Feline SCCs of the Head and Neck Display Partial Epithelial-Mesenchymal Transition and Harbor Stem Cell-like Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material
2.2. Tumor Grading
2.3. Immunohistochemical Staining (IHC)
2.4. Immunofluorescent Staining (IF)
2.5. Statistics
3. Results
3.1. All but One Feline HNSCCs Corresponded to Invasive Lesions
3.2. Immunohistochemical Staining Results
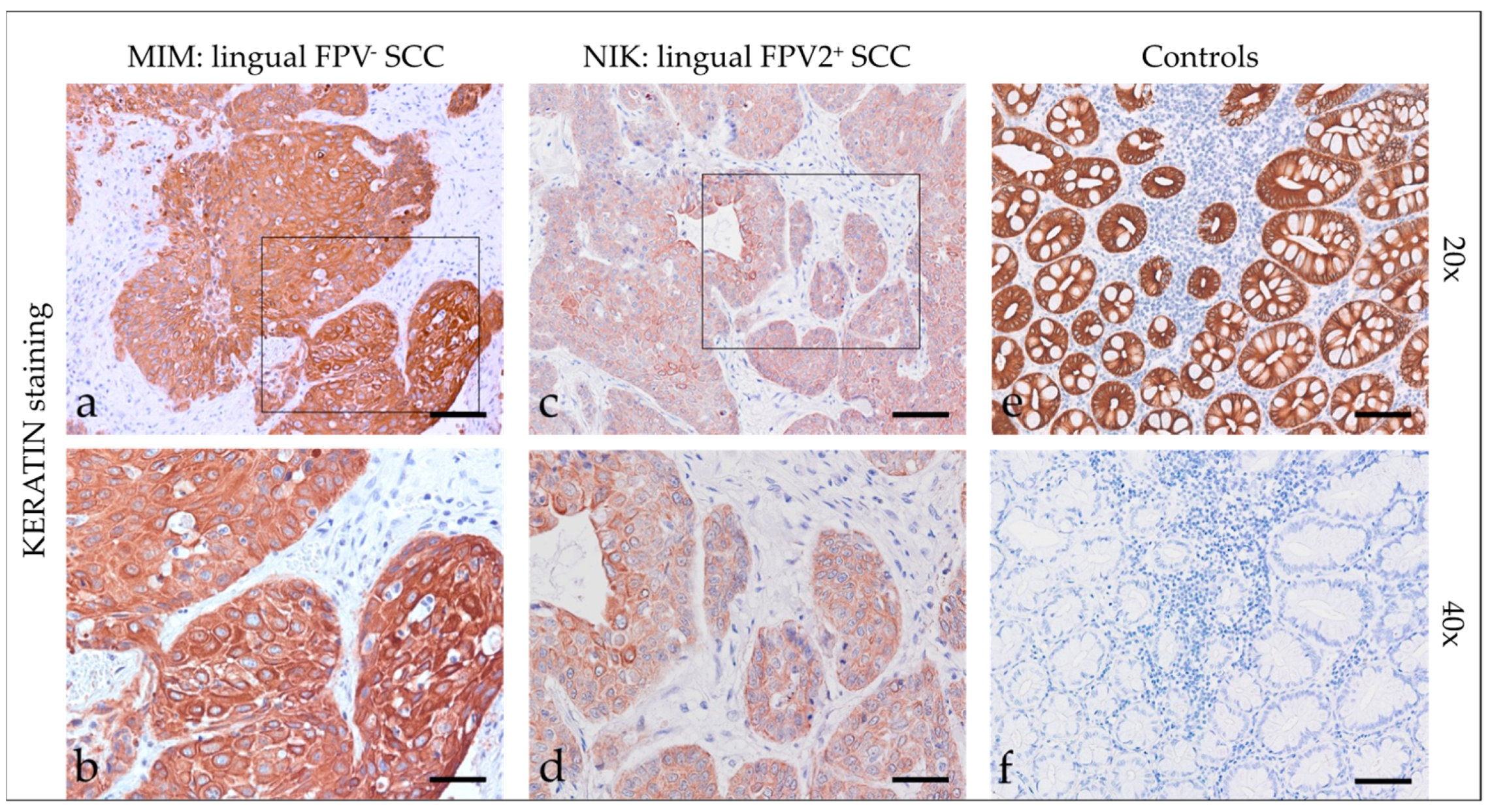
3.2.1. Keratins
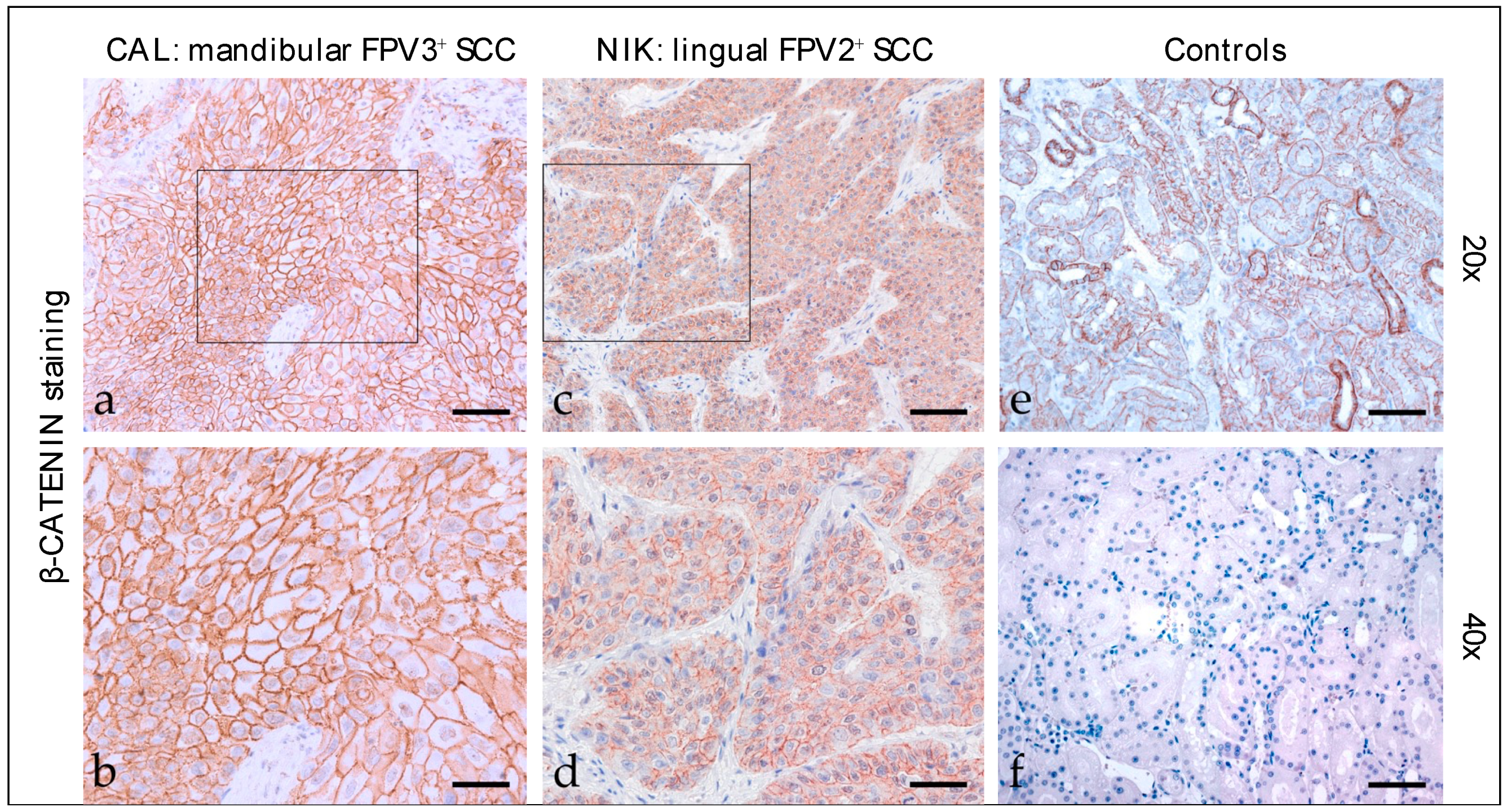
3.2.2. β-Catenin
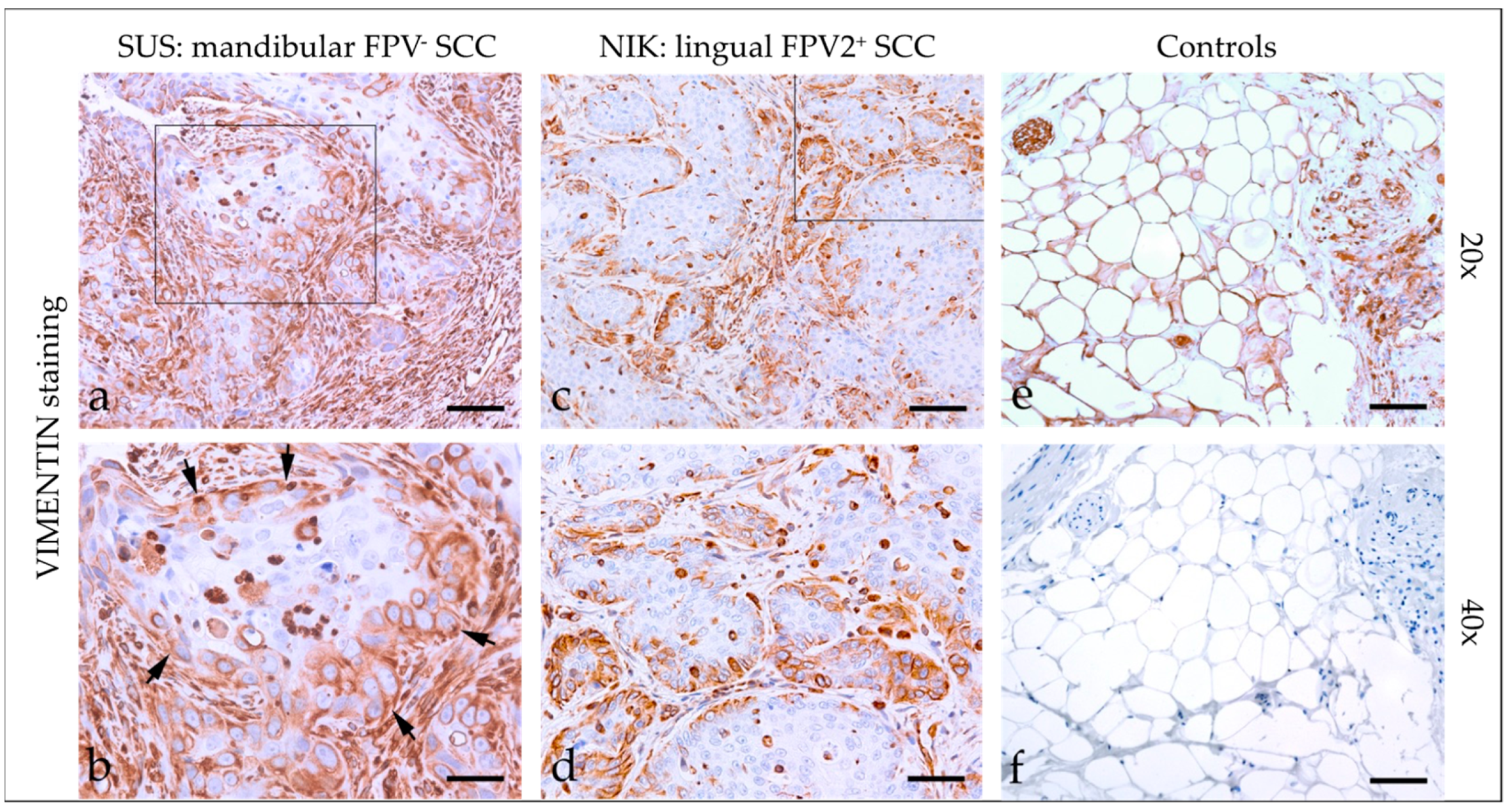
3.2.3. Vimentin
3.2.4. CD146
3.2.5. Cyclooxygenase-2 (COX-2)
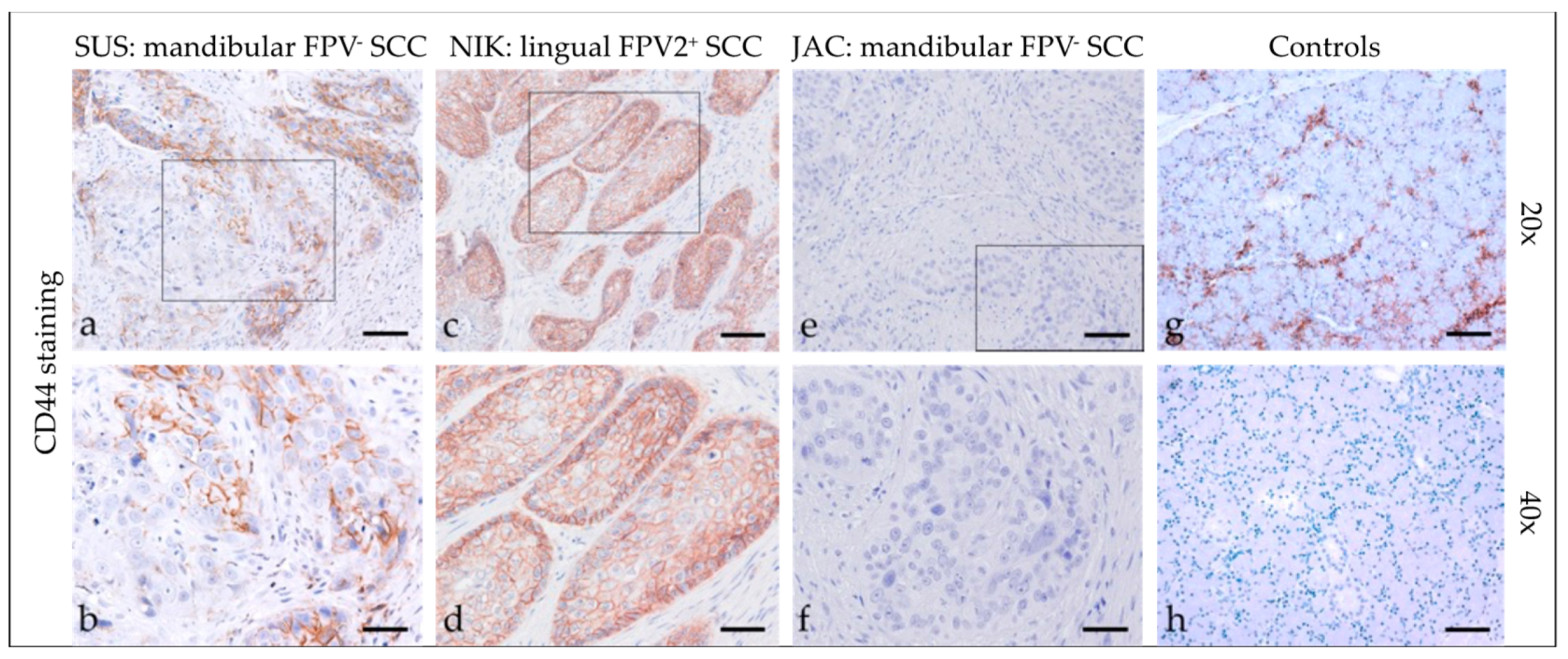
3.2.6. CD44
3.2.7. CD271 (p75NTR)
3.2.8. Feline HNSCCs Scored Positive for EMT and CSC Markers
| Code | Tumor Localization | Keratin | β-Catenin | Vimentin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | D | % +Cells | I | D | % +Cells | I | D | % +Cells | ||
| CAL * | mandibular | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <10 |
| JAC * | mandibular | 2 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <10 |
| MAX * | oromandibular | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <50 |
| MIM | lingual | 3 | diffuse | 100 | 2 | diffuse | 100 | 1–3 | patchy | >50 |
| MIN | mandibular | 1–2 | diffuse | 100 | 2 | diffuse | 100 | 3 | patchy | <50 |
| MMI | mandibular | 3 | diffuse | 100 | 1 | Patchy | >50 | 3 | patchy | <10 |
| NEL | lingual | 1–2 | diffuse | 100 | 3 | diffuse | 100 | 2–3 | patchy | <50 |
| NIK * | lingual, labial | 1–2 | diffuse | 100 | 2 | diffuse | 100 | 3 | patchy | <50 |
| PAG | oral | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <10 |
| PUP | nasopharyngeal, orbital | 2–3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <10 |
| SHA | mandibular | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <50 |
| SNO | mandibular | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | <50 |
