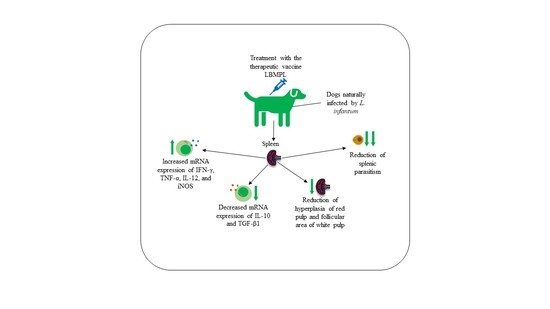
LBMPL Vaccine Therapy Induces Progressive Organization of the Spleen Microarchitecture, Improved Th1 Adaptative Immune Response and Control of Parasitism in Leishmania infantum Naturally Infected Dogs
Abstract
1. Introduction
2. Results
2.1. Histopathological Alterations Were Less Pronounced in the Splenic White and Red Pulp of Animals Treated with the LBMPL Vaccine
2.2. Subtype Th1 Cytokine Expression Is Higher in the Spleen of Dogs Treated with LBMPL Vaccine
2.3. LBMPL Vaccine Promoted a Strong Reduction in Splenic Parasite Burden in Treated Dogs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Ethics Statement
5.2. Treatment Protocols
5.3. Euthanasia Protocol and Necropsy
5.4. Histopathology
5.5. RNA Extraction and Quantification of the Gene Expression of iNOS and Cytokines (IFN-γ, TNF-α, IL-12, IL-10 and TGF-β1) by qRT-PCR
5.6. Splenic DNA Extraction and Quantification of Parasite Load by Quantitative PCR
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Global Leishmaniasis Surveillance. 2017–2018, and First Report on 5 Additional Indicators. Wkly. Epidemiol. Rec. 2020, 95, 265–280. [Google Scholar]
- Harhay, M.O.; Olliaro, P.L.; Costa, D.L.; Costa, C.H.N. Urban Parasitology: Visceral Leishmaniasis in Brazil. Trends Parasitol. 2011, 27, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Coura-Vital, W.; Ker, H.G.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Leal, G.G.D.A.; Moreira, N.D.D.; Oliveira, L.A.M.; Machado, E.M.D.M.; Morais, M.H.F.; Corrêa-Oliveira, R.; et al. Evaluation of Change in Canine Diagnosis Protocol Adopted by the Visceral Leishmaniasis Control Program in Brazil and a New Proposal for Diagnosis. PLoS ONE 2014, 9, e91009. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Carvalho, M.G.; Mayrink, W.; França-Silva, J.C.; Giunchetti, R.C.; Genaro, O.; Corrêa-Oliveira, R. Parasite Density and Impaired Biochemical/Hematological Status Are Associated with Severe Clinical Aspects of Canine Visceral Leishmaniasis. Res. Vet. Sci. 2006, 81, 68–75. [Google Scholar] [CrossRef]
- Arenas, R.; Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J. Leishmaniasis: A Review. F1000 Res. 2017, 6, 750. [Google Scholar] [CrossRef]
- Cavalcanti, A.S.; Ribeiro-Alves, M.; Pereira, L.d.O.R.; Mestre, G.L.; Ferreira, A.B.R.; Morgado, F.N.; Boité, M.C.; Cupolillo, E.; Moraes, M.O.; Porrozzi, R. Parasite Load Induces Progressive Spleen Architecture Breakage and Impairs Cytokine MRNA Expression in Leishmania Infantum-Naturally Infected Dogs. PLoS ONE 2015, 10, e0123009. [Google Scholar] [CrossRef] [PubMed]
- Saporito, L.; Giammanco, G.M.; De Grazia, S.; Colomba, C. Visceral Leishmaniasis: Host-Parasite Interactions and Clinical Presentation in the Immunocompetent and in the Immunocompromised Host. Int. J. Infect. Dis. 2013, 17, e572–e576. [Google Scholar] [CrossRef]
- De Melo, C.V.B.; Hermida, M.D.E.R.; Mesquita, B.R.; Fontes, J.L.M.; Koning, J.J.; da Silva Solcà, M.; Benevides, B.B.; Mota, G.B.S.; Freitas, L.A.R.; Mebius, R.E.; et al. Phenotypical Characterization of Spleen Remodeling in Murine Experimental Visceral Leishmaniasis. Front. Immunol. 2020, 11, 653. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Werneck, G.L.; Costa, D.L.; Holanda, T.A.; Aguiar, G.B.; Carvalho, A.S.; Cavalcanti, J.C.; Santos, L.S. Is Severe Visceral Leishmaniasis a Systemic Inflammatory Response Syndrome?—A Case Control Study. Rev. Soc. Bras. Med. Trop. 2010, 43, 386–392. [Google Scholar] [CrossRef]
