Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques
Abstract
1. Introduction
2. Results
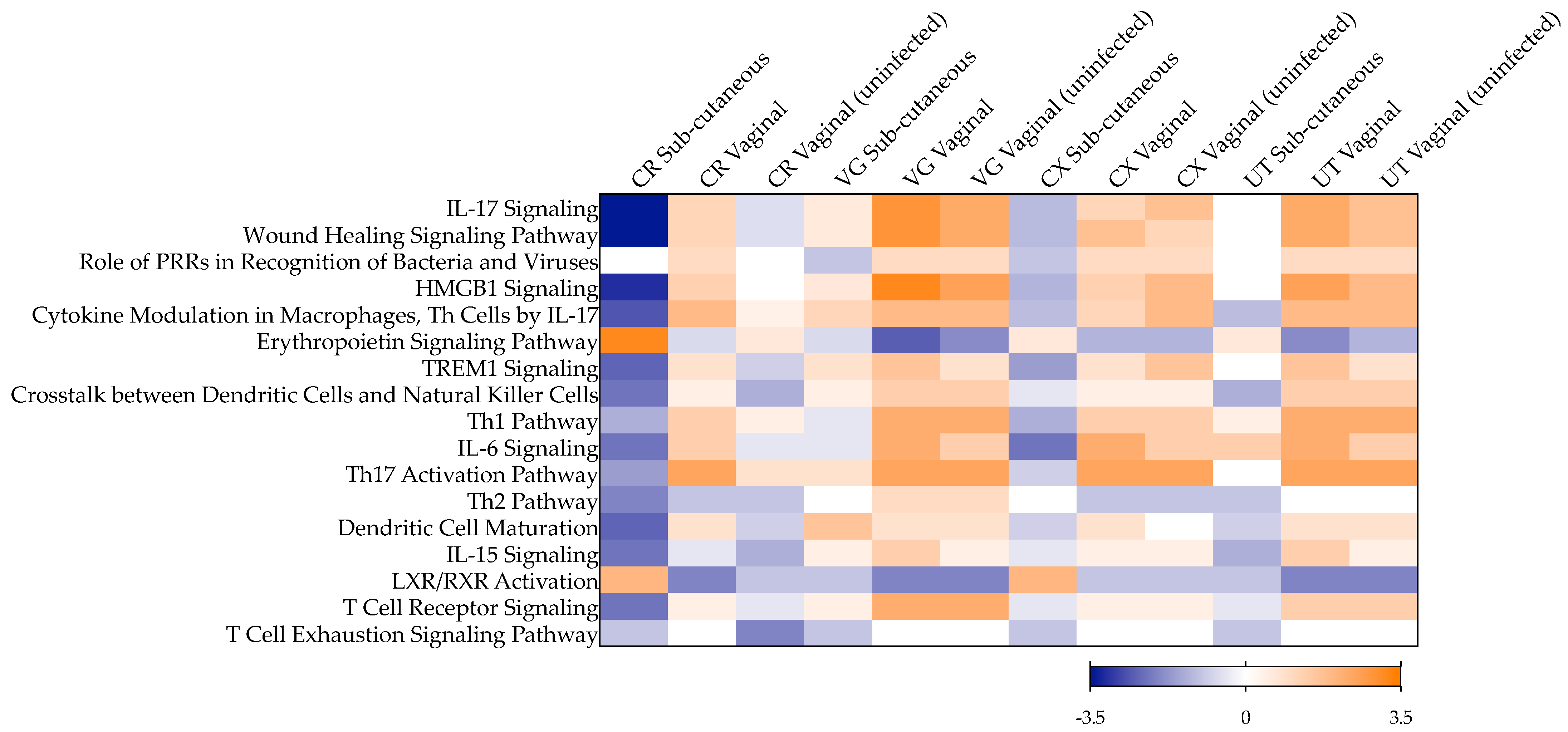
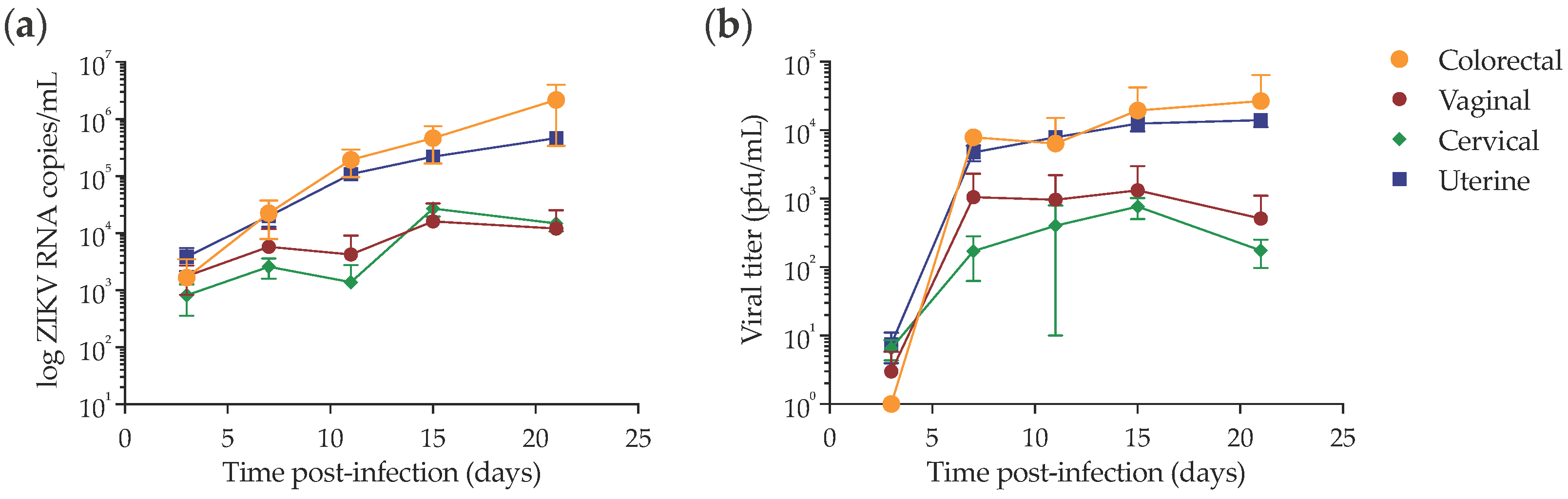
2.1. Late Mucosal Responses to Sub-Cutaneous ZIKV Challenge
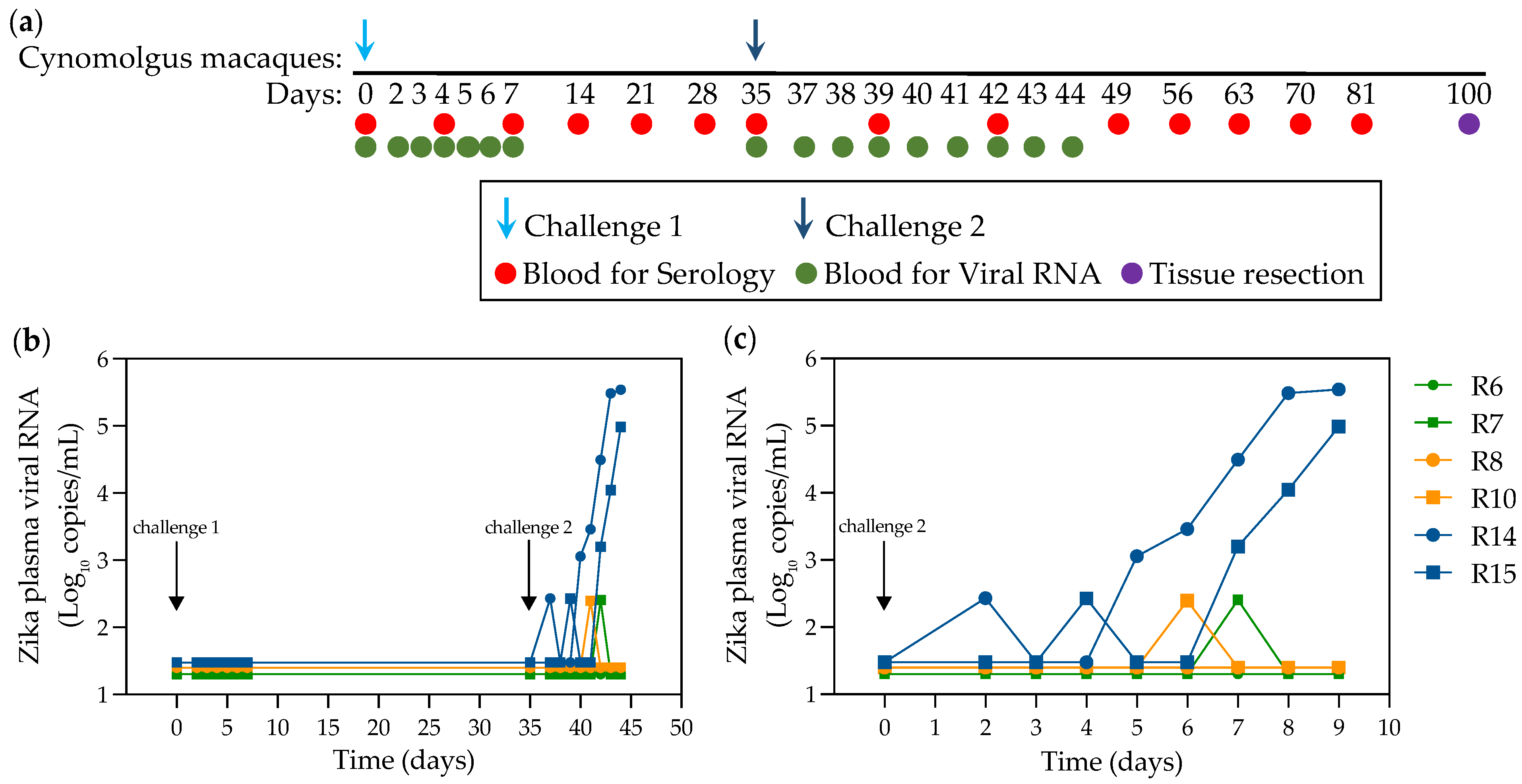
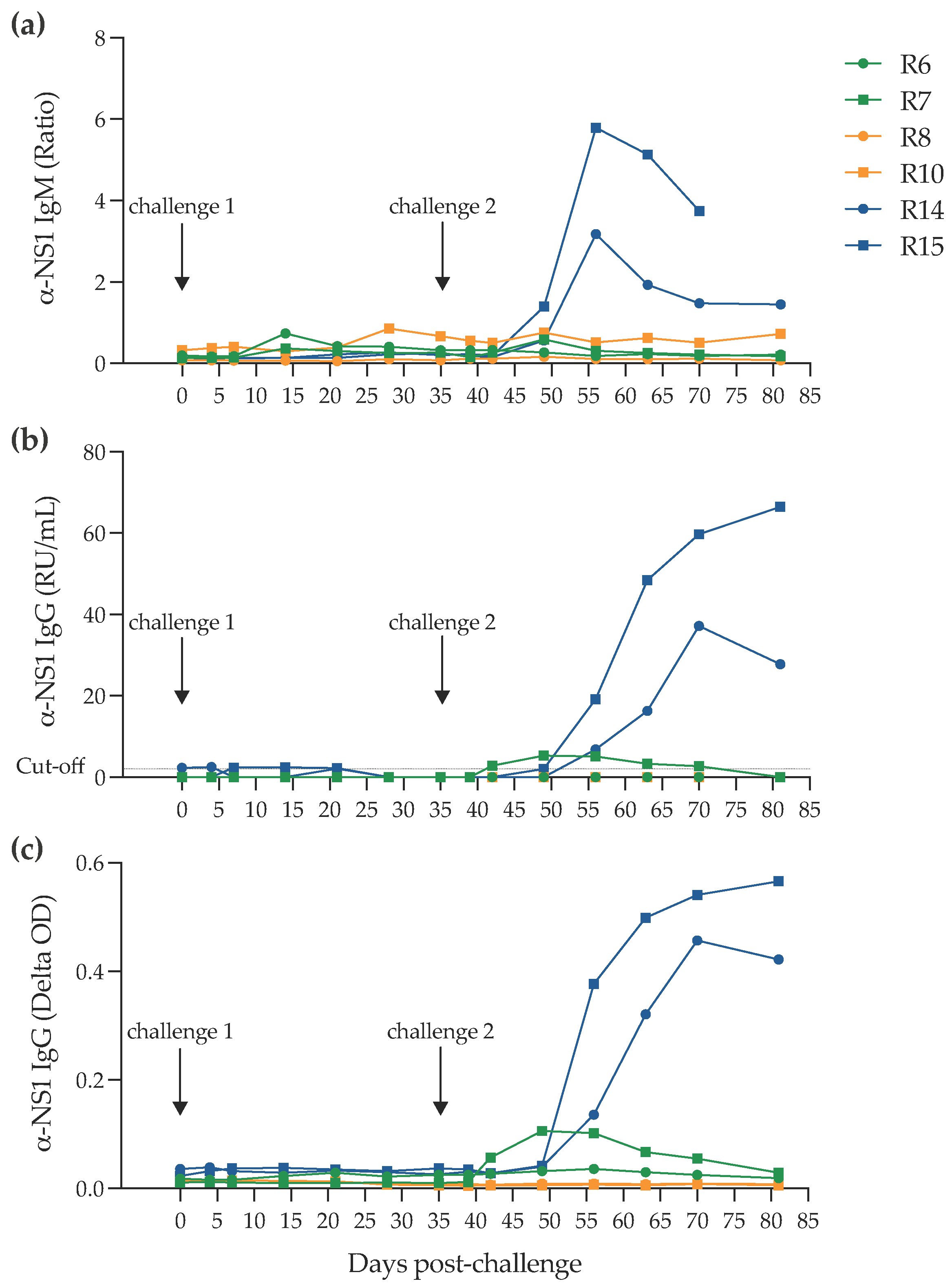
2.2. Infection of NHPs via Intravaginal Challenge
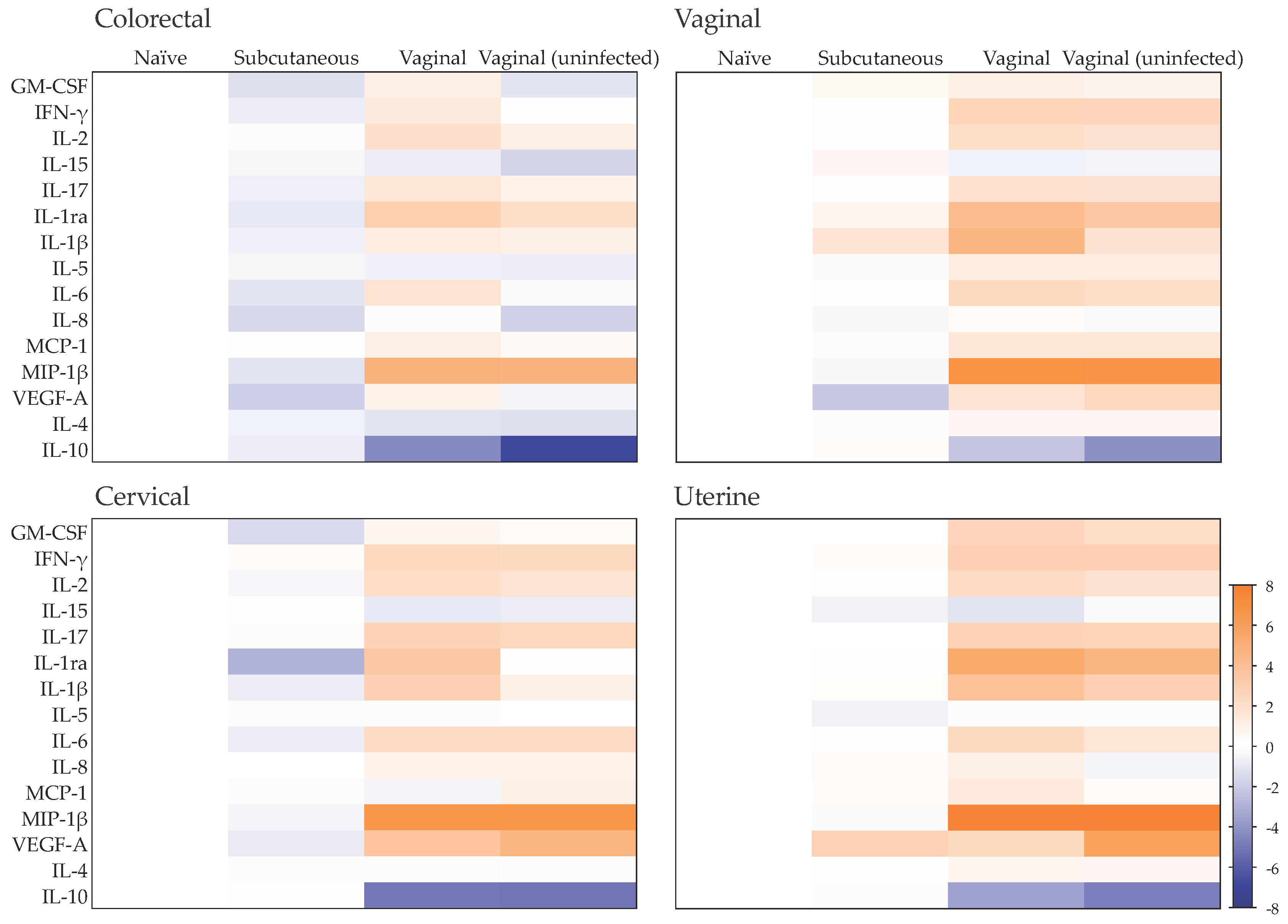
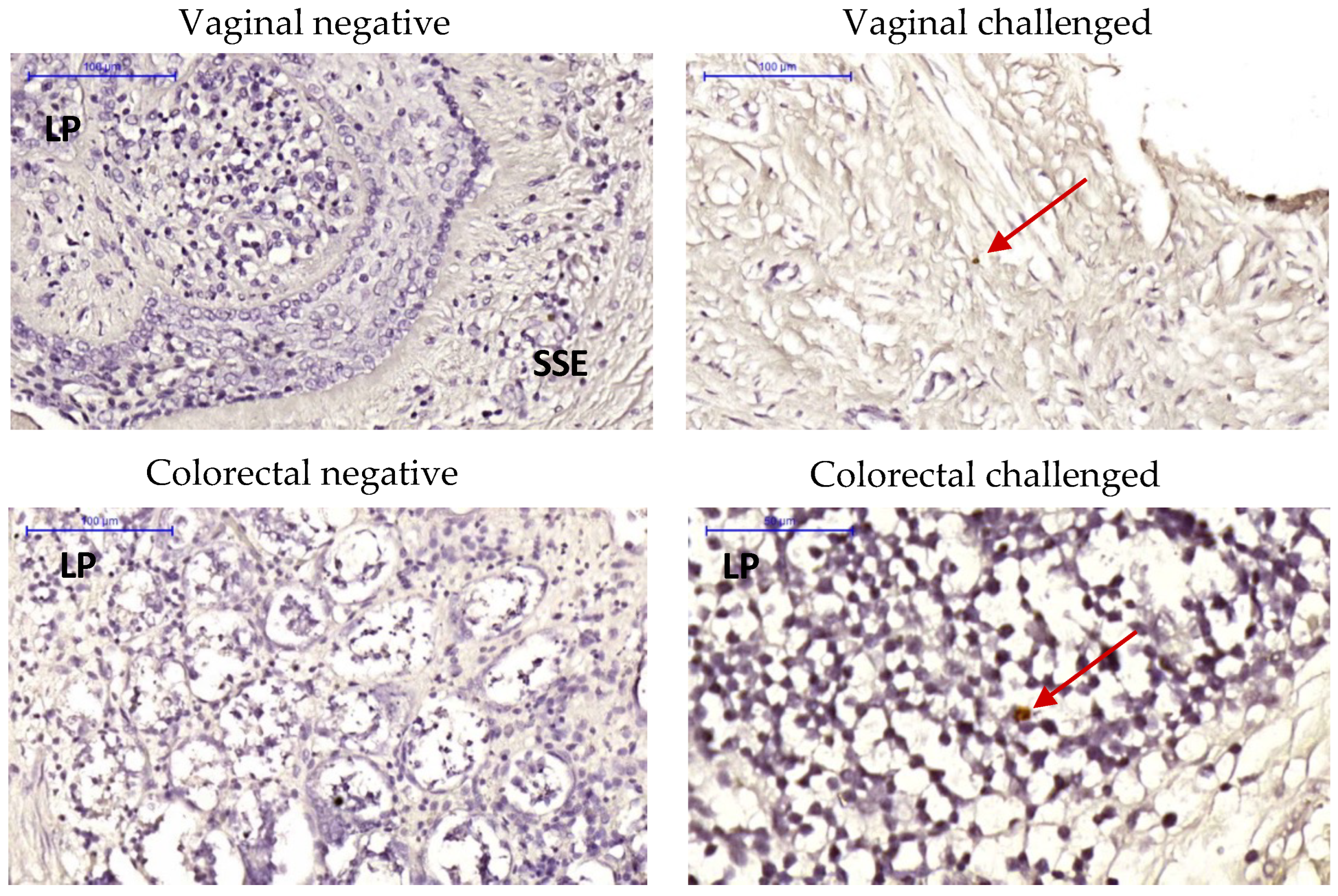
2.3. Late Mucosal Responses to Vaginal ZIKV Challenge
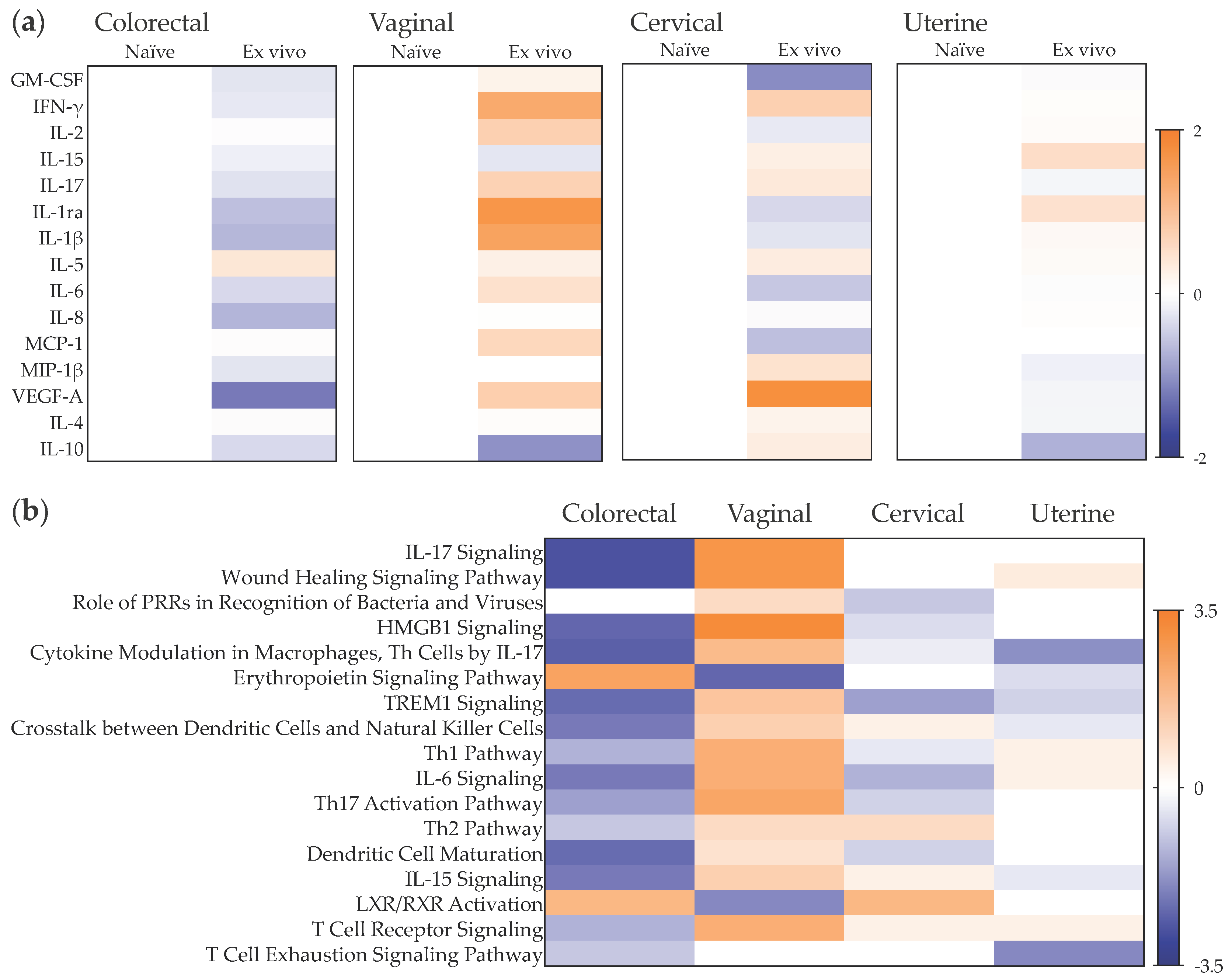
2.4. Early Responses to ZIKV Challenge in a Mucosal Tissue Model
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Virus and In Vivo Vaginal Challenge
4.3. Serology
4.4. Cell, Tissue Explant, and Virus Culture Conditions
4.5. Infectivity Assay
4.6. Plaque Assay
4.7. Quantification of Viral RNA by RT-qPCR
4.8. RNAscope In Situ Hybridization (ISH) and Immunohistochemistry
4.9. Multiplex Cytokine Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, A.V.C.; Crovella, S. Microcephaly Prevalence in Infants Born to Zika Virus-Infected Women: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 1714. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor, L.d.C. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C.; Vazquez-Calvo, A.; Blazquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martin-Acebes, M.A. Zika Virus: The Latest Newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, K.M.A.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 2016, 22, 1256–1259. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e1244. [Google Scholar] [CrossRef]
- Althaus, C.L.; Low, N. How Relevant Is Sexual Transmission of Zika Virus? PLoS Med. 2016, 13, e1002157. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Freour, T.; Mirallie, S.; Hubert, B.; Splingart, C.; Barriere, P.; Maquart, M.; Leparc-Goffart, I. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Eurosurveillance 2016, 21, 30254. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.M. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Hills, S.L.; Oster, A.M.; Porse, C.C.; Danyluk, G.; Cone, M.; Brooks, R.; Scotland, S.; Schiffman, E.; Fredette, C.; et al. Male-to-Female Sexual Transmission of Zika Virus-United States, January-April 2016. Clin. Infect. Dis. 2017, 64, 211–213. [Google Scholar] [CrossRef]
- Gaskell, K.M.; Houlihan, C.; Nastouli, E.; Checkley, A.M. Persistent Zika Virus Detection in Semen in a Traveler Returning to the United Kingdom from Brazil, 2016. Emerg. Infect. Dis. 2017, 23, 137–139. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 2016, 16, 405. [Google Scholar] [CrossRef]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Eurosurveillance 2016, 21, 30314. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Gubern, C.G.; et al. Persistence of Zika Virus in Body Fluids - Final Report. N. Engl. J. Med. 2018, 379, 1234–1243. [Google Scholar] [CrossRef]
