First Data on Campylobacter spp. Presence in Shellfish in Croatia
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Panciker, G.; Bej, A.K. Detection of pathogenic bacteria in shellfish using multiplex PCR followed by CovaLinkTM NH microwell plate sandwich hybridization. J. Microbiol. Methods 2003, 53, 199–209. [Google Scholar] [CrossRef]
- Walters, S.P.; Gannon, V.P.J.; Field, K.G. Detection of Bacteroidales fecal indicators and the zoonotic pathogens E. coli O157: H7, Salmonella, and Campylobacter in river water. Environ. Sci. Technol. 2007, 41, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Džafić, N. Effect of Systematic Monitoring of Hygiene Quality in Mussels (Mytilus galloprovincialis) on Territory of Istra County. Master’s Thesis, Veterinary Faculty University of Zagreb, Zagre, Croatia, 2012. [Google Scholar]
- Škoko, I. Detection and Phylogenetic Analysis of Norovirus from Bivalve Molluscs at Production Areas in the Republic of Croatia. Ph.D. Thesis, Veterinary Faculty University of Zagreb, Zagreb, Croatia, 2015. [Google Scholar]
- Escobedo-Hinojosa, W.; Pardo-López, L. Analysis of bacterial metagenomes from the Southwestern gulf of Mexico for pathogens detection. Pathog. Dis. 2017, 75, ftx058. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Yee, E.; Chapman, M.H.; Smith, T.P.L.; Bono, J.L.; Huynh, S.; Parker, C.; Vandamme, P.; Luong, K.; Korlach, J. Comparative genomics of the Campylobacter lari group. Genome Biol. Evol. 2014, 6, 3252–3266. [Google Scholar] [CrossRef]
- Pitkanen, T.; Hanninen, M.-L. Members of the family Campylobacteraceae: Campylobacter jejuni, Campylobacter coli. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project), Part 3: Specific Excreted Pathogens: Environmental and Epidemiology Aspects—Section 2: Bacteria; Rose, J.B., Jiménez-Cisneros, B., Pruden, A., Ashbolt, N., Miller, J., Eds.; Michigan State University: East Lansing, MI, USA, 2017; Available online: http://www.waterpathogens.org/book/campylobacter (accessed on 1 July 2022).
- Martinez-Urtaza, J.; Lozano-Leon, A.; DePaola, A.; Ishibashi, M.; Shimada, K.; Nishibuchi, M.; Liebana, E. Characterization of pathogenic Vibrio parahaemolyticus isolates from clinical sources in Spain and comparison with Asian and North American pandemic isolates. J. Clin. Microbiol. 2004, 42, 4672–4678. [Google Scholar] [CrossRef]
- Van Dyke, M.I.; Morton, V.K.; McLellan, N.L.; Huck, P.M. The occurrence of Campylobacter in river water and waterfowl within a watershed in southern Ontario, Canada. J. Appl. Microbiol. 2010, 109, 1053–1066. [Google Scholar] [CrossRef]
- Wilkes, G.; Edge, T.A.; Gannon, V.P.J.; Jokinen, C.; Lyautey, E.; Neumann, N.F.; Ruecker, N.; Scott, A.; Sunohara, M.; Topp, E.; et al. Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res. 2011, 45, 5807–5825. [Google Scholar] [CrossRef]
- Abeyta, C.; Deeter, F.G.; Kaysner, C.A.; Stott, R.F.; Wekell, M.M. Campylobacter jejuni in a Washington state shellfish growing bed associated with illness. J. Food Prot. 1993, 56, 323–325. [Google Scholar] [CrossRef]
- Yoder, J.S.; Hlavsa, M.C.; Craun, G.F.; Hill, V.; Roberts, V.; Yu, P.A.; Hicks, L.A.; Newton, A.; Hilborn, E.D.; Wade, T.J. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events–United States, 2005–2006. MMWR Surveill. Summ. 2008, 57, 1–29. [Google Scholar] [CrossRef]
- The Rhode Island Department of Health (RIDOH). Potters Pond Closed to Shellfish Harvesting. Available online: https://www.ri.gov/press/view/42081 (accessed on 1 July 2022).
- Croatian Health Statistics Yearbook 2020. Croatian Institute of Public Health: Zagreb, Croatia, 2020. Available online: https://www.hzjz.hr/hrvatski-zdravstveno-statisticki-ljetopis/hrvatski-zdravstveno-statisticki-ljetopis-za-2020-tablicni-podaci/ (accessed on 1 July 2022).
- Humski, A.; Mikulić, M.; Stojević, D.; Jurinović, L.; Džafić, N.; Vučković, D. Quantitative data on Campylobacter spp. in broiler neck skins in different regions of Croatia. In Proceedings of the 20th International workshop on Campylobacter, Helicobacter and Related Organisms, Belfast, UK, 8–11 September 2019. [Google Scholar]
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. Official Journal of the European Communities OJ L 139/2004. Consolidated version 28/10/2021 (in Croatian). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02004R0853-20211028 (accessed on 1 July 2022).
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Official Journal of the European Union L 338/2005. Consolidated Version 08/03/2020 (in Croatian). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2073-20200308 (accessed on 1 July 2022).
- Willson, I.G.; Moore, J.E. Presence of Salmonella spp. and Campylobacter spp. in shellfish. Epidemiol. Infect. 1996, 116, 147–153. [Google Scholar] [CrossRef]
- Rincé, A.; Balière, C.; Hervio-Heath, D.; Cozien, J.; Lozach, S.; Parnaudeau, S.; Le Guyader, F.S.; Le Hello, S.; Giard, J.-C.; Sauvageot, N.; et al. Occurrence of bacterial pathogens and human Noroviruses in shellfish-harvesting areas and Their catchments in France. Front. Microbiol. 2018, 9, 2443. [Google Scholar] [CrossRef]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; Del Castillo, E.; Polz, M.F.; Collier, T.K.; Strom, M.S.; et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef]
- Bouchriti, N.; El Marrakchi, A.; Goyal, S.M.; Boutaib, R. Bacterial loads in Moroccan mussels from harvest to sale. J. Food Protect. 1995, 58, 509–512. [Google Scholar] [CrossRef]
- Waldenström, J.; Broman, T.; Carlsson, I.; Hasselquist, D.; Achterberg, R.P.; Wagenaar, J.A.; Olsen, B. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in Different Ecological Guilds and Taxa of Migrating Birds. Appl. Environ Microbiol. 2002, 68, 5911–5917. [Google Scholar] [CrossRef]
- Jurinović, L.; Duvnjak, S.; Kompes, G.; Šoprek, S.; Šimpraga, B.; Krstulović, F.; Mikulić, M.; Humski, A. Occurrence of Campylobacter jejuni in gulls feeding on Zagreb Rubbish Tip, Croatia; Their diversity and antimicrobial susceptibility in Perspective with human and broiler isolates. Pathogens 2020, 9, 695. [Google Scholar] [CrossRef]
- Kralj, J.; Barišić, S.; Ćiković, D.; Tutiš, V.; van Swelm, N.D. Extensive post-breeding movements of Adriatic Yellow-legged