| SUR * | nasopharyngeal | 2–3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | >50 |
| SUS | mandibular | 3 | diffuse | 100 | 3 | diffuse | 100 | 3 | patchy | >50 |
| Code | CD146 | COX-2 | CD44 | CD271 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | D | % +Cells | I | D | % +Cells | I | D | % +Cells | I | D | % +Cells | |
| CAL * | 1 | diffuse | 100 | 0–3 | patchy | <50 | 0–3 | patchy | <50 | 1–3 | diffuse | 100 |
| JAC * | 1–2 | patchy | >50 | 0–3 | patchy | >50 | 0–2 | patchy | <10 | 2–3 | diffuse | 100 |
| MAX * | 1 | diffuse | 100 | 0–2 | patchy | <50 | 0 | - | - | 3 | diffuse | 100 |
| MIM | 1 | diffuse | 100 | 0–3 | patchy | <50 | 0–2 | patchy | >50 | 2–3 | diffuse | 100 |
| MIN | 1–2 | diffuse | 100 | 0–2 | patchy | <10 | 0–3 | patchy | <50 | 1–3 | diffuse | 100 |
| MMI | 0 | - | 0 | 0–2 | patchy | <10 | 0–1 | patchy | <10 | 2 | patchy | <50 |
| NEL | 1–2 | diffuse | 100 | 1–3 | patchy | >50 | 0–3 | patchy | <50 | 1–2 | diffuse | 100 |
| NIK * | 0 | - | 0 | 0–3 | patchy | <10 | 0–3 | patchy | >50 | 1–2 | diffuse | 100 |
| PAG | 1 | diffuse | 100 | 0–2 | patchy | <10 | 0–2 | patchy | >50 | 2 | diffuse | 100 |
| PUP | 1 | diffuse | 100 | 0–2 | patchy | >50 | 0–3 | patchy | <50 | 1–3 | diffuse | 100 |
| SHA | 1 | diffuse | 100 | 0–3 | patchy | <50 | 3 | diffuse | 100 | 2–3 | diffuse | 100 |
| SNO | 1 | diffuse | 100 | 0–2 | patchy | <50 | 0–3 | patchy | <50 | 2–3 | diffuse | 100 |
| SUR * | 1–2 | diffuse | 100 | 0–3 | patchy | >50 | 3 | patchy | <50 | 1–2 | diffuse | 100 |
| SUS | 1 | diffuse | 100 | 0–3 | patchy | >50 | 0–3 | patchy | <50 | 1–3 | diffuse | 100 |
3.3. IF Double Staining Results Are Suggestive of pEMT and the Presence of CSCs in Feline HNSCCs
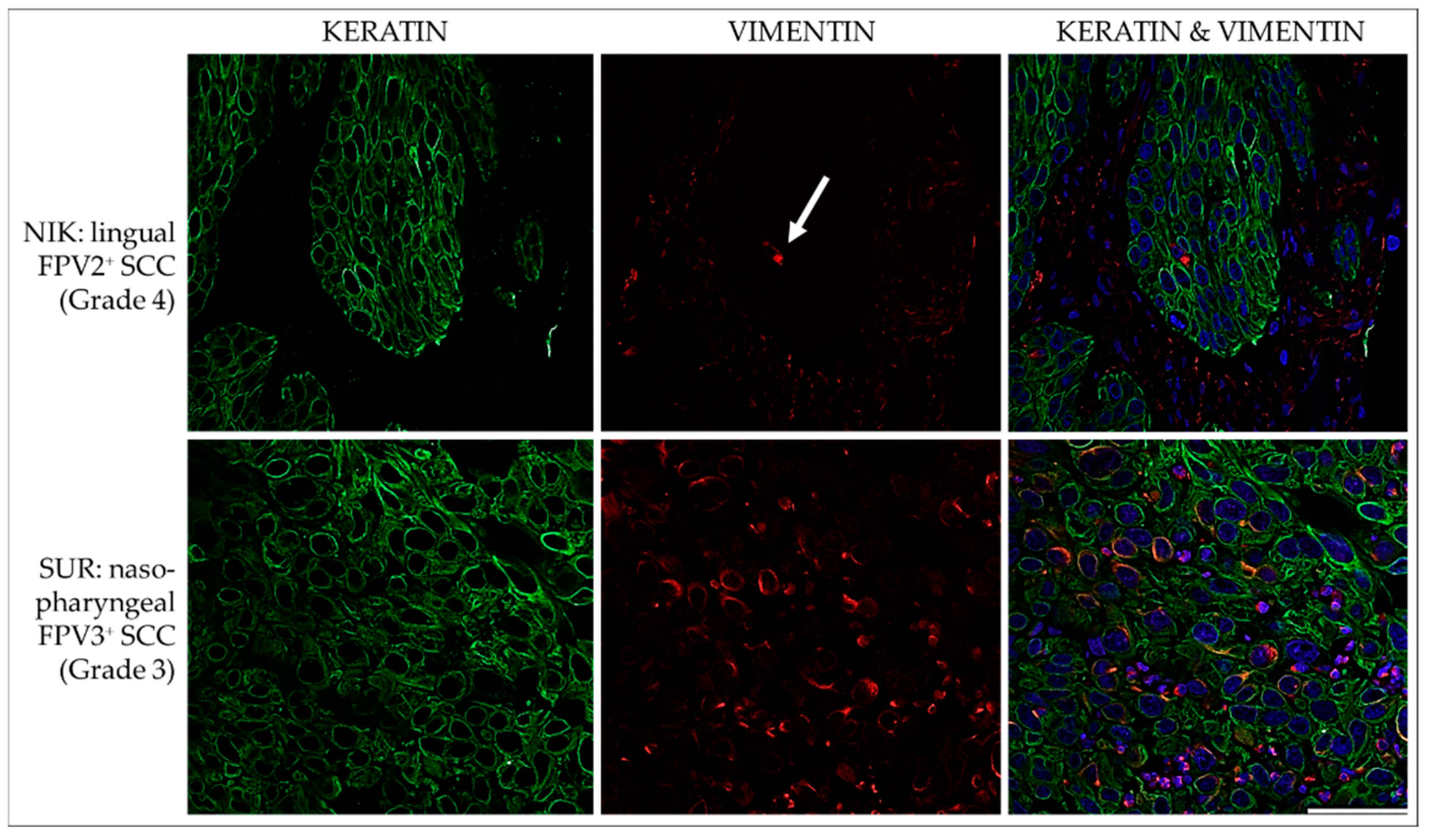
3.3.1. Keratin and Vimentin
3.3.2. E-Cadherin and N-Cadherin
3.3.3. CD44 and CD271
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Withrow, S.J.; Vail, D.M.; Page, R.L. (Eds.) Squamous cell carcinoma. In Withrow & MacEwen’s Small Animal Clinical Oncology, 5th ed.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 310–312. [Google Scholar]