- Khan, S.; Woodruff, E.M.; Trapecar, M.; Fontaine, K.A.; Ezaki, A.; Borbet, T.C.; Ott, M.; Sanjabi, S. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J. Exp. Med. 2016, 213, 2913–2929. [Google Scholar] [CrossRef]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.; Bowen, R.A.; Brault, A.C. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Nalca, A.; Rossi, F.D.; Miller, L.J.; Wiley, M.R.; Perez-Sautu, U.; Washington, S.C.; Norris, S.L.; Wollen-Roberts, S.E.; Shamblin, J.D.; et al. High Infection Rates for Adult Macaques after Intravaginal or Intrarectal Inoculation with Zika Virus. Emerg. Infect. Dis. 2017, 23, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Perez-Sautu, U.; Wiley, M.R.; Miller, L.J.; Kimmel, A.E.; Principe, L.M.; Wollen-Roberts, S.E.; Shamblin, J.D.; Valdez, S.M.; Cazares, L.H.; et al. Modeling mosquito-borne and sexual transmission of Zika virus in an enzootic host, the African green monkey. PLoS Negl. Trop. Dis. 2020, 14, e0008107. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Cottrell, M.L.; Prybylski, J.; Kashuba, A.D.M.; Veazey, R.S.; Garcia-Perez, J.; Olejniczak, N.; McCoy, C.F.; Ziprin, P.; Richardson-Harman, N.; et al. The ex vivo pharmacology of HIV-1 antiretrovirals differs between macaques and humans. iScience 2022, 25, 104409. [Google Scholar] [CrossRef]
- Herrera, C.; McRaven, M.D.; Laing, K.G.; Dennis, J.; Hope, T.J.; Shattock, R.J. Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines (Basel) 2021, 9, 231. [Google Scholar] [CrossRef]
- Berry, N.; Ferguson, D.; Ham, C.; Hall, J.; Jenkins, A.; Giles, E.; Devshi, D.; Kempster, S.; Rose, N.; Dowall, S.; et al. High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci. Rep. 2019, 9, 14495. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sanchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Bradley, M.P.; Nagamine, C.M. Animal Models of Zika Virus. Comp. Med. 2017, 67, 242–252. [Google Scholar]
- Dong, S.; Liang, Q. Recent Advances in Animal Models of Zika Virus Infection. Virol. Sin. 2018, 33, 125–130. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Newman, C.M.; Golos, T.G.; Friedrich, T.C.; O’Connor, D.H. Using Macaques to Address Critical Questions in Zika Virus Research. Annu. Rev. Virol. 2019, 6, 481–500. [Google Scholar] [CrossRef]
- Cranage, M.; Sharpe, S.; Herrera, C.; Cope, A.; Dennis, M.; Berry, N.; Ham, C.; Heeney, J.; Rezk, N.; Kashuba, A.; et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008, 5, e157. [Google Scholar] [CrossRef] [PubMed]