- Poulaki, A.; Piperaki, E.T.; Voulgarelis, M. Effects of Visceralising Leishmania on the Spleen, Liver, and Bone Marrow: A Pathophysiological Perspective. Microorganisms 2021, 9, 759. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and Function of the Spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Santana, C.C.; Vassallo, J.; De Freitas, L.A.R.; Oliveira, G.G.S.; Pontes-De-Carvalho, L.C.; Dos-Santos, W.L.C. Inflammation and Structural Changes of Splenic Lymphoid Tissue in Visceral Leishmaniasis: A Study on Naturally Infected Dogs. Parasite Immunol. 2008, 30, 515–524. [Google Scholar] [CrossRef]
- Silva, J.S.; Andrade, A.C.; Santana, C.C.; Santos, L.Q.; de Oliveira, C.I.; Veras, P.S.T.; Vassallo, J.; dos-Santos, W.L.C. Low CXCL13 Expression, Splenic Lymphoid Tissue Atrophy and Germinal Center Disruption in Severe Canine Visceral Leishmaniasis. PLoS ONE 2012, 7, e29103. [Google Scholar] [CrossRef] [PubMed]
- Hermida, M.D.-R.; De Melo, C.V.B.; Lima, I.D.S.; Oliveira, G.G.D.S.; Dos-Santos, W.L.C. Histological Disorganization of Spleen Compartments and Severe Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.A.; Dutra, L.F.; Reis, M.D.O.; Lambert, R.C. A Esplenectomia Na Leishmaniose Visceral Refratária Ao Tratamento Clínico: Relato de Caso. Rev. Soc. Bras. Med. Trop. 2012, 45, 130–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.; Silveira, P.; Melo-Júnior, O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; Aguiar-Soares, R.D.; Reis, L.E.; Cardoso, J.M.; Mathias, F.A.; de Brito, R.C.; da Silva, S.M.; Gontijo, N.F.; Ferreira, S.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- Menezes-Souza, D.; Guerra-Sá, R.; Carneiro, C.M.; Vitoriano-Souza, J.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Silveira-Lemos, D.; Oliveira, G.C.; Corrêa-Oliveira, R.; Reis, A.B. Higher Expression of CCL2, CCL4, CCL5, CCL21, and CXCL8 Chemokines in the Skin Associated with Parasite Density in Canine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2012, 6, e1566. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, W.L.; Tafuri, W.L.; Barbosa, A.J.A.; Michalick, M.S.M.; Genaro, O.; França-Silva, J.C.; Mayrink, W.; Nascimento, E. Histopathology and Immunocytochemical Study of Type 3 and Type 4 Complement Receptors in the Liver and Spleen of Dogs Naturally and Experimentally Infected with Leishmania (Leishmania) Chagasi. Rev. Inst. Med. Trop. Sao Paulo 1996, 38, 81–89. [Google Scholar] [CrossRef]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W.; Tafuri, W.L.; Corrêa-Oliveira, R. Systemic and Compartmentalized Immune Response in Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef]
- Bagues, N.C.T.; de Pinheiro, C.G.M.; Bastos, L.A.; Fraga, D.B.M.; Veras, P.S.T.; Pontes-de-Carvalho, L.C.; dos-Santos, W.L.C.; Oliveira, G.G.D.S. Parasitic Load and Histological Aspects in Different Regions of the Spleen of Dogs with Visceral Leishmaniasis. Comp. Immunol. Microbiol. Infect. Dis. 2018, 56, 14–19. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.V.A.; Figueiredo, F.B.; Menezes, R.C.; Mendes-Junior, A.A.; De Miranda, L.H.M.; Cupolillo, E.; Porrozzi, R.; Morgado, F.N. Morphophysiological Changes in the Splenic Extracellular Matrix of Leishmania Infantum—Naturally Infected Dogs Is Associated with Alterations in Lymphoid Niches and the CD4 + T Cell Frequency in Spleens. PLoS Negl. Trop. Dis. 2018, 2, e0006445. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Mirá, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet Guidelines for the Practical Management of Canine Leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Kip, A.E.; Balasegaram, M.; Beijnen, J.H.; Schellens, J.H.M.; De Vries, P.J.; Dorloa, T.P.C. Systematic Review of Biomarkers to Monitor Therapeutic Response in Leishmaniasis. Antimicrob. Agents Chemother. 2015, 59, 1–14. [Google Scholar] [CrossRef]
