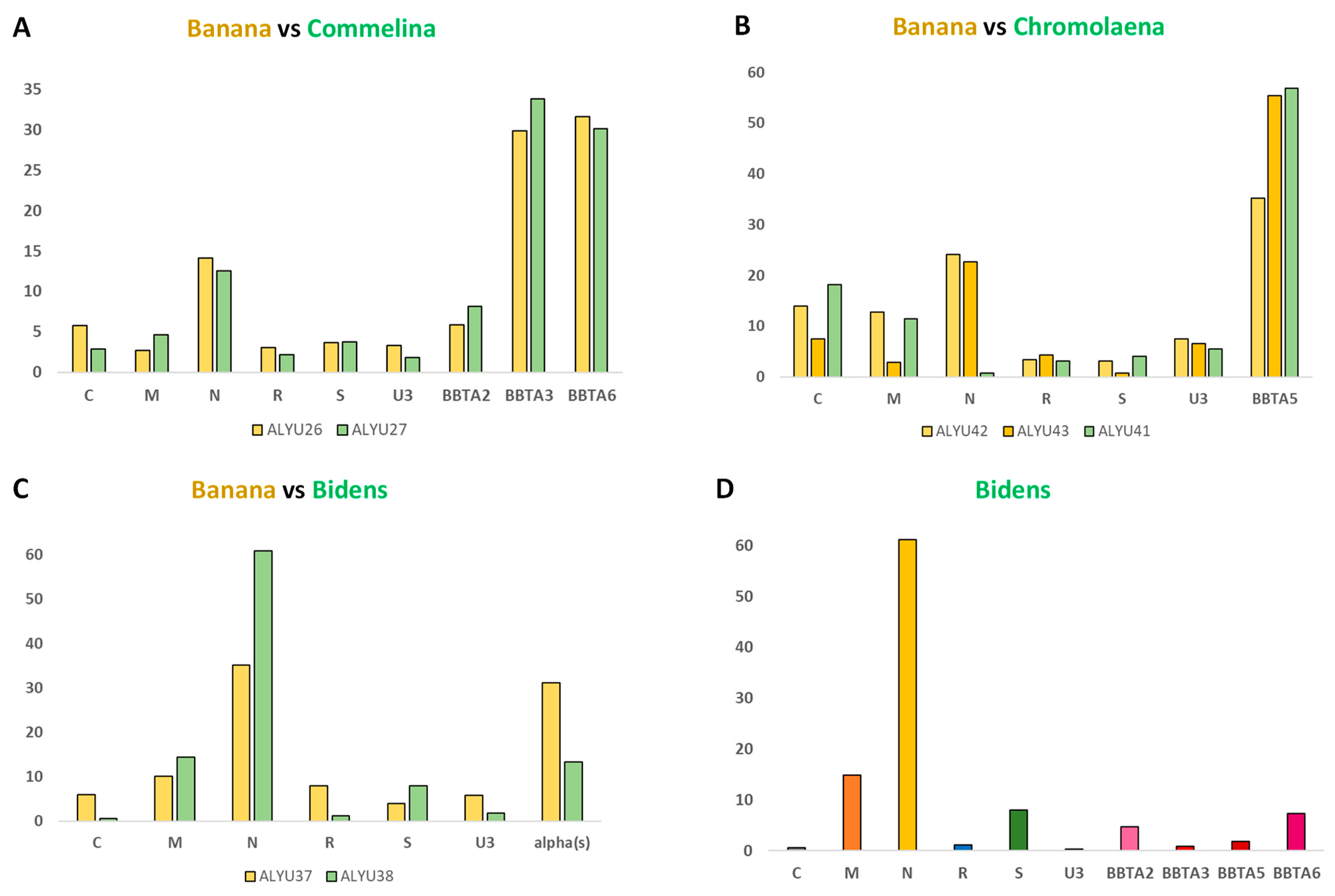
2. Results and Discussion
3. Materials and Methods
3.1. Surveys in Vietnam, Laos, and China
3.2. Total DNA Extraction from Banana Leaf Samples
3.3. Rolling Circle Amplification (RCA)
3.4. Polymerase Chain Reaction (PCR) Analysis
3.5. Illumina Sequencing of RCA Products and De Novo Reconstruction of Viral Genomes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dale, J.L. Banana bunchy top: An economically important tropical plant virus disease. Advances in Virus Research 33: 301–325. Banana bunchy top: An economically important tropical plant virus disease. Adv. Virus Res. 1987, 33, 301–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.L.; Selvarajan, R.; Iskra-Caruana, M.-L.; Chabannes, M.; Hanna, R. Biology, Etiology, and Control of Virus Diseases of Banana and Plantain. Adv. Virus Res. 2015, 91, 229–269. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Greenwell, A.M.; Bressan, A. Localization, concentration, and transmission efficiency of banana bunchy top virus in four asexual lineages of Pentalonia aphids. Viruses 2013, 5, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.M.; Harding, R.M.; Dale, J.L. The genome organization of banana bunchy top virus: Analysis of six ssDNA components. J. Gen. Virol. 1995, 76, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Wanitchakorn, R.; Hafner, G.J.; Harding, R.M.; Dale, J.L. Functional analysis of proteins encoded by banana bunchy top virus DNA-4 to -6. J. Gen. Virol. 2000, 81, 299–306. [Google Scholar] [CrossRef]
- Qazi, J. Banana bunchy top virus and the bunchy top disease. J. Gen. Plant Pathol. 2016, 82, 2–11. [Google Scholar] [CrossRef]
- Horser, C.L.; Harding, R.M.; Dale, J.L. Banana bunchy top nanovirus DNA-1 encodes the ‘master’ replication initiation protein. J. Gen. Virol. 2001, 82, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Dale, J.L.; Ha, C.V.; Vu, M.T.; Revill, P.A. Characterisation of Rep-encoding components associated with banana bunchy top nanovirus in Vietnam. Arch. Virol. 2002, 147, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Guyot, V.; Rajeswaran, R.; Chu, H.C.; Karthikeyan, C.; Laboureau, N.; Galzi, S.; Mukwa, L.F.T.; Krupovic, M.; Kumar, P.L.; Iskra-Caruana, M.L.; et al. A newly emerging alphasatellite affects banana bunchy top virus replication, transcription, siRNA production and transmission by aphids. PLoS Pathog. 2022, 18, e1010448. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.E.; Iskra-Caruana, M.L. Diseases caused by viruses. In Diseases of Banana, Abaca and Enset; Jones, D.R., Ed.; GABI: Wallingford, UK, 2000; pp. 241–253. [Google Scholar]