- Rowland-Jones, S.; Sutton, J.; Ariyoshi, K.; Dong, T.; Gotch, F.; McAdam, S.; Whitby, D.; Sabally, S.; Gallimore, A.; Corrah, T.; et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1995, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.M.; Pezzuto, P.; Silveira, P.P.; Melo, F.O.; Ferreira, T.A.; Oliveira-Szejnfeld, P.S.; Leal, J.I.; Amorim, M.M.; Hamilton, S.; Rawlinson, W.D.; et al. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann. Neurol. 2017, 81, 152–156. [Google Scholar] [CrossRef]
- Carroll, T.; Lo, M.; Lanteri, M.; Dutra, J.; Zarbock, K.; Silveira, P.; Rourke, T.; Ma, Z.M.; Fritts, L.; O’Connor, S.; et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017, 13, e1006537. [Google Scholar] [CrossRef]
- Woollard, S.M.; Olwenyi, O.A.; Dutta, D.; Dave, R.S.; Mathews, S.; Gorantla, S.; Johnson, N.; Giavedoni, L.; Norgren, R.B., Jr.; Byrareddy, S.N. Preliminary Studies on Immune Response and Viral Pathogenesis of Zika Virus in Rhesus Macaques. Pathogens 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Coombs, K.M. ZIKV Infection Induces DNA Damage Response and Alters the Proteome of Gastrointestinal Cells. Viruses 2020, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Jeannin, P.; Burlaud-Gaillard, J.; Roingeard, P.; Gessain, A.; Ceccaldi, P.E.; Vidy, A. Evidence That Zika Virus Is Transmitted by Breastfeeding to Newborn A129 (Ifnar1 Knock-Out) Mice and Is Able to Infect and Cross a Tight Monolayer of Human Intestinal Epithelial Cells. Front. Microbiol. 2020, 11, 524678. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.; de Oliveira Santos, I.; Braz-de-Melo, H.A.; de Sant’Ana, L.P.; das Neves Almeida, R.; Pasquarelli-do-Nascimento, G.; Prado, P.S.; Kobinger, G.P.; Maurice, C.F.; Magalhaes, K.G. Gut microbiota modulation induced by Zika virus infection in immunocompetent mice. Sci. Rep. 2021, 11, 1421. [Google Scholar] [CrossRef]
- de Alwis, R.; Zellweger, R.M.; Chua, E.; Wang, L.F.; Chawla, T.; Sessions, O.M.; Marlier, D.; Connolly, J.E.; von Messling, V.; Anderson, D.E. Systemic inflammation, innate immunity and pathogenesis after Zika virus infection in cynomolgus macaques are modulated by strain-specificity within the Asian lineage. Emerg. Microbes Infect. 2021, 10, 1457–1470. [Google Scholar] [CrossRef]
- Herrera, C.; Gallagher, A.; Ferguson, D.; Fen, Y.; Stein, M.; Ham, C.; Giotis, E.S.; Skinner, M.A.; Kempster, M.; Hall, J.; et al. Mucosal responses to HIV-1 co-infection with an emerging pathogen, Zika virus. J. Int. AIDS Soc. 2021, 24 (Suppl. 1), e25659. [Google Scholar] [CrossRef]
