What Has Happened to Heartworm Disease in Europe in the Last 10 Years?
Abstract
1. Introduction
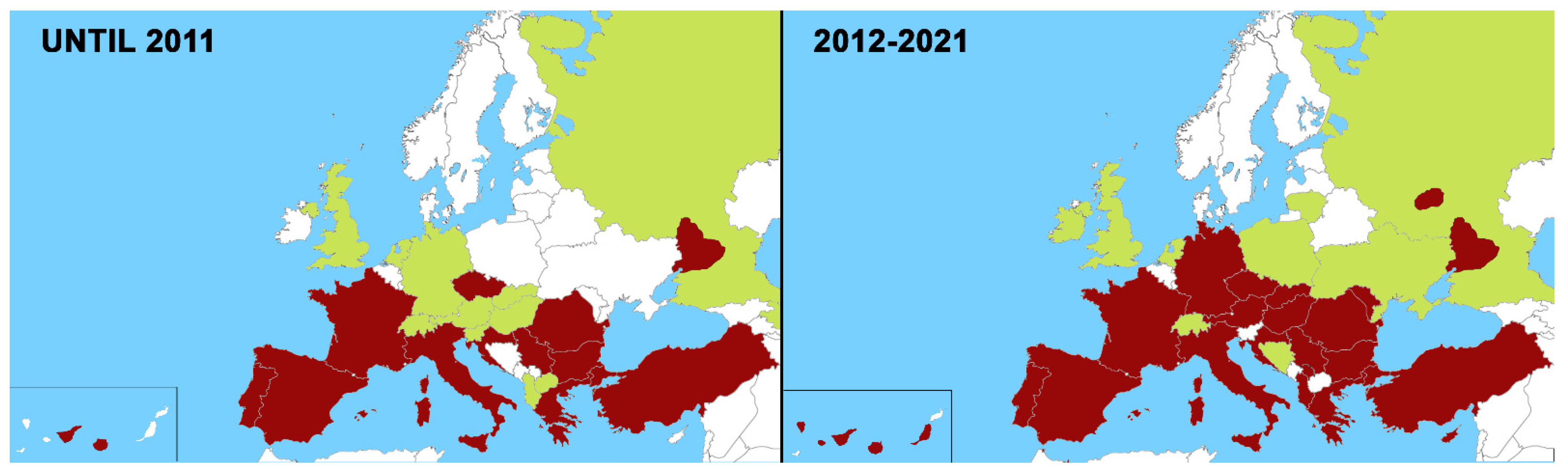
2. Changes in the Distribution of Heartworm in Europe in the Last 10 Years (2012–2021)
2.1. Canine Heartworm
2.2. Feline Heartworm
2.3. Heartworm in Wild Carnivores
2.4. Heartworm Vectors
| European Countries | Until 2011 | 2012–2021 |
|---|---|---|
| Portugal [2,145,146,147] | Cx. theileri | Ae. dentritus Ae. caspius An. maculipennis Cx. pipiens Cx. quinquefasciatus Cx. theileri |
| Spain [2,148,149] | Cx. pipiens Cx. theileri | Cx. pipiens |
| Italy [2,34,150,151,152,153] | Ae. albopictus Ae. caspius An. maculipennis Cq. richiardii Cx. pipiens | Ae. albopictus Ae. koreicus Cq. richiardii Cs. annulate Cx. pipiens Och. caspius |
| France [154] | - | Cx. pipiens |
| Turkey [2] | Ae. vexans Cx. pipiens | - |
| Germany [155] | - | Cx. pipiens/torrentium |
| Serbia [162] | - | Cq. richiardii Cx. pipens Och. caspius |
| Slovakia [160,161] | - | Ae. vexans Cq. richiardii Cx. pipiens pipiens Och. sticus |
| Belarus [159] | Cx. pipiens/torrentium | |
| Moldova [159] | - | Ae. behningi An. maculipennis An. pseudopictus Cx. pipiens/torrentium |
| Romania [141,158] | - | An. hyrcanus An. maculipennis Cq. richiardii Cx. pipiens |
| Hungary [156,157] | - | Ae. vexans Cq. richiardii Cx. modestus Cx. pipiens Och. caspius |
| Russia [163,164] | - | Ae. albopictus Ae. cantans Ae. cataphylla Ae. cexans, Ae. cinereus Ae. communis Ae. excrucians Ae. geniculatus Ae. intrudens Ae. vexans Cq. richiardii, Cx. modestus Cx. pipiens An. messeae Och. cantans |
3. Factors Contributing to the Spread of Heartworm
4. New Studies to Assess the Risk of Heartworm Infection in Europe
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe—New Distribution Trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Carretón, E.; García-Guasch, L.; Expósito, J.; Armario, B.; Morchón, R.; Simón, F. First epidemiological report of feline heartworm infection in the Barcelona metropolitan area (Spain). Parasit. Vectors 2014, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef]
- European Society of Dirofilariosis and Angiostrongylosis. Guidelines; ESDA: Las Palmas de Gran Canaria, Spain. Available online: http://www.esda.vet/index.php/guidelines (accessed on 1 August 2022).
- American Heartworm Society. Heartworm Guidelines; AHS: Raleigh, NC, USA. Available online: https://www.heartwormsociety.org/veterinary-resources/american-heartworm-society-guidelines (accessed on 1 August 2022).