- Sequeira, I.; Pires, M.D.A.; Leitao, J.; Henriques, J.; Viegas, C.; Requicha, J. Feline Oral Squamous Cell Carcinoma: A Critical Review of Etiologic Factors. Vet. Sci. 2022, 9, 558. [Google Scholar] [CrossRef]
- Altamura, G.; Cuccaro, B.; Eleni, C.; Strohmayer, C.; Brandt, S.; Borzacchiello, G. Investigation of multiple Felis catus papillomavirus types (-1/-2/-3/-4/-5/-6) DNAs in feline oral squamous cell carcinoma: A multicentric study. J. Vet. Med. Sci. 2022, 84, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Salo, T. Identity matters: Cancer stem cells and tumour plasticity in head and neck squamous cell carcinoma. Expert Rev. Mol. Med. 2023, 25, E8. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, R.; Ortiz-Sarabia, G.; Molina-Frechero, N.; Salas-Pacheco, J.M.; Salas-Pacheco, S.M.; Lavalle-Carrasco, J.; Lopez-Verdin, S.; Tremillo-Maldonado, O.; Bologna-Molina, R. Epithelial-Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review. Cancers 2021, 13, 3027. [Google Scholar] [CrossRef]
- Hujanen, R.; Almahmoudi, R.; Salo, T.; Salem, A. Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches. Cancers 2021, 13, 4747. [Google Scholar] [CrossRef]
- Oshimori, N. Cancer stem cells and their niche in the progression of squamous cell carcinoma. Cancer Sci. 2020, 111, 3985–3992. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liu, L.; Zhang, S.; Yang, X.; Wang, Y. Cancer stem cell biomarkers for head and neck squamous cell carcinoma: A bioinformatic analysis. Oncol. Rep. 2018, 40, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Cirillo, N. The molecular markers of cancer stem cells in head and neck tumors. J. Cell Physiol. 2020, 235, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, Q.; An, J.; Long, Q.; Wang, H.; Xiang, M.; Xiang, M.; Zhao, Y.; Liu, Y.; Liu, J.; et al. Partial EMT in Squamous Cell Carcinoma: A Snapshot. Int. J. Biol. Sci. 2021, 17, 3036–3047. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef]