- Scott, J.M.; Lebratti, T.J.; Richner, J.M.; Jiang, X.; Fernandez, E.; Zhao, H.; Fremont, D.H.; Diamond, M.S.; Shin, H. Cellular and Humoral Immunity Protect against Vaginal Zika Virus Infection in Mice. J. Virol. 2018, 92, e00038-18. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Nunez, N.V.; Follain, G.; Delalande, F.; Hirschler, A.; Partiot, E.; Hale, G.L.; Bollweg, B.C.; Roels, J.; Chazal, M.; Bakoa, F.; et al. Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells. Nat. Commun. 2019, 10, 4430. [Google Scholar] [CrossRef] [PubMed]
- Cumberworth, S.L.; Barrie, J.A.; Cunningham, M.E.; de Figueiredo, D.P.G.; Schultz, V.; Wilder-Smith, A.J.; Brennan, B.; Pena, L.J.; Freitas de Oliveira Franca, R.; Linington, C.; et al. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected. Acta Neuropathol. Commun. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu-Kayisli, O.; Guo, X.; Tang, Z.; Semerci, N.; Ozmen, A.; Larsen, K.; Mutluay, D.; Guller, S.; Schatz, F.; Kayisli, U.A.; et al. Zika Virus-Infected Decidual Cells Elicit a Gestational Age-Dependent Innate Immune Response and Exaggerate Trophoblast Zika Permissiveness: Implication for Vertical Transmission. J. Immunol. 2020, 205, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Ilan, O.; Tal, Y.; Hershko, A.Y.; Shamriz, O.; Bohbot, E.; Tayeb, S.; Regev, D.; Panet, A.; Eliashar, R. A Novel Tool for Nasal Polyp Investigation: An Ex vivo Organ Culture System. Isr. Med. Assoc. J. 2020, 22, 48–52. [Google Scholar]
- Jurado, K.A.; Simoni, M.K.; Tang, Z.; Uraki, R.; Hwang, J.; Householder, S.; Wu, M.; Lindenbach, B.D.; Abrahams, V.M.; Guller, S.; et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 2016, 1, e88461. [Google Scholar] [CrossRef]
- Kuassivi, O.N.; Abiven, H.; Satie, A.P.; Cartron, M.; Mahe, D.; Aubry, F.; Mathieu, R.; Rebours, V.; Le Tortorec, A.; Dejucq-Rainsford, N. Human Testicular Germ Cells, a Reservoir for Zika Virus, Lack Antiviral Response Upon Zika or Poly(I:C) Exposure. Front. Immunol. 2022, 13, 909341. [Google Scholar] [CrossRef]
- Langerak, T.; Broekhuizen, M.; Unger, P.A.; Tan, L.; Koopmans, M.; van Gorp, E.; Danser, A.H.J.; Rockx, B. Transplacental Zika virus transmission in ex vivo perfused human placentas. PLoS Negl. Trop. Dis. 2022, 16, e0010359. [Google Scholar] [CrossRef]
- Limonta, D.; Branton, W.; Wong, C.P.; Saito, L.; Power, C.; Hobman, T.C. Use of Primary Human Fetal Astrocytes and Tissue Explants as Ex Vivo Models to Study Zika Virus Infection of the Developing Brain. Methods Mol. Biol. 2020, 2142, 251–259. [Google Scholar] [CrossRef]