- Savić, S.; Stosic, M.Z.; Marcic, D.; Hernández, I.; Potkonjak, A.; Otasevic, S.; Ruzic, M.; Morchón, R. Seroepidemiological Study of Canine and Human Dirofilariasis in the Endemic Region of Northern Serbia. Front. Vet. Sci. 2020, 7, 571. [Google Scholar] [CrossRef]
- Zumaquero, L.; Simón, F.; Carretón, E.; Hernández, I.; Sandoval, C.; Morchón, R. Prevalence of canine and human dirofilariosis in Puebla, Mexico. Vet. Parasitol. 2020, 282, 109098. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasit. Vectors 2013, 6, 16. [Google Scholar] [CrossRef]
- Cardoso, L.; Mendão, C.; Madeira de Carvalho, L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal—A national serological study. Parasit. Vectors 2012, 5, 62. [Google Scholar] [CrossRef]
- Maia, C.; Coimbra, M.; Ramos, C.; Cristóvão, J.M.; Cardoso, L.; Campino, L. Serological investigation of Leishmania infantum, Dirofilaria immitis and Angiostrongylus vasorum in dogs from southern Portugal. Parasit. Vectors 2015, 8, 152. [Google Scholar] [CrossRef]
- Alho, A.M.; Landum, M.; Ferreira, C.; Meireles, J.; Gonçalves, L.; Madeira de Carvalho, L.; Belo, S. Prevalence and seasonal variations of canine dirofilariosis in Portugal. Vet. Parasitol. 2014, 206, 99–105. [Google Scholar] [CrossRef]
- Vieira, A.L.; Vieira, M.J.; Oliveira, J.M.; Simões, A.R.; Diez-Baños, P.; Gestal, J. Prevalence of canine heartworm (Dirofilaria immitis) disease in dogs of central Portugal. Parasite 2014, 21, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Landum, M.; Ferreira, C.C.; Calado, M.; Alho, A.M.; Maurício, I.L.; Meireles, J.S.; de Carvalho, L.M.; Cunha, C.; Belo, S. Detection of Wolbachia in Dirofilaria infected dogs in Portugal. Vet. Parasitol. 2014, 204, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Afonso, A.; Calado, M.; Maurício, I.; Alho, A.M.; Meireles, J.; Madeira de Carvalho, L.; Belo, S. Molecular characterization of Dirofilaria spp. circulating in Portugal. Parasit. Vectors 2017, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; García-Rodríguez, S.N.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Matos, J.I.; Rodríguez Escolar, I.; Carretón, E. Expansion of Canine Heartworm in Spain. Animals 2022, 12, 1268. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Morchón, R.; Silveira-Viera, L.; Falcón, Y.; Simón, F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet. Parasitol. 2016, 216, 66–71. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef]
- Pérez Pérez, P.; Rodríguez-Escolar, I.; Carretón, E.; Sánchez Agudo, J.Á.; Lorenzo-Morales, J.; Montoya-Alonso, J.A.; Morchón, R. Serological Survey of Canine Vector-Borne Infections in North-Center Spain. Front. Vet. Sci. 2021, 8, 784331. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Simón, L.; González-Miguel, J.; García-Guasch, L.; Morchón, R.; Simón, F. Prevalence of Dirofilaria immitis in dogs from Barcelona: Validation of a geospatial prediction model. Vet. Parasitol. 2015, 212, 456–459. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; Falcón-Cordón, Y.; Falcón-Cordón, S.; Simón, F.; Carretón, E. Prevalence of heartworm in dogs and cats of Madrid, Spain. Parasit. Vectors 2017, 10, 354. [Google Scholar] [CrossRef]
- Morchón, R.; Mellado, I.; González-Miguel, J.; Vicente, M.; Vicente, L.; Simón, F. Prevalencia de la dirofilariosis cardiopulmonar canina. Argos 2011, 126, 30. [Google Scholar]
- Diosdado, A.; Gómez, P.J.; González-Miguel, J.; Simón, F.; Morchón, R. Current status of canine dirofilariosis in an endemic area of western Spain. J. Helminthol. 2018, 92, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, G.; Carreri, L. An Epidemiologic Assessment of Canine Heartworm in Northern Italy. In Proceedings of the 13th Trienial State of the Heartworm Symposium, Memphis, TN, USA, April 16–18 2020; pp. 41–42. [Google Scholar]
- Traversa, D.; Aste, G.; Milillo, P.; Capelli, G.; Pampurini, F.; Tunesi, C.; Santori, D.; Paoletti, B.; Boari, A. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet Parasitol. 2010, 169, 128–132. [Google Scholar] [CrossRef]
- Mortarino, M.; Musella, V.; Costa, V.; Genchi, C.; Cringoli, G.; Rinaldi, L. GIS modeling for canine dirofilariosis risk assessment in central Italy. Geospat. Health 2008, 2, 253–261. [Google Scholar] [CrossRef]
- Fioretti, D.P.; Diaferia, M.; Grelloni, V.; Maresca, C. Canine filariosis in Umbria: An update of the occurrence one year after the first observation of autochthonous foci. Parassitologia 2003, 45, 79–83. [Google Scholar] [PubMed]
- Colombo, M.; Morelli, S.; Simonato, G.; Di Cesare, A.; Veronesi, F.; Frangipane di Regalbono, A.; Grassi, L.; Russi, I.; Tiscar, P.G.; Morganti, G.; et al. Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Guardone, L.; Mignone, W.; Monni, G.; Tozzini, G.; Prati, M.C.; Macchioni, F. Canine filarial infections in Liguria, north-west Italy. J. Helminthol. 2016, 90, 121–124. [Google Scholar] [CrossRef]
- Vascellari, M.; Ravagnan, S.; Carminato, A.; Cazzin, S.; Carli, E.; Da Rold, G.; Lucchese, L.; Natale, A.; Otranto, D.; Capelli, G. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasit. Vectors 2016, 9, 369. [Google Scholar] [CrossRef]
- Sauda, F.; Malandrucco, L.; Macrì, G.; Scarpulla, M.; De Liberato, C.; Terracciano, G.; Fichi, G.; Berrilli, F.; Perrucci, S. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite 2018, 25, 2. [Google Scholar] [CrossRef]
- Macchioni, F.; Sed, G.; Cecchi, F. Canine filarial infections in an area of Central Italy (Tuscany-Latium border) historically free from the disease. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100404. [Google Scholar] [CrossRef]