- Kisoda, S.; Mouri, Y.; Kitamura, N.; Yamamoto, T.; Miyoshi, K.; Kudo, Y. The role of partial-EMT in the progression of head and neck squamous cell carcinoma. J. Oral. Biosci. 2022, 64, 176–182. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–929. [Google Scholar] [CrossRef]
- Okuyama, K.; Suzuki, K.; Yanamoto, S. Relationship between Tumor Budding and Partial Epithelial-Mesenchymal Transition in Head and Neck Cancer. Cancers 2023, 15, 1111. [Google Scholar] [CrossRef]
- Pang, L.Y.; Bergkvist, G.T.; Cervantes-Arias, A.; Yool, D.A.; Muirhead, R.; Argyle, D.J. Identification of tumour initiating cells in feline head and neck squamous cell carcinoma and evidence for gefitinib induced epithelial to mesenchymal transition. Vet. J. 2012, 193, 46–52. [Google Scholar] [CrossRef]
- Harris, K.; Gelberg, H.B.; Kiupel, M.; Helfand, S.C. Immunohistochemical Features of Epithelial-Mesenchymal Transition in Feline Oral Squamous Cell Carcinoma. Vet. Pathol. 2019, 56, 826–839. [Google Scholar] [CrossRef]
- Strohmayer, C.; Klang, A.; Kummer, S.; Walter, I.; Jindra, C.; Weissenbacher-Lang, C.; Redmer, T.; Kneissl, S.; Brandt, S. Tumor Cell Plasticity in Equine Papillomavirus-Positive Versus-Negative Squamous Cell Carcinoma of the Head and Neck. Pathogens 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Anneroth, G.; Batsakis, J.; Luna, M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand. J. Dent. Res. 1987, 95, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013, 330, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Wood, N.H.; Khammissa, R.A.; Lemmer, J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 2: Human papillomavirus associated oral and oropharyngeal squamous cell carcinoma. Head Face Med. 2010, 6, 15. [Google Scholar] [CrossRef]