- Pena, L.J.; Miranda Guarines, K.; Duarte Silva, A.J.; Sales Leal, L.R.; Mendes Felix, D.; Silva, A.; de Oliveira, S.A.; Junqueira Ayres, C.F.; Junior, A.S.; de Freitas, A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Petitt, M.; Tabata, T.; Puerta-Guardo, H.; Harris, E.; Pereira, L. Zika virus infection of first-trimester human placentas: Utility of an explant model of replication to evaluate correlates of immune protection ex vivo. Curr. Opin. Virol. 2017, 27, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.K.; Jurado, K.A.; Abrahams, V.M.; Fikrig, E.; Guller, S. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol. 2017, 77, e12613. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Sanchez, E.; Burciaga-Flores, M.; Zapata-Cuellar, L.; Camacho-Villegas, T.A.; Elizondo-Quiroga, D.E. Possible Routes for Zika Virus Vertical Transmission in Human Placenta: A Comprehensive Review. Viral Immunol. 2022, 35, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.A.; Harms, M.; Kruger, F.; Gross, R.; Joas, S.; Hayn, M.; Dietz, A.N.; Lippold, S.; von Einem, J.; Schubert, A.; et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat. Commun. 2018, 9, 2207. [Google Scholar] [CrossRef]
- Berry, N.; Herrera, C.; Cranage, M. Detection, quantification, and characterisation of HIV/SIV. Methods Mol. Biol. 2011, 665, 133–160. [Google Scholar] [CrossRef]
- Grivel, J.C.; Margolis, L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 2009, 4, 256–269. [Google Scholar] [CrossRef]
- Introini, A.; Vanpouille, C.; Grivel, J.C.; Margolis, L. An ex vivo Model of HIV-1 Infection in Human Lymphoid Tissue and Cervico-vaginal Tissue. Bio. Protoc. 2014, 4, e1047. [Google Scholar] [CrossRef]
- Arakelyan, A.; Fitzgerald, W.; Grivel, J.C.; Vanpouille, C.; Margolis, L. Histocultures (tissue explants) in human retrovirology. Methods Mol. Biol. 2014, 1087, 233–248. [Google Scholar] [CrossRef]
- Calenda, G.; Villegas, G.; Reis, A.; Millen, L.; Barnable, P.; Mamkina, L.; Kumar, N.; Roberts, K.; Kalir, T.; Martinelli, E.; et al. Mucosal Susceptibility to Human Immunodeficiency Virus Infection in the Proliferative and Secretory Phases of the Menstrual Cycle. AIDS Res. Hum. Retroviruses 2019, 35, 335–347. [Google Scholar] [CrossRef]
- Saba, E.; Origoni, M.; Taccagni, G.; Ferrari, D.; Doglioni, C.; Nava, A.; Lisco, A.; Grivel, J.C.; Margolis, L.; Poli, G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013, 6, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.R.; Brewers, L.M.; Kashuba, A.D.M.; Sykes, C. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS 2018, 32, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, K.M.; Barry, S.M.; Anderson, M.R.; Hope, T.J. Enhanced cellular responses and environmental sampling within inner foreskin explants: Implications for the foreskin’s role in HIV transmission. Mucosal Immunol. 2010, 3, 410–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penn, M.L.; Grivel, J.C.; Schramm, B.; Goldsmith, M.A.; Margolis, L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 1999, 96, 663–668. [Google Scholar] [CrossRef]