- Giangaspero, A.; Marangi, M.; Latrofa, M.S.; Martinelli, D.; Traversa, D.; Otranto, D.; Genchi, C. Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol. Res. 2013, 112, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, L.; Maurelli, M.P.; Pennacchio, S.; Bosco, A.; Musella, V.; Ciuca, L.; Cringoli, G.; Rinaldi, L. Dirofilaria immitis and Angiostrongylus vasorum: The contemporaneous detection in kennels. BMC Vet. Res. 2015, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, A.; Ferrara, G.; Iovane, G.; Schettini, R.; Ciarcia, R.; Caputo, V.; Pompameo, M.; Pagnini, U.; Montagnaro, S. Seroprevalence of Ehrlichia spp.; Anaplasma spp.; Borreliaburgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Gori, F.; Colombo, M.; Traversa, D.; Sarrocco, G.; Simonato, G.; Nespeca, C.; Di Cesare, A.; Frangipane di Regalbono, A.; Veronesi, F.; et al. Simultaneous Exposure to Angiostrongylus vasorum and Vector-Borne Pathogens in Dogs from Italy. Pathogens 2021, 10, 1200. [Google Scholar] [CrossRef]
- Santoro, M.; Miletti, G.; Vangone, L.; Spadari, L.; Reccia, S.; Fusco, G. Heartworm Disease (Dirofilaria immitis) in Two Roaming Dogs From the Urban Area of Castel Volturno, Southern Italy. Front. Vet. Sci. 2019, 6, 270. [Google Scholar] [CrossRef]
- Pipia, A.P.; Varcasia, A.; Tosciri, G.; Seu, S.; Manunta, M.L.; Mura, M.C.; Sanna, G.; Tamponi, C.; Brianti, E.; Scala, A. New insights onto cardiopulmonary nematodes of dogs in Sardinia, Italy. Parasitol. Res. 2014, 113, 1505–1509. [Google Scholar] [CrossRef]
- Brianti, E.; Panarese, R.; Napoli, E.; De Benedetto, G.; Gaglio, G.; Bezerra-Santos, M.A.; Mendoza-Roldan, J.A.; Otranto, D. Dirofilaria immitis infection in the Pelagie archipelago: The southernmost hyperendemic focus in Europe. Transbound Emerg. Dis. 2021, 69, 1274–1280. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasit. Vectors 2020, 13, 193. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Ringot, D.; Watier-Grillot, S.; Davoust, B.; Mediannikov, O. A cardiac and subcutaneous canine dirofilariosis outbreak in a kennel in central France. Parasite 2019, 26, 72. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Marie, J.L.; Tahir, D.; Watier-Grillot, S.; Mediannikov, O.; Davoust, B. Detection of Canine Vector-Borne Filariasis and Their Wolbachia Endosymbionts in French Guiana. Microorganisms 2020, 8, 770. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Otranto, D.; Stolowy, N.; Amrane, S.; Santhakumari Manoj, R.R.; Polette, L.; Watier-Grillot, S.; Mediannikov, O.; Davoust, B.; L’Ollivier, C. Human and Animal Dirofilariasis in Southeast of France. Microorganisms 2021, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
- Tahir, D.; Bittar, F.; Barré-Cardi, H.; Sow, D.; Dahmani, M.; Mediannikov, O.; Raoult, D.; Davoust, B.; Parola, P. Molecular survey of Dirofilaria immitis and Dirofilaria repens by new real-time TaqMan® PCR assay in dogs and mosquitoes (Diptera: Culicidae) in Corsica (France). Vet. Parasitol. 2017, 235, 1–7. [Google Scholar] [CrossRef]
- Pantchev, N.; Schaper, R.; Limousin, S.; Norden, N.; Weise, M.; Lorentzen, L. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: Results of a countrywide serologic survey. Parasitol. Res. 2009, 105, S101–S114. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.P.; Morelli, S.; Unterköfler, M.S.; Bajer, A.; Bakran-Lebl, K.; Dwużnik-Szarek, D.; Farkas, R.; Grandi, G.; Heddergott, M.; Jokelainen, P.; et al. Dirofilaria spp. and Angiostrongylus vasorum: Current Risk of Spreading in Central and Northern Europe. Pathogens 2021, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.P.; Auer, H.; Leschnik, M.; Silbermayr, K.; Duscher, G.; Joachim, A. Dirofilaria in Humans, Dogs, and Vectors in Austria (1978–2014)-From Imported Pathogens to the Endemicity of Dirofilaria repens. PLoS Negl. Trop. Dis. 2016, 10, e0004547. [Google Scholar] [CrossRef]
- Sonnberger, K.; Duscher, G.G.; Fuehrer, H.P.; Leschnik, M. Current trends in canine dirofilariosis in Austria-do we face a pre-endemic status? Parasitol. Res. 2020, 119, 1001–1009. [Google Scholar] [CrossRef]
- Sonnberger, K.; Fuehrer, H.P.; Sonnberger, B.W.; Leschnik, M. The Incidence of Dirofilaria immitis in Shelter Dogs and Mosquitoes in Austria. Pathogens 2021, 10, 550. [Google Scholar] [CrossRef]
- Vrhovec, M.G.; Pantchev, N.; Failing, K.; Bauer, C.; Travers-Martin, N.; Zahner, H. Retrospective Analysis of Canine Vector-borne Diseases (CVBD) in Germany with Emphasis on the Endemicity and Risk Factors of Leishmaniosis. Parasitol. Res. 2017, 116, 131–144. [Google Scholar] [CrossRef][Green Version]
- Liesner, J.M.; Krücken, J.; Schaper, R.; Pachnicke, S.; Kohn, B.; Müller, E.; Schulze, C.; von Samson-Himmelstjerna, G. Vector-borne pathogens in dogs and red foxes from the federal state of Brandenburg, Germany. Vet. Parasitol. 2016, 224, 44–51. [Google Scholar] [CrossRef]
- Schäfer, I.; Volkmann, M.; Beelitz, P.; Merle, R.; Müller, E.; Kohn, B. Retrospective analysis of vector-borne infections in dogs after travelling to endemic areas (2007–2018). Vet. Parasitol. 2019, 276S, 100015. [Google Scholar] [CrossRef]
- Maerz, I. Clinical and diagnostic imaging findings in 37 rescued dogs with heartworm disease in Germany. Vet. Parasitol. 2020, 283, 109156. [Google Scholar] [CrossRef]
- Sassnau, R.; Czajka, C.; Kronefeld, M.; Werner, D.; Genchi, C.; Tannich, E.; Kampen, H. Dirofilaria repens and Dirofilaria immitis DNA findings in mosquitoes in Germany: Temperature data allow autochthonous extrinsic development. Parasitol. Res. 2014, 113, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Bowman, D.; Drake, J. Canine heartworm disease (Dirofilaria immitis) in Western Europe: Survey of veterinary awareness and perceptions. Parasit. Vectors 2014, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- López-Bailén, E. Case study: Canine heartworm disease diagnosed in Ireland. Vet. Ireland J. 2020, 10, 251–256. [Google Scholar]
- Volgina, N.S.; Romashov, B.V.; Romashova, N.B.; Shtannikov, A. Prevalence of borreliosis, anaplasmosis, ehrlichiosis and Dirofilaria immitis in dogs and vectors in Voronezh Reserve (Russia). Comp. Immunol. Microbiol. Infect Dis. 2013, 36, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kartashev, V.; Batashova, I.; Kartashov, S.; Ermakov, A.; Mironova, A.; Kuleshova, Y.; Ilyasov, B.; Kolodiy, I.; Klyuchnikov, A.; Ryabikina, E.; et al. Canine and human dirofilariosis in the rostov region (southern Russia). Vet. Med. Int. 2011, 2011, 685713. [Google Scholar] [CrossRef] [PubMed]