- Thomas, J.E.; Geering, A.D.W.; Dahal, G.; Lockhart, B.E.L.; Thottappilly, G. Banana and Plantain. In Virus and Virus-like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 477–496. [Google Scholar] [CrossRef]
- Pinili, M.S.; Nagashima, I.; Dizon, T.O.; Natsuaki, K.T. Cross-Transmission and New Alternate Hosts of Banana bunchy top virus. Trop. Agric. Dev. 2013, 57, 1–7. [Google Scholar] [CrossRef]
- Hamim, I.; Green, J.C.; Borth, W.B.; Melzer, M.J.; Wang, Y.N.; Hu, J.S. First report of Banana bunchy top virus in Heliconia spp. on Hawaii. Plant Dis. 2017, 101, 2153. [Google Scholar] [CrossRef]
- Rahayuniati, R.F.; Subandiyah, S.; Hartono, S.; Somowiyarjo, S.; Kurniawan, R.E.K.; Prakoso, A.B.; Crew, K.; Vance, M.E.; Ray, J.D.; Thomas, J.E. Recent distribution and diversity analysis on banana bunchy top virus of banana and alternative host in Indonesia. Trop. Plant Pathol. 2021, 46, 506–517. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Trees: An Identification and Information Guide; GABI: Wallingford, UK, 1994; p. 466. [Google Scholar]
- Bagariang, W.; Hidayat, P.; Hidayat, S.H. Morphometric Analysis and Host Range of the Genus Pentalonia Coquerel (Hemiptera: Aphididae) Infesting Banana in Java. J. Perlindungan Tanam. Indones. 2019, 23, 171. [Google Scholar] [CrossRef]
- Seguin, J.; Otten, P.; Baerlocher, L.; Farinelli, L.; Pooggin, M.M. MISIS-2: A bioinformatics tool for in-depth analysis of small RNAs and representation of consensus master genome in viral quasispecies. J. Virol. Methods 2016, 233, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, I.; Vetten, H.J.; Commandeur, U.; Ziebell, H.; Gronenborn, B.; Timchenko, T. Nanovirus DNA-N encodes a protein mandatory for aphid transmission. Virology 2018, 522, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, J.; Vernerey, M.-S.; Yvon, M.; Pirolles, E.; Villegas, M.; Gaafar, Y.; Ziebell, H.; Michalakis, Y.; Zeddam, J.-L.; Blanc, S. Route of a Multipartite Nanovirus across the Body of Its Aphid Vector. J. Virol. 