- Herrera, C.; Shattock, R.J. Candidate microbicides and their mechanisms of action. Curr. Top. Microbiol. Immunol. 2014, 383, 1–25. [Google Scholar]
- Anton, P.A.; Cranston, R.D.; Kashuba, A.; Hendrix, C.W.; Bumpus, N.N.; Richardson-Harman, N.; Elliott, J.; Janocko, L.; Khanukhova, E.; Dennis, R.; et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res. Hum. Retroviruses 2012, 28, 1412–1421. [Google Scholar] [CrossRef]
- Fox, J.; Tiraboschi, J.M.; Herrera, C.; Else, L.; Egan, D.; Dickinson, L.; Jackson, A.; Olejniczak, N.; Back, D.; Khoo, S.; et al. Brief Report: Pharmacokinetic/Pharmacodynamic Investigation of Single-Dose Oral Maraviroc in the Context of HIV-1 Pre-exposure Prophylaxis. J. Acquir. Immune Defic. Syndr. 2016, 73, 252–257. [Google Scholar] [CrossRef]
- McGowan, I.; Cranston, R.D.; Duffill, K.; Siegel, A.; Engstrom, J.C.; Nikiforov, A.; Jacobson, C.; Rehman, K.K.; Elliott, J.; Khanukhova, E.; et al. A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study). PLoS One 2015, 10, e0125363. [Google Scholar]
- Richardson-Harman, N.; Hendrix, C.W.; Bumpus, N.N.; Mauck, C.; Cranston, R.D.; Yang, K.; Elliott, J.; Tanner, K.; McGowan, I.; Kashuba, A.; et al. Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS One 2014, 9, e111507. [Google Scholar]
- Richardson-Harman, N.; Mauck, C.; McGowan, I.; Anton, P. Dose-response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res. Hum. Retroviruses 2012, 28, 1422–1433. [Google Scholar] [CrossRef]
- Herrera, C.; Lwanga, J.; Lee, M.; Mantori, S.; Amara, A.; Else, L.; Penchala, S.D.; Egan, D.; Challenger, E.; Dickinson, L.; et al. Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP). J. Antimicrob. Chemother. 2021, 76, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Nash, S.; Dietrich, J.; Ssemata, A.S.; Herrera, C.; O’Hagan, K.; Else, L.; Chiodi, F.; Kelly, C.; Shattock, R.; Chirenje, M.; et al. Combined HIV Adolescent Prevention Study (CHAPS): Comparison of HIV pre-exposure prophylaxis regimens for adolescents in sub-Saharan Africa-study protocol for a mixed-methods study including a randomised controlled trial. Trials 2020, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.A.; Panther, L.; Marzinke, M.A.; Hendrix, C.W.; Hoesley, C.J.; van der Straten, A.; Husnik, M.J.; Soto-Torres, L.; Nel, A.; Johnson, S.; et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J. Acquir. Immune. Defic. Syndr. 2015, 70, 242–249. [Google Scholar] [CrossRef]
- McGowan, I.; Dezzutti, C.S.; Siegel, A.; Engstrom, J.; Nikiforov, A.; Duffill, K.; Shetler, C.; Richardson-Harman, N.; Abebe, K.; Back, D.; et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): An open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016, 3, e569–e578. [Google Scholar] [CrossRef]