- Świątalska, A.; Demiaszkiewicz, A.W. First autochthonous case of Dirofilaria immitis invasion in dog in Poland. Žycie Weterynaryjne 2012, 87, 685–686. [Google Scholar]
- Krämer, F.; Schaper, R.; Schunack, B.; Połozowski, A.; Piekarska, J.; Szwedko, A.; Jodies, R.; Kowalska, D.; Schüpbach, D.; Pantchev, N. Serological detection of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis antibodies and Dirofilaria immitis antigen in a countrywide survey in dogs in Poland. Parasitol. Res. 2014, 113, 3229–3239. [Google Scholar] [CrossRef]
- Hamel, D.; Silaghi, C.; Zapadynska, S.; Kudrin, A.; Pfister, K. Vector-borne pathogens in ticks and EDTA-blood samples collected from client-owned dogs, Kiev, Ukraine. Ticks Tick Borne Dis. 2013, 4, 152–155. [Google Scholar] [CrossRef]
- Dumitrache, M.O.; D’Amico, G.; Voiniţchi, E.; Maximenco, S.; Mircean, V.; Ionică, A.M. An epidemiological survey of Dirofilaria spp. and Acanthocheilonema spp. in dogs from the Republic of Moldova. Parasit. Vectors 2021, 14, 390. [Google Scholar] [CrossRef]
- Tiškina, V.; Jokelainen, P. Vector-borne parasitic infections in dogs in the Baltic and Nordic countries: A questionnaire study to veterinarians on canine babesiosis and infections with Dirofilaria immitis and Dirofilaria repens. Vet. Parasitol. 2017, 244, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sabūnas, V.; Radzijevskaja, J.; Sakalauskas, P.; Paulauskas, A. First Report of Heartworm (Dirofilaria Immitis) Infection in an Imported Dog in Lithuania. Helminthologia 2019, 56, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mrljak, V.; Kuleš, J.; Mihaljević, Ž.; Torti, M.; Gotić, J.; Crnogaj, M.; Živičnjak, T.; Mayer, I.; Šmit, I.; Bhide, M.; et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector Borne Zoonotic Dis. 2017, 17, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Jurković, D.; Beck, A.; Huber, D.; Mihaljević, Ž.; Polkinghorne, A.; Martinković, F.; Lukačević, D.; Pilat, M.; Brezak, R.; Bosnić, S.; et al. Seroprevalence of vector-borne pathogens in dogs from Croatia. Parasitol. Res. 2019, 118, 347–352. [Google Scholar] [CrossRef]
- Tasić, A.; Tasić-Otašević, S.; Gabrielli, S.; Miladinović-Tasić, N.; Ignjatović, A.; Dorđević, J.; Dimitrijević, S.; Cancrini, G. Canine Dirofilaria infections in two uninvestigated areas of Serbia: Epidemiological and genetic aspects. Vector Borne Zoonotic Dis. 2012, 12, 1031–1035. [Google Scholar] [CrossRef]
- Krstić, M.; Gabrielli, S.; Ignjatović, M.; Savić, S.; Cancrini, G.; Ranđelović, G.; Momčilović, S.; Stojnev, S.; Otašević, S. An appraisal of canine and human cases reveals an endemic status of dirofilariosis in parts of Serbia. Mol. Cell Probes 2017, 31, 37–41. [Google Scholar] [CrossRef]
- Marcic, D.; Potkonjak, A.; Zekic Stosic, M.; Spasojevic-Kosic, L.; Pusic, I.; Savic, S. Prevalence of Dirofilaria immitis in Dogs from Shelters in Vojvodina, Serbia. Acta Sci. Vet. 2020, 48. [Google Scholar] [CrossRef]
- Potkonjak, A.; Savić, S.; Spasojević Kosić, L.; Tasić Otašević, T.; Tomanović, S.; Kovacevic Filipovic, M. Consensus statement on epidemiological situation and expected frequency of canine vector-borne diseases in Serbia. Veterinarski Glasnik 2020, 74, 211–215. [Google Scholar] [CrossRef]
- Otašević, S.; Savić, S.; Jurhar-Pavlova, M. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals 2022, 12, 911. [Google Scholar] [CrossRef]
- Sinani, A.; Aliu, H.; Latifi, F.; Haziri, I.; Xhekaj, B.; Kampen, H.; Sherifi, K. First serological evidence of infections with selected vector-borne pathogens in dogs in Kosovo. Parasitol. Res. 2020, 119, 3863–3868. [Google Scholar] [CrossRef]
- Omeragić, J.; Šerić-Haračić, S.; Klarić Soldo, D.; Kapo, N.; Fejzić, N.; Škapur, V.; Medlock, J. Distribution of ticks in Bosnia and Herzegovina. Ticks Tick Borne Dis. 2022, 13, 101870. [Google Scholar] [CrossRef] [PubMed]
- Mircean, V.; Dumitrache, M.O.; Györke, A.; Pantchev, N.; Jodies, R.; Mihalca, A.D.; Cozma, V. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Dis. 2012, 12, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Ionică, A.M.; Matei, I.A.; Mircean, V.; Dumitrache, M.O.; D’Amico, G.; Győrke, A.; Pantchev, N.; Annoscia, G.; Albrechtová, K.; Otranto, D.; et al. Current surveys on the prevalence and distribution of Dirofilaria spp. and Acanthocheilonema reconditum infections in dogs in Romania. Parasitol. Res. 2015, 114, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Giubega, S.; Imre, M.; Ilie, M.S.; Imre, K.; Luca, I.; Florea, T.; Dărăbuș, G.; Morariu, S. Identity of Microfilariae Circulating in Dogs from Western and South-Western Romania in the Last Decade. Pathogens 2021, 10, 1400. [Google Scholar] [CrossRef]
- Cazan, C.D.; Ionică, A.M.; Matei, I.A.; D’Amico, G.; Muñoz, C.; Berriatua, E.; Dumitrache, M.O. Detection of Leishmania infantum DNA and antibodies against Anaplasma spp.; Borrelia burgdorferi s.l. and Ehrlichia canis in a dog kennel in South-Central Romania. Acta Vet. Scand. 2020, 62, 42. [Google Scholar] [CrossRef]
- Ciucă, L.; Musella, V.; Miron, L.D.; Maurelli, M.P.; Cringoli, G.; Bosco, A.; Rinaldi, L. Geographic distribution of canine heartworm (Dirofilaria immitis) infection in stray dogs of eastern Romania. Geospat. Health 2016, 11, 499. [Google Scholar] [CrossRef]
- Ciucă, L.; Genchi, M.; Kramer, L.; Mangia, C.; Miron, L.D.; Del Prete, L.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. Heat treatment of serum samples from stray dogs naturally exposed to Dirofilaria immitis and Dirofilaria repens in Romania. Vet. Parasitol. 2016, 225, 81–85. [Google Scholar] [CrossRef]
- Iliev, P.T.; Kirkova, Z.T.; Tonev, A.S. Preliminary Study on the Prevalence of Endoparasite Infections and Vector-borne Diseases in Outdoor Dogs in Bulgaria. Helminthologia 2020, 57, 171–178. [Google Scholar] [CrossRef]