- Fleming, J.C.; Woo, J.; Moutasim, K.; Mellone, M.; Frampton, S.J.; Mead, A.; Ahmed, W.; Wood, O.; Robinson, H.; Ward, M.; et al. HPV, tumour metabolism and novel target identification in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 356–367. [Google Scholar] [CrossRef]
- Yamashita-Kawanishi, N.; Chang, C.Y.; Chambers, J.K.; Uchida, K.; Sugiura, K.; Kukimoto, I.; Chang, H.W.; Haga, T. Comparison of prevalence of Felis catus papillomavirus type 2 in squamous cell carcinomas in cats between Taiwan and Japan. J. Vet. Med. Sci. 2021, 83, 1229–1233. [Google Scholar] [CrossRef]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Arrindell, J.; Desnues, B. Vimentin: From a cytoskeletal protein to a critical modulator of immune response and a target for infection. Front. Immunol. 2023, 14, 1224352. [Google Scholar] [CrossRef]
- Kudo, Y.; Kitajima, S.; Ogawa, I.; Hiraoka, M.; Sargolzaei, S.; Keikhaee, M.R.; Sato, S.; Miyauchi, M.; Takata, T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin. Cancer Res. 2004, 10, 5455–5463. [Google Scholar] [CrossRef]
- Armando, F.; Godizzi, F.; Razzuoli, E.; Leonardi, F.; Angelone, M.; Corradi, A.; Meloni, D.; Ferrari, L.; Passeri, B. Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Stenner, M.; Yosef, B.; Huebbers, C.U.; Preuss, S.F.; Dienes, H.P.; Speel, E.J.; Odenthal, M.; Klussmann, J.P. Nuclear translocation of beta-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: An early event in human papillomavirus-related tumour progression? Histopathology 2011, 58, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Andreani, G.; Rocchigiani, G.; Lorenzi, D.; Poli, A. Correlation Between Cyclo-oxygenase-2 and Vascular Endothelial Growth Factor Expression in Canine and Feline Squamous Cell Carcinomas. J. Comp. Pathol. 2016, 154, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Toomey, D.; Conroy, H.; Jarnicki, A.G.; Higgins, S.C.; Sutton, C.; Mills, K.H. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine 2008, 26, 3540–3549. [Google Scholar] [CrossRef]