2020, 94, e01998-19. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Bressan, A. Tropism, compartmentalization and retention of banana bunchy top virus (Nanoviridae) in the aphid vector Pentalonia nigronervosa. J. Gen. Virol. 2013, 94, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Wang, M.; Sether, D.; Xie, W.; Leonhardt, K.W. Use of polymerase chain reaction (PCR) to study transmission of banana bunchy top virus by the banana aphid (Pentalonia nigronervosa). Ann. Appl. Biol. 1996, 128, 55–64. [Google Scholar] [CrossRef]
- Magee, C.J.P. Transmission Studies on the Banana Bunchy-top Virus. J. Aust. Inst. Agric. Sci. 1940, 6, 109–110. [Google Scholar]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Wheeler, D.J. A Block-Sorting Lossless Data Compression Algorithm; Technical Report 124; Digital Equipment Corporation, Digital Equipment Corporation Press: Palo Alto, CA, USA, 1994; pp. 1–24. [Google Scholar]
| Sample/Isolate | Country | Plant Species/Genome/Cultivar | BBTV DNA-R PCR | BBTV Genome Illumina | No. of Alpha-Satellites | BBTA2 | BBTA3 | BBTA5 | BBTA6 | Defective (d) Molecules | Other Viruses |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALYU-25 | Vietnam | Musa itinerans | (+) | full | 2 | 1 | 1 | Badnavirus | |||
| ALYU-26 | Vietnam | Musa sp. | (+) | full | 3 | 1 | 1 | 1 | |||
| ALYU-27 | Vietnam | Commelina sp. | (+) | full | 3 | 1 | 1 | 1 | |||
| ALYU-28 | Vietnam | Ipomoea aquatica | (+) | no * | |||||||
| ALYU-29 | Vietnam | Musa AA Pisang mas? | (+) | full | 3 | 1 | 1 | 1 | dA5, dA2, dR | ||
| ALYU-30 | Vietnam | Phyllanthus sp. | (+) | no * | |||||||
| ALYU-31 | Vietnam | Arachis hypogaea | (+) | no* | |||||||
| ALYU-32 | Vietnam | Musa sp. sweet banana | (+) | full | 2 | 1 | 1 | dA2 | |||
| ALYU-33 | Vietnam | Musa AAB Chuoi Ngop | (+) | full | 2 | 1 | 1 | Badnavirus | |||
| ALYU-34 | Vietnam | Musa sp. | (+) | full | 2 | 1 | 1 | ||||
| ALYU-35 | Vietnam | Musa sp. | (+) | full | 1 | 1 | |||||
| ALYU-36 | Vietnam | Musa sp. | (+) | full | 1 | 1 | |||||
| ALYU-37 | Vietnam | Musa sp. | (+) | full | 1 | 1 | |||||
| ALYU-38 | Vietnam | Bidens pilosa | (+) | full | 4 | 1 | 1 | 1 | 1 | Circo-, Microvirus | |
| ALYU-39 | Vietnam | Musa sp. | (+) | full | 2 | 1 | 1 | ||||
| ALYU-40 | Vietnam | Musa AAA red banana | (+) | full | 1 | 1 | |||||