- Manev, I. Serological survey of vector-borne pathogens in stray dogs from Sofia area, Bulgaria. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100441. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.; Šnábel, V.; Dakova, V.; Čabanová, V.; Cavallero, S.; Trifonova, A.; Mirchev, R.; Hurníková, Z.; Vasilková, Z.; Miterpáková, M. Dirofilaria Immitis in Bulgaria: The First Genetic Baseline Data and an Overview of the Current Status. Helminthologia 2020, 57, 211–218. [Google Scholar] [CrossRef]
- Rafailov, R. Prevalence of Dirofilaria immitis in dogs in Bulgaria. Trad. Modern. Vet. Med. 2020, 5, 57–64. [Google Scholar]
- Arnaudov, A. Seroprevalence of some vector-borne diseases in dogs from Plovdiv, Bulgaria. Trakia J. Sci. 2021, 19, 236–242. [Google Scholar] [CrossRef]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current Surveys of the Seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in Dogs in Bulgaria. Parasitol. Res. 2015, 114, S117–S130. [Google Scholar] [CrossRef] [PubMed]
- Iliev, P.; Kirkova, Z.; Ivanov, A.; Kalkanov, I. Retrospective analysis on helminthic and protozoan infections in dogs and cats in Bulgaria. Bulg. J. Vet. Med. 2017, 20, 389–393. [Google Scholar]
- Radev, V.; Lalkovski, N.; Zhelyazkov, P.; Kostova, T.; Sabev, P.; Nedelchev, N.; Vassileva, R. Prevalence of gastrointestinal parasites and Dirofilaria spp. in stray dogs from some regions in Bulgaria. Bulg. J. Vet. Med. 2016, 19, 57–62. [Google Scholar] [CrossRef]
- Stoyanova, H.; Carretón, E.; Montoya-Alonso, J.A. Stray Dogs of Sofia (Bulgaria) Could be an Important Reservoir of Heartworm (Dirofilaria Immitis). Helminthologia 2019, 56, 329–333. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.S.; Mirchev, R.L.; Trifonova, A.P. Dirofilaria immitis infection in carnivores from Bulgaria: 2012–2013 update. Bulgarian J. Vet. Med. 2016, 19, 153–162. [Google Scholar] [CrossRef]
- Schüle, C.; Rehbein, S.; Shukullari, E.; Rapti, D.; Reese, S.; Silaghi, C. Police dogs from Albania as indicators of exposure risk to Toxoplasma gondii, Neospora caninum and vector-borne pathogens of zoonotic and veterinary concern. Vet. Parasitol. Reg. Stud. Rep. 2015, 1, 35–46. [Google Scholar] [CrossRef]
- Hamel, D.; Shukullari, E.; Rapti, D.; Silaghi, C.; Pfister, K.; Rehbein, S. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol. Res. 2016, 115, 489–499. [Google Scholar] [CrossRef]
- Diakou, A.; Kapantaidakis, E.; Tamvakis, A.; Giannakis, V.; Strus, N. Dirofilaria infections in dogs in different areas of Greece. Parasit. Vectors 2016, 9, 508. [Google Scholar] [CrossRef]
- Diakou, A.; Soubasis, N.; Chochlios, T.; Oikonomidis, I.L.; Tselekis, D.; Koutinas, C.; Karaiosif, R.; Psaralexi, E.; Tsouloufi, T.K.; Brellou, G.; et al. Canine and feline dirofilariosis in a highly enzootic area: First report of feline dirofilariosis in Greece. Parasitol. Res. 2019, 118, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, L.V.; Kontos, V.I.; Kritsepi Konstantinou, M.; Polizopoulou, Z.S.; Rousou, X.A.; Christodoulopoulos, G. Cross-Sectional Serosurvey and Factors Associated with Exposure of Dogs to Vector-Borne Pathogens in Greece. Vector Borne Zoonotic. Dis. 2019, 19, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit. Vectors 2019, 1, 283. [Google Scholar] [CrossRef] [PubMed]
- Guven, E.; Avcioglu, H.; Cengiz, S.; Hayirli, A. Vector-Borne Pathogens in Stray Dogs in Northeastern Turkey. Vector Borne Zoonotic Dis. 2017, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Ciftci, A.T. Serological and Molecular Detection of Dirofilaria Species in Stray Dogs and Investigation of Wolbachia DNA by PCR in Turkey. J. Arthropod. Borne Dis. 2016, 10, 445–453. [Google Scholar]
- Köse, M.; Erdoğan, M. Serological screening of canine heartworm (Dirofilaria immitis) infections in Turkey. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 503–508. [Google Scholar]
- Çetinkaya, H.; Matur, E.; Akyazi, İ.; Ekiz, E.E.; Aydin, L.; Toparlak, M. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis. 2016, 7, 706–714. [Google Scholar] [CrossRef]
- Ural, K.; Gultekin, M.; Atasoy, A.; Ulutas, B. Spatial distribution of vector borne disease agents in dogs in Aegean region, Turkey. Revista MVZ Córdoba 2014, 19, 2014. [Google Scholar] [CrossRef]
- Kokkinos, P.; Dimzas, D.; Pantchev, N.; Tamvakis, A.; Balzer, J.; Diakou, A. Filarial infections in dogs in Cyprus, an apparently heartworm free island. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100330. [Google Scholar] [CrossRef]
- Attipa, C.; Solano-Gallego, L.; Leutenegger, C.M.; Papasouliotis, K.; Soutter, F.; Balzer, J.; Carver, S.; Buch, J.S.; Tasker, S. Associations between clinical canine leishmaniosis and multiple vector-borne co-infections: A case-control serological study. BMC Vet. Res. 2019, 15, 331. [Google Scholar] [CrossRef]
- Martina, M.; Zuzana, H.; Daniela, V.; Lenka, B. Different epidemiological pattern of canine dirofilariosis in two neighboring countries in Central Europe-the Czech Republic and Slovakia. Parasitol. Res. 2021, 120, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Iglódyová, A.; Miterpáková, M.; Hurníková, Z.; Antolová, D.; Dubinský, P.; Letková, V. Canine dirofilariosis under specific environmental conditions of the Eastern Slovak Lowland. Ann. Agric. Environ. Med. 2012, 19, 57–60. [Google Scholar] [PubMed]
- Víchová, B.; Miterpáková, M.; Iglódyová, A. Molecular detection of co-infections with Anaplasma phagocytophilum and/or Babesia canis canis in Dirofilaria-positive dogs from Slovakia. Vet. Parasitol. 2014, 203, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Čabanová, V.; Pantchev, N.; Hurníková, Z.; Miterpáková, M. Recent study on canine vector-borne zoonoses in southern Slovakia—Serologic survey. Acta Parasitol. 2015, 60, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Valentová, D.; Čabanová, V.; Berešíková, Ľ. Heartworm on the rise-new insights into Dirofilaria immitis epidemiology. Parasitol. Res. 2018, 117, 2347–2350. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Zborovská, H.; Bielik, B.; Halán, M. The Fatal Case of an Autochthonous Heartworm Disease in a Dog from a Non-endemic Region of South-eastern Slovakia. Helminthologia 2020, 57, 154–157. [Google Scholar] [CrossRef]