- Frejborg, E.; Salo, T.; Salem, A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 9246. [Google Scholar] [CrossRef]
- DiBernardi, L.; Dore, M.; Davis, J.A.; Owens, J.G.; Mohammed, S.I.; Guptill, C.F.; Knapp, D.W. Study of feline oral squamous cell carcinoma: Potential target for cyclooxygenase inhibitor treatment. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 245–250. [Google Scholar] [CrossRef]
- Janakiraman, H.; House, R.P.; Talwar, S.; Courtney, S.M.; Hazard, E.S.; Hardiman, G.; Mehrotra, S.; Howe, P.H.; Gangaraju, V.; Palanisamy, V. Repression of caspase-3 and RNA-binding protein HuR cleavage by cyclooxygenase-2 promotes drug resistance in oral squamous cell carcinoma. Oncogene 2017, 36, 3137–3148. [Google Scholar] [CrossRef]
- Saka-Herran, C.; Jane-Salas, E.; Estrugo-Devesa, A.; Lopez-Lopez, J. Head and neck cancer and non-steroidal anti-inflammatory drugs: Systematic review and meta-analysis. Head. Neck 2021, 43, 1664–1682. [Google Scholar] [CrossRef]
- Sadasivam, S.; Subramanian, R. A perspective on challenges and opportunities in characterizing oral cancer stem cells. Front. Biosci. (Landmark Ed) 2020, 25, 1011–1021. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Chesa, P.G.; Rettig, W.J.; Thomson, T.M.; Old, L.J.; Melamed, M.R. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. J. Histochem. Cytochem. 1988, 36, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Thomson, T.M.; Rettig, W.J.; Chesa, P.G.; Green, S.H.; Mena, A.C.; Old, L.J. Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp. Cell Res. 1988, 174, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Viejo, M.; Menendez-Menendez, Y.; Otero-Hernandez, J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J. Stem Cells 2015, 7, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Redmer, T. Decoding the Role of CD271 in Melanoma. Cancers 2020, 12, 2460. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Huczynski, A. Salinomycin and its derivatives - A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–227. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Luo, Q.; Ju, Y.; Song, G. Salinomycin Suppresses Tumorigenicity of Liver Cancer Stem Cells and Wnt/Beta-catenin Signaling. Curr. Stem. Cell Res. Ther. 2021, 16, 630–637. [Google Scholar] [CrossRef]
- Mai, T.T.; Hamai, A.; Hienzsch, A.; Caneque, T.; Muller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumour Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 2012, 287, 32800–32824. [Google Scholar] [CrossRef]
- Reategui, E.P.; de Mayolo, A.A.; Das, P.M.; Astor, F.C.; Singal, R.; Hamilton, K.L.; Goodwin, W.J.; Carraway, K.L.; Franzmann, E.J. Characterization of CD44v3-containing isoforms in head and neck cancer. Cancer Biol. Ther. 2006, 5, 1163–1168. [Google Scholar] [CrossRef][Green Version]
- Wang, S.J.; Wreesmann, V.B.; Bourguignon, L.Y. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck 2007, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]





| No. | Code | Breed | Age in Years | Sex | Tumor Localization | FPV Infection Status |
|---|---|---|---|---|---|---|
| 1 | CAL | Norwegian Forest | 14 | fs | mandibular | FPV3 |
| 2 | JAC | ESH | 14 | m | mandibular | FPV1 |
| 5 | MAX | ESH | 8 | mc | mandibular | FPV3 |
| 8 | MIM | ESH | 12 | fs | lingual | negative |
| 6 | MIN | ESH | 13 | fs | mandibular | negative |
| 10 | MMI | ESH | 15 | fs | mandibular | negative |
| 7 | NEL | Persian | 12 | fs | lingual | negative |
| 12 | NIK | ESH | 3 | mc | lingual, labial | FPV2 |
| 9 | PAG | Persian | 14 | fs | oral | negative |
| 14 | PUP | ESH | 14 | fs | nasopharyngeal, orbital | negative |
| 3 | SHA | Abyssinian | 12 | mc | mandibular | negative |
| 4 | SNO | ESH | 15 | fs | mandibular | negative |
| 11 | SUR | ESH | 11 | fs | nasopharyngeal | FPV3 |
| 13 | SUS | ESH | 14 | fs | mandibular | negative |