| ALYU-41 | Laos | Chromolaena odorata | (+) | no N * | 1 | 1 | |||||
| ALYU-42 | Laos | Musa AAA Cavendish | (+) | full | 1 | 1 | |||||
| ALYU-43 | Laos | Musa AAA Cavendish | (+) | full | 1 | 1 | |||||
| ALYU-44 | Laos | Musa ornata | (+) | full | |||||||
| ALYU-45 | Laos | Musa sp. | (+) | full | |||||||
| ALYU-46 | Laos | Musa ABB Klue Tiparot | (−) | no ** | |||||||
| ALYU-47 | Laos | Musa yunnenensis | (+) | full | |||||||
| ALYU-48 | Laos | Musa sp. | (+) | full | |||||||
| ALYU-49 | Laos | Musa sp. | (+) | full | |||||||
| ALYU-50 | Laos | Musa AA Kouay niew mung | (+) | full | |||||||
| ALYU-51 | Laos | Musa ABB Pisang Awak? | (+) | full | |||||||
| ALYU-52 | China | Musa acuminata wild | (−) | no ** | |||||||
| ALYU-53 | China | Musa yunnanensis | (+) | full | |||||||
| ALYU-54 | China | Musa AAA Cavendish | (+) | full | 2 | 1 | 1 | dA6 | |||
| ALYU-55 | China | Musa AAA Cavendish | (+) | full | 3 | 1 | 1 | 1 | A5-U3 chimera | ||
| ALYU-56 | China | Musa AAA Cavendish | (+) | full | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyot, V.; Ly, N.-S.; Trieu, T.-D.; Insisiengmay, O.; Zhang, T.; Iskra-Caruana, M.-L.; BforBB Consortium; Pooggin, M.M. Evidence for Dicot Plants as Alternative Hosts of Banana Bunchy Top Virus and Its Alphasatellites in South-East Asia. Pathogens 2023, 12, 1289. https://doi.org/10.3390/pathogens12111289
Guyot V, Ly N-S, Trieu T-D, Insisiengmay O, Zhang T, Iskra-Caruana M-L, BforBB Consortium, Pooggin MM. Evidence for Dicot Plants as Alternative Hosts of Banana Bunchy Top Virus and Its Alphasatellites in South-East Asia. Pathogens. 2023; 12(11):1289. https://doi.org/10.3390/pathogens12111289
Chicago/Turabian StyleGuyot, Valentin, Ngoc-Sam Ly, Tien-Dung Trieu, Oudomphone Insisiengmay, Ting Zhang, Marie-Line Iskra-Caruana, BforBB Consortium, and Mikhail M. Pooggin. 2023. "Evidence for Dicot Plants as Alternative Hosts of Banana Bunchy Top Virus and Its Alphasatellites in South-East Asia" Pathogens 12, no. 11: 1289. https://doi.org/10.3390/pathogens12111289
APA StyleGuyot, V., Ly, N.-S., Trieu, T.-D., Insisiengmay, O., Zhang, T., Iskra-Caruana, M.-L., BforBB Consortium, & Pooggin, M. M. (2023). Evidence for Dicot Plants as Alternative Hosts of Banana Bunchy Top Virus and Its Alphasatellites in South-East Asia. Pathogens, 12(11), 1289. https://doi.org/10.3390/pathogens12111289