- Jacsó, O.; Mándoki, M.; Majoros, G.; Pétsch, M.; Mortarino, M.; Genchi, C.; Fok, E. First autochthonous Dirofilaria immitis (Leidy, 1856) infection in a dog in Hungary. Helminthologia 2009, 46, 159–161. [Google Scholar] [CrossRef]
- Farkas, R.; Gyurkovszky, M.; Lukács, Z.; Aladics, B.; Solymosi, N. Seroprevalence of some vector-borne infections of dogs in Hungary. Vector Borne Zoonotic Dis. 2014, 14, 256–260. [Google Scholar] [CrossRef]
- Farkas, R.; Mag, V.; Gyurkovszky, M.; Takács, N.; Vörös, K.; Solymosi, N. The current situation of canine dirofilariosis in Hungary. Parasitol. Res. 2020, 119, 129–135. [Google Scholar] [CrossRef]
- Tolnai, Z.; Széll, Z.; Sproch, Á.; Szeredi, L.; Sréter, T. Dirofilaria immitis: An emerging parasite in dogs, red foxes and golden jackals in Hungary. Vet. Parasitol. 2014, 203, 339–342. [Google Scholar] [CrossRef]
- Bacsadi, Á.; Papp, A.; Szeredi, L.; Tóth, G.; Nemes, C.; Imre, V.; Tolnai, Z.; Széll, Z.; Sréter, T. Retrospective study on the distribution of Dirofilaria immitis in dogs in Hungary. Vet. Parasitol. 2016, 220, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Trájer, A.; Rengei, A.; Farkas-Iványi, K.; Bede-Fazekas, Á. Impacts of urbanisation level and distance from potential natural mosquito breeding habitats on the abundance of canine dirofilariosis. Acta Vet. 2016, 64, 340–359. [Google Scholar] [CrossRef] [PubMed]
- Széll, Z.; Bacsadi, Á.; Szeredi, L.; Nemes, C.; Fézer, B.; Bakcsa, E.; Kalla, H.; Tolnai, Z.; Sréter, T. Rapid spread and emergence of heartworm resulting from climate and climate-driven ecological changes in Hungary. Vet. Parasitol. 2020, 280, 109067. [Google Scholar] [CrossRef] [PubMed]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Grimm, F.; Beatrice, L.; Riond, B.; Bley, T.; Jordi, R.; Dennler, M.; Hofmann-Lehmann, R. Clinical and molecular investigation of a canine distemper outbreak and vector-borne infections in a group of rescue dogs imported from Hungary to Switzerland. BMC Vet. Res. 2015, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; García Rodríguez, S.N.; Carretón, E.; Rodríguez Escolar, I.; Costa-Rodríguez, N.; Matos, J.I.; Morchón, R. Seroprevalence of Feline Heartworm in Spain: Completing the Epidemiological Puzzle of a Neglected Disease in the Cat. Front. Vet. Sci. 2022, 9, 900371. [Google Scholar] [CrossRef]
- Villanueva-Saz, S.; Giner, J.; Verde, M.; Yzuel, A.; González, A.; Lacasta, D.; Marteles, D.; Fernández, A. Prevalence of microfilariae, antigen and antibodies of feline dirofilariosis infection (Dirofilaria immitis) in the Zaragoza metropolitan area, Spain. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100541. [Google Scholar] [CrossRef]
- Morchón, R.; Ferreira, A.C.; Martín-Pacho, J.R.; Montoya, A.; Mortarino, M.; Genchi, C.; Simón, F. Specific IgG antibody response against antigens of Dirofilaria immitis and its Wolbachia endosymbiont bacterium in cats with natural and experimental infections. Vet. Parasitol. 2004, 125, 313–321. [Google Scholar] [CrossRef]
- Morchón, R.; Hernández de la Fuente, I.; Calvo-López, A.; Lambea, A.; Falcón-Cordón, Y.; Carretón, E. Cituación actual de la dirofilariosis feline. Argos 2019, 21, 4–9. [Google Scholar]
- Maia, C.; Ramos, C.; Coimbra, M.; Cardoso, L.; Campino, L. Prevalence of Dirofilaria immitis antigen and antibodies to Leishmania infantum in cats from southern Portugal. Parasitol. Int. 2015, 64, 154–156. [Google Scholar] [CrossRef]
- Neves, M.; Lopes, A.P.; Martins, C.; Fino, R.; Paixão, C.; Damil, L.; Lima, C.; Alho, A.M.; Schallig, H.; Dubey, J.P.; et al. Survey of Dirofilaria immitis antigen and antibodies to Leishmania infantum and Toxoplasma gondii in cats from Madeira Island, Portugal. Parasit. Vectors 2020, 13, 117. [Google Scholar] [CrossRef]
- Magi, M.; Prati, M.C.; Sebastiani, B.; Bandecchi, P.; Guberti, V. Seroprevalence of feline heartworm disease in Tuscany. Vet. Rec. 2002, 150, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Venco, L.; Ferrari, N.; Mortarino, M.; Genchi, M. Feline heartworm (Dirofilaria immitis) infection: A statistical elaboration of the duration of the infection and life expectancy in asymptomatic cats. Vet. Parasitol. 2008, 158, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Panarese, R.; Iatta, R.; Beugnet, F.; Otranto, D. Incidence of Dirofilaria immitis and Leishmania infantum infections in sheltered dogs from Southern Italy. Transb. Emerg. Dis. 2022, 69, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Favole, P.; Cauduro, A.; Opreni, M.; Zanzani, S.; Albonico, F.; Manfredi, M.; Cantile, C.; Lorenzo, V. Epidural dirofilariosis in a paraparetic cat: Case report of Dirofilaria immitis infection. J. Fel. Med. Surg. 2013, 15, 1160–1164. [Google Scholar] [CrossRef]
- Biasato, I.; Tursi, M.; Zanet, S.; Longato, E.; Capucchio, M.T. Pulmonary artery dissection causing haemothorax in a cat: Potential role of Dirofilaria immitis infection and literature review. J. Vet. Cardiol. 2017, 19, 82–87. [Google Scholar] [CrossRef]
- Schäfer, I.; Kohn, B.; Volkmann, M.; Müller, E. Retrospective evaluation of vector-borne pathogens in cats living in Germany (2012–2020). Parasit. Vectors 2021, 14, 123. [Google Scholar] [CrossRef]
- Kulmer, L.M.; Unterköfler, M.S.; Fuehrer, H.P.; Janovska, V.; Pagac, M.; Svoboda, M.; Venco, L.; Leschnik, M. First Autochthonous Infection of a Cat with Dirofilaria immitis in Austria. Pathogens 2021, 10, 1104. [Google Scholar] [CrossRef]
- Tonev, A.S.; Kirkova, Z.; Iliev, P.T.; Roussenov, A.; Chaprazov, T.; Roydev, R.; Pirovski, N. Clinical Case of Life-threatening Co-infection Due to Dirofilaria Immitis and Aelurostrongylus Abstrusus in a Cat: First Report of Feline Heartworm Disease in Bulgaria. Helminthologia 2021, 58, 106–114. [Google Scholar] [CrossRef]