- Ghimire, P.G.; Ghimire, P.; Adhikari, J.; Chapagain, A. A Case Report of Visceral Leishmaniasis and Malaria Co-Infection with Pancytopenia and Splenomegaly—A Diagnostic Challenge. BMC Infect. Dis. 2019, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of INOS Gene Expression in Lps Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.R.; Vieira, P.M.A.; Correa-Oliveira, R.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B.; Malaquias, L.C.C. Qualitative and Quantitative Immunohistochemical Evaluation of INOS Expression in the Spleen of Dogs Naturally Infected with Leishmania Chagasi. Parasitol. Res. 2011, 108, 1397–1403. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Ramer-tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic Indicators of Clinical Progression during Canine Leishmania Infantum Infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef]
- Lage, R.S.; Oliveira, G.C.; Busek, S.U.; Guerra, L.L.; Giunchetti, R.C.; Corrêa-Oliveira, R.; Reis, A.B. Analysis of the Cytokine Profile in Spleen Cells from Dogs Naturally Infected by Leishmania Chagasi. Vet. Immunol. Immunopathol. 2007, 115, 135–145. [Google Scholar] [CrossRef]
- Giunchetti, R.C.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; de Souza, J.V.; das Dores Moreira, N.; Malaquias, L.C.; Mota e Castro, L.L.; et al. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine 2007, 25, 7674–7686. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roatt, B.M.; de Oliveira Cardoso, J.M.; Reis, L.E.S.; Moreira, G.J.L.; Gonçalves, L.C.; de Souza Marques, F.; das Dores Moreira, N.; de Abreu Vieira, P.M.; de Oliveira Aguiar-Soares, R.D.; Giunchetti, R.C.; et al. LBMPL Vaccine Therapy Induces Progressive Organization of the Spleen Microarchitecture, Improved Th1 Adaptative Immune Response and Control of Parasitism in Leishmania infantum Naturally Infected Dogs. Pathogens 2022, 11, 974. https://doi.org/10.3390/pathogens11090974
Roatt BM, de Oliveira Cardoso JM, Reis LES, Moreira GJL, Gonçalves LC, de Souza Marques F, das Dores Moreira N, de Abreu Vieira PM, de Oliveira Aguiar-Soares RD, Giunchetti RC, et al. LBMPL Vaccine Therapy Induces Progressive Organization of the Spleen Microarchitecture, Improved Th1 Adaptative Immune Response and Control of Parasitism in Leishmania infantum Naturally Infected Dogs. Pathogens. 2022; 11(9):974. https://doi.org/10.3390/pathogens11090974
Chicago/Turabian StyleRoatt, Bruno Mendes, Jamille Mirelle de Oliveira Cardoso, Levi Eduardo Soares Reis, Gabriel José Lucas Moreira, Letícia Captein Gonçalves, Flávia de Souza Marques, Nádia das Dores Moreira, Paula Melo de Abreu Vieira, Rodrigo Dian de Oliveira Aguiar-Soares, Rodolfo Cordeiro Giunchetti, and et al. 2022. "LBMPL Vaccine Therapy Induces Progressive Organization of the Spleen Microarchitecture, Improved Th1 Adaptative Immune Response and Control of Parasitism in Leishmania infantum Naturally Infected Dogs" Pathogens 11, no. 9: 974. https://doi.org/10.3390/pathogens11090974
APA StyleRoatt, B. M., de Oliveira Cardoso, J. M., Reis, L. E. S., Moreira, G. J. L., Gonçalves, L. C., de Souza Marques, F., das Dores Moreira, N., de Abreu Vieira, P. M., de Oliveira Aguiar-Soares, R. D., Giunchetti, R. C., & Reis, A. B. (2022). LBMPL Vaccine Therapy Induces Progressive Organization of the Spleen Microarchitecture, Improved Th1 Adaptative Immune Response and Control of Parasitism in Leishmania infantum Naturally Infected Dogs. Pathogens, 11(9), 974. https://doi.org/10.3390/pathogens11090974