- Robinson, J.A.; Marzinke, M.A.; Bakshi, R.P.; Fuchs, E.J.; Radebaugh, C.L.; Aung, W.; Spiegel, H.M.; Coleman, J.S.; Rohan, L.C.; Hendrix, C.W. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B). AIDS Res. Hum. Retroviruses 2017, 33, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Fonsart, J.; Saragosti, S.; Taouk, M.; Peytavin, G.; Bushman, L.; Charreau, I.; Hance, A.; Goldwirt, L.; Morel, S.; Mammano, F.; et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: A sub-study of the ANRS IPERGAY trial. J. Antimicrob. Chemother. 2017, 72, 478–485. [Google Scholar] [CrossRef]
- Sekabira, R.; McGowan, I.; Yuhas, K.; Brand, R.M.; Marzinke, M.A.; Manabe, Y.C.; Frank, I.; Eron, J.; Landovitz, R.J.; Anton, P.; et al. Higher colorectal tissue HIV infectivity in cisgender women compared with MSM before and during oral preexposure prophylaxis. AIDS 2021, 35, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Wilkin, T.; Landovitz, R.J.; Wu, C.; Chen, Y.; Marzinke, M.A.; Hendrix, C.W.; Richardson, P.; Eshleman, S.H.; Andrade, A.; et al. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 2019, 33, 237–246. [Google Scholar] [CrossRef]
- McGowan, I.M.; Kunjara Na Ayudhya, R.P.; Brand, R.M.; Marzinke, M.A.; Hendrix, C.W.; Johnson, S.; Piper, J.; Holtz, T.H.; Curlin, M.E.; Chitwarakorn, A.; et al. An Open-Label Pharmacokinetic and Pharmacodynamic Assessment of Tenofovir Gel and Oral Emtricitabine/Tenofovir Disoproxil Fumarate. AIDS Res. Hum. Retroviruses 2022, 38, 279–287. [Google Scholar] [CrossRef]
- Leyva, F.; Fuchs, E.J.; Bakshi, R.; Carballo-Dieguez, A.; Ventuneac, A.; Yue, C.; Caffo, B.; Du, Y.; Torbenson, M.; Li, L.; et al. Simultaneous Evaluation of Safety, Acceptability, Pericoital Kinetics, and Ex Vivo Pharmacodynamics Comparing Four Rectal Microbicide Vehicle Candidates. AIDS Res. Hum. Retroviruses 2015, 31, 1089–1097. [Google Scholar] [CrossRef]
- Anton, P.A.; Saunders, T.; Elliott, J.; Khanukhova, E.; Dennis, R.; Adler, A.; Cortina, G.; Tanner, K.; Boscardin, J.; Cumberland, W.G.; et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One 2011, 6, e23243. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Harman, N.; Lackman-Smith, C.; Fletcher, P.S.; Anton, P.A.; Bremer, J.W.; Dezzutti, C.S.; Elliott, J.; Grivel, J.C.; Guenthner, P.; Gupta, P.; et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J. Clin. Microbiol. 2009, 47, 3530–3539. [Google Scholar] [CrossRef]