- Pană, D.; Rădulescu, A.; Mitrea, I.L.; Ionita, M. First Report on Clinical Feline Heartworm (Dirofilaria Immitis) Infection in Romania. Helminthologia 2020, 57, 49–56. [Google Scholar] [CrossRef]
- Penezić, A.; Moriano, R.; Spasić, M.; Ćirović, D. First report of a naturally patent infection with Dirofilaria immitis in an otter (Lutra lutra). Parasitol. Res. 2018, 117, 929–931. [Google Scholar] [CrossRef]
- Potkonjak, A.; Rojas, A.; Gutiérrez, R.; Nachum-Biala, Y.; Kleinerman, G.; Savić, S.; Polaček, V.; Pušić, I.; Harrus, S.; Baneth, G. Molecular survey of Dirofilaria species in stray dogs, red foxes and golden jackals from Vojvodina, Serbia. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101409. [Google Scholar] [CrossRef] [PubMed]
- Gavrilović, P.; Blitva-Robertson, G.; Özvegy, J.; Kiskároly, F.; Becskei, Z. Case Report of dirofilariasis in grey wolf in Serbia. Acta Parasitol. 2014, 60, 175–178. [Google Scholar] [PubMed]
- Gavrilović, P.; Dobrosavljević, I.; Vasković, N.; Todorović, I.; Živulj, A.; Kureljušić, B.; Pavlović, I. Cardiopulmonary parasitic nematodes of the red fox (Vulpes vulpes) in Serbia. Acta Vet. 2019, 67, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Penezić, A.; Selaković, S.; Pavlović, I.; Ćirović, D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Laidoudi, Y.; Marié, J.L.; Fenollar, F.; Davoust, B.; Mediannikov, O. Molecular investigation of vector-borne pathogens in red foxes (Vulpes vulpes) from southern France. J. Wildl. Dis. 2020, 56, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Marcelino, I.; Colella, V.; Flanagan, C.; Silva, N.; Correia, J.J.; Latrofa, M.S.; Otranto, D.; Madeira de Carvalho, L. Dirofilaria immitis in pinnipeds and a new host record. Parasit. Vectors 2017, 10, 142. [Google Scholar] [CrossRef]
- Ionică, A.M.; Matei, I.A.; D’Amico, G.; Daskalaki, A.A.; Juránková, J.; Ionescu, D.T.; Mihalca, A.D.; Modrý, D.; Gherman, C.M. Role of golden jackals (Canis aureus) as natural reservoirs of Dirofilaria spp. in Romania. Parasit. Vectors 2016, 9, 240. [Google Scholar] [CrossRef]
- Ionică, A.M.; Matei, I.A.; D’Amico, G.; Bel, L.V.; Dumitrache, M.O.; Modrý, D.; Mihalca, A.D. Dirofilaria immitis and D. repens show circadian co-periodicity in naturally co-infected dogs. Parasit. Vectors 2017, 10, 116. [Google Scholar] [CrossRef][Green Version]
- Kravchenko, V.; Itin, G.; Kartashev, V.; Ermakov, A.; Kartashov, S.; Diosdado, A.; González-Miguel, J.; Simón, F. Dirofilaria immitis and D. repens in sylvatic reservoirs of Krasnodar Krai (Russian Federation). Vet. Parasitol. Reg. Stud. Rep. 2016, 6, 35–38. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Meneguz, P.G.; Orusa, R.; Zoppi, S.; Robetto, S.; Marucco, F.; Tizzani, P. Dirofilaria immitis in wolves recolonizing northern Italy: Are wolves competent hosts? Parasit. Vectors 2020, 13, 482. [Google Scholar] [CrossRef]
- Mirchev, R.; Trifonova, A.A.; Panayotova Pencheva, M. Dirofilaria immitis in foxes (Vulpes Vulpes, LINNAEUS, 1758) in different areas from Bulgaria. Intern. Sci. Online J. Sci. Tech. 2013, 3, 38–42. [Google Scholar]
- Ferreira, C.A.; de Pinho Mixão, V.; Novo, M.T.; Palmeiro Calado, M.M.; Pires Gonçalves, L.A.; Duarte Belo, S.M.; Gouveia de Almeida, A.P. First molecular identification of mosquito vectors of Dirofilaria immitis in continental Portugal. Parasit. Vectors 2015, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- DE Pinho Mixão, V.; Mendes, A.M.; Maurício, I.L.; Calado, M.M.; Novo, N.T.; Belo, S.; Almeida, A.P.G. Molecular detection of Wolbachia pipientis in natural populations of mosquito vectors of Dirofilaria immitis from continental Portugal: First detection in Culex theileri. Med. Vet. Entomol. 2016, 30, 301–309. [Google Scholar] [CrossRef]
- Manoj, R.; Latrofa, M.S.; Cavalera, M.A.; Mendoza-Roldan, J.A.; Maia, C.; Otranto, D. Molecular detection of zoonotic filarioids in Culex spp. from Portugal. Med. Vet. Entomol. 2021, 35, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Puente, J.; Ferraguti, M.; Jiménez-Peñuela, J.; Ruiz, S.; Martínez, J.; Roiz, D.; Soriguer, R.; Figuerola, J. Filarial worm circulation by mosquitoes along an urbanization gradient in southern Spain. Transbound Emerg. Dis. 2019, 66, 1752–1757. [Google Scholar] [CrossRef]
- Bravo-Barriga, D.; Parreira, R.; Almeida, A.P.; Calado, M.; Blanco-Ciudad, J.; Serrano-Aguilera, F.J.; Pérez-Martín, J.E.; Sánchez-Peinado, J.; Pinto, J.; Reina, D.; et al. Culex pipiens as a potential vector for transmission of Dirofilaria immitis and other unclassified Filarioidea in Southwest Spain. Vet. Parasitol. 2016, 223, 173–180. [Google Scholar] [CrossRef]
- Capelli, G.; Frangipane di Regalbono, A.; Simonato, G.; Cassini, R.; Cazzin, S.; Cancrini, G.; Otranto, D.; Pietrobelli, M. Risk of canine and human exposure to Dirofilaria immitis infected mosquitoes in endemic areas of Italy. Parasit. Vectors 2013, 6, 60. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Latrofa, M.S.; Zatelli, A.; Ćupina, A.I.; Montarsi, F.; Pombi, M.; Mendoza-Roldan, J.A.; Beugnet, F.; Otranto, D. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Intern. J. Parasitol. 2020, 50, 555–559. [Google Scholar] [CrossRef]
- Montarsi, F.; Ciocchetta, S.; Devine, G.; Ravagnan, S.; Mutinelli, F.; Frangipane di Regalbono, A.; Otranto, D.; Capelli, G. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasit. Vectors 2015, 8, 177. [Google Scholar] [CrossRef]
- Suter, T.; Flacio, E.; Fariña, B.F.; Engeler, L.; Tonolla, M.; Müller, P. First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasit. Vectors 2015, 8, 402. [Google Scholar] [CrossRef]
- Younes, L.; Barré-Cardi, H.; Bedjaoui, S.; Ayhan, N.; Varloud, M.; Mediannikov, O.; Otranto, D.; Davoust, B. Dirofilaria immitis and Dirofilaria repens in mosquitoes from Corsica Island, France. Parasit. Vectors 2021, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Kronefeld, M.; Kampen, H.; Sassnau, R.; Werner, D. Molecular detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in mosquitoes from Germany. Parasit. Vectors 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Zittra, C.; Kocziha, Z.; Pinnyei, S.; Harl, J.; Kieser, K.; Laciny, A.; Eigner, B.; Silbermayr, K.; Duscher, G.G.; Fok, É.; et al. Screening blood-fed mosquitoes for the diagnosis of filarioid helminths and avian malaria. Parasit. Vectors 2015, 8, 16. [Google Scholar] [CrossRef]