| Code | KRT | NPM | Mitotic Cells/HPF | Invasion Pattern | Invasion Stage | LPI | Total Score | Grade |
|---|---|---|---|---|---|---|---|---|
| CAL * | 1 | 2 | 1 | 2 | 4 | 4 | 14 | 2 |
| JAC * | 2 | 3 | 4 | 4 | 4 | 4 | 21 | 4 |
| MAX | 4 | 2 | 4 | 3 | 3 | 2 | 18 | 3 |
| MIM | 4 | 3 | 4 | 4 | 4 | 3 | 22 | 4 |
| MIN | 3 | 3 | 4 | 3 | 4 | 1 | 18 | 3 |
| MMI | 4 | 2 | ne * | 4 | 4 | 4 | 19+ | 3–4 |
| NEL | 4 | 3 | 4 | 4 | 4 | 2 | 21 | 4 |
| NIK * | 4 | 3 | 4 | 4 | 4 | 2 | 21 | 4 |
| PAG | 3 | 2 | ne * | 4 | 3 | 4 | 17+ | 3 |
| PUP | 3 | 2 | 1 | 4 | 4 | 4 | 18 | 3 |
| SHA* | 4 | 4 | 4 | 2 | 4 | 4 | 22 | 4 |
| SNO | 3 | 3 | ne * | 4 | 4 | 3 | 18+ | 3 |
| SUR * | 3 | 3 | 4 | 2 | 4 | 3 | 19 | 3 |
| SUS | 4 | 4 | 4 | 4 | 4 | 3 | 23 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kummer, S.; Klang, A.; Strohmayer, C.; Walter, I.; Jindra, C.; Kneissl, S.; Brandt, S. Feline SCCs of the Head and Neck Display Partial Epithelial-Mesenchymal Transition and Harbor Stem Cell-like Cancer Cells. Pathogens 2023, 12, 1288. https://doi.org/10.3390/pathogens12111288
Kummer S, Klang A, Strohmayer C, Walter I, Jindra C, Kneissl S, Brandt S. Feline SCCs of the Head and Neck Display Partial Epithelial-Mesenchymal Transition and Harbor Stem Cell-like Cancer Cells. Pathogens. 2023; 12(11):1288. https://doi.org/10.3390/pathogens12111288
Chicago/Turabian StyleKummer, Stefan, Andrea Klang, Carina Strohmayer, Ingrid Walter, Christoph Jindra, Sibylle Kneissl, and Sabine Brandt. 2023. "Feline SCCs of the Head and Neck Display Partial Epithelial-Mesenchymal Transition and Harbor Stem Cell-like Cancer Cells" Pathogens 12, no. 11: 1288. https://doi.org/10.3390/pathogens12111288
APA StyleKummer, S., Klang, A., Strohmayer, C., Walter, I., Jindra, C., Kneissl, S., & Brandt, S. (2023). Feline SCCs of the Head and Neck Display Partial Epithelial-Mesenchymal Transition and Harbor Stem Cell-like Cancer Cells. Pathogens, 12(11), 1288. https://doi.org/10.3390/pathogens12111288