- Herrera, C.; Cranage, M.; McGowan, I.; Anton, P.; Shattock, R.J. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob. Agents Chemother. 2009, 53, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Abraha, A.; Nankya, I.L.; Gibson, R.; Demers, K.; Tebit, D.M.; Johnston, E.; Katzenstein, D.; Siddiqui, A.; Herrera, C.; Fischetti, L.; et al. CCR5- and CXCR4-tropic subtype C human immunodeficiency virus type 1 isolates have a lower level of pathogenic fitness than other dominant group M subtypes: Implications for the epidemic. J. Virol. 2009, 83, 5592–5605. [Google Scholar] [CrossRef] [PubMed]
- Nugeyre, M.T.; Tchitchek, N.; Adapen, C.; Cannou, C.; Contreras, V.; Benjelloun, F.; Ravel, J.; Le Grand, R.; Marlin, R.; Menu, E. Dynamics of Vaginal and Rectal Microbiota Over Several Menstrual Cycles in Female Cynomolgus Macaques. Front. Cell. Infect. Microbiol. 2019, 9, 188. [Google Scholar] [CrossRef]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Fletcher, P.S.; Elliott, J.; Grivel, J.C.; Margolis, L.; Anton, P.; McGowan, I.; Shattock, R.J. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. Aids 2006, 20, 1237–1245. [Google Scholar] [CrossRef]
- Crowley, A.R.; Ackerman, M.E. Mind the Gap: How Interspecies Variability in IgG and Its Receptors May Complicate Comparisons of Human and Non-human Primate Effector Function. Front. Immunol. 2019, 10, 697. [Google Scholar] [CrossRef]
- Hu, Q.; Frank, I.; Williams, V.; Santos, J.J.; Watts, P.; Griffin, G.E.; Moore, J.P.; Pope, M.; Shattock, R.J. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 2004, 199, 1065–1075. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Clarke, S.; Berry, N.; Almond, N. Attenuated SIV causes persisting neuroinflammation in the absence of a chronic viral load and neurotoxic antiretroviral therapy. AIDS 2016, 30, 2439–2448. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berry, N.; Stein, M.; Ferguson, D.; Ham, C.; Hall, J.; Giles, E.; Kempster, S.; Adedeji, Y.; Almond, N.; Herrera, C. Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques. Pathogens 2022, 11, 1033. https://doi.org/10.3390/pathogens11091033
Berry N, Stein M, Ferguson D, Ham C, Hall J, Giles E, Kempster S, Adedeji Y, Almond N, Herrera C. Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques. Pathogens. 2022; 11(9):1033. https://doi.org/10.3390/pathogens11091033
Chicago/Turabian StyleBerry, Neil, Monja Stein, Deborah Ferguson, Claire Ham, Jo Hall, Elaine Giles, Sarah Kempster, Yemisi Adedeji, Neil Almond, and Carolina Herrera. 2022. "Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques" Pathogens 11, no. 9: 1033. https://doi.org/10.3390/pathogens11091033
APA StyleBerry, N., Stein, M., Ferguson, D., Ham, C., Hall, J., Giles, E., Kempster, S., Adedeji, Y., Almond, N., & Herrera, C. (2022). Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques. Pathogens, 11(9), 1033. https://doi.org/10.3390/pathogens11091033