- Kurucz, K.; Kiss, V.; Zana, B.; Jakab, F.; Kemenesi, G. Filarial nematode (order, Spirurida) surveillance in urban habitats, in the city of Pécs (Hungary). Parasitol. Res. 2018, 117, 3355–3360. [Google Scholar] [CrossRef] [PubMed]
- Tomazatos, A.; Cadar, D.; Török, E.; Maranda, I.; Horváth, C.; Keresztes, L.; Spinu, M.; Jansen, S.; Jöst, H.; Schmidt-Chanasit, J.; et al. Circulation of Dirofilaria immitis and Dirofilaria repens in the Danube Delta Biosphere Reserve, Romania. Parasit. Vectors 2018, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Șuleșco, T.; Volkova, T.; Yashkova, S.; Tomazatos, A.; von Thien, H.; Lühken, R.; Tannich, E. Detection of Dirofilaria repens and Dirofilaria immitis DNA in mosquitoes from Belarus. Parasitol. Res. 2016, 115, 3535–3541. [Google Scholar] [CrossRef]
- Bocková, E.; Iglódyová, A.; Kočišová, A. Potential mosquito (Diptera:Culicidae) vector of Dirofilaria repens and Dirofilaria immitis in urban areas of Eastern Slovakia. Parasitol. Res. 2015, 114, 4487–4492. [Google Scholar] [CrossRef]
- Čabanová, V.; Miterpáková, M.; Valentová, D.; Blažejová, H.; Rudolf, I.; Stloukal, E.; Hurníková, Z.; Dzidová, M. Urbanization impact on mosquito community and the transmission potential of filarial infection in central Europe. Parasit. Vectors 2018, 11, 261. [Google Scholar] [CrossRef]
- Kurucz, K.; Kepner, A.; Krtinic, B.; Zana, B.; Földes, F.; Bányai, K.; Oldal, M.; Jakab, F.; Kemenesi, G. First molecular identification of Dirofilaria spp. (Onchocercidae) in mosquitoes from Serbia. Parasitol. Res. 2016, 115, 3257–3260. [Google Scholar] [CrossRef]
- Shaikevich, E.; Bogacheva, A.; Ganushkina, L. Dirofilaria and Wolbachia in mosquitoes (Diptera: Culicidae) in central European Russia and on the Black Sea coast. Parasite 2019, 26, 2. [Google Scholar] [CrossRef]
- Bogacheva, A.S.; Ganushkina, L.A.; Lopatina, Y.V. Infection of blood-sucking mosquitoes (diptera: Culicidae) with dirofilariae (Spirurida, Onchocercidae) in the tula región. Med. Parazitol. 2016, 2, 8–12. [Google Scholar]
- Cancrini, G.; Scaramozzino, P.; Gabrielli, S.; Di Paolo, M.; Toma, L.; Romi, R. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in central Italy. J. Med. Entomol. 2007, 44, 1064–1066. [Google Scholar] [CrossRef]
- Brooks, D.R.; Hoberg, E.P. How will global climate change affect parasite-host assemblages? Trends Parasitol. 2007, 23, 571–574. [Google Scholar] [CrossRef]
- Genchi, C.; Mortarino, M.; Rinaldi, L.; Cringoli, G.; Traldi, G.; Genchi, M. Changing climate and changing vector-borne disease distribution: The example of Dirofilaria in Europe. Vet. Parasitol. 2011, 176, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L. Subcutaneous dirofilariosis (Dirofilaria repens): An infection spreading throughout the old world. Parasit. Vectors 2017, 10, 517. [Google Scholar] [CrossRef]
- Cringoli, G.; Rinaldi, L.; Veneziano, V.; Musella, V. Disease mapping and risk assessment in veterinary parasitology: Some case studies. Parassitologia 2005, 47, 9–25. [Google Scholar]
- Rinaldi, L.; Musella, V.; Biggeri, A.; Cringoli, G. New insights into the application of geographical information systems and remote sensing in veterinary parasitology. Geospat. Health 2006, 1, 33–47. [Google Scholar] [CrossRef]
- Franco, A.O.; Davies, C.R.; Mylne, A.; Dedet, J.P.; Gállego, M.; Ballart, C.; Gramiccia, M.; Gradoni, L.; Molina, R.; Gálvez, R.; et al. Predicting the distribution of canine leishmaniasis in western Europe based on environmental variables. Parasitology 2011, 138, 1878–1891. [Google Scholar] [CrossRef]
- Petrić, M.; Ducheyne, E.; Gossner, C.M.; Marsboom, C.; Nicolas, G.; Venail, R.; Hendrickx, G.; Schaffner, F. Seasonality and timing of peak abundance of Aedes albopictus in Europe: Implications to public and animal health. Geospat. Health 2021, 16. [Google Scholar] [CrossRef]
- Cringoli, G.; Rinaldi, L.; Veneziano, V.; Capelli, G. A prevalence survey and risk analysis of filariosis in dogs from the Mt. Vesuvius area of southern Italy. Vet. Parasitol. 2001, 102, 243–252. [Google Scholar] [CrossRef]
- Rinaldi, L.; Genchi, C.; Musella, V.; Genchi, M.; Cringoli, G. Geographical information systems as a tool in the control of heartworm infections in dogs and cats. Vet. Parasitol. 2011, 176, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Rinaldi, L.; Cascone, C.; Mortarino, M.; Cringoli, G. Is heartworm disease really spreading in Europe? Vet. Parasitol. 2005, 133, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Afonin, A.; López-Díez, L.I.; González-Miguel, J.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A.; Kartashev, V.; Simón, F. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet. Parasitol. 2014, 200, 257–264. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morchón, R.; Montoya-Alonso, J.A.; Rodríguez-Escolar, I.; Carretón, E. What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens 2022, 11, 1042. https://doi.org/10.3390/pathogens11091042
Morchón R, Montoya-Alonso JA, Rodríguez-Escolar I, Carretón E. What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens. 2022; 11(9):1042. https://doi.org/10.3390/pathogens11091042
Chicago/Turabian StyleMorchón, Rodrigo, José Alberto Montoya-Alonso, Iván Rodríguez-Escolar, and Elena Carretón. 2022. "What Has Happened to Heartworm Disease in Europe in the Last 10 Years?" Pathogens 11, no. 9: 1042. https://doi.org/10.3390/pathogens11091042
APA StyleMorchón, R., Montoya-Alonso, J. A., Rodríguez-Escolar, I., & Carretón, E. (2022). What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens, 11(9), 1042. https://doi.org/10.3390/pathogens11091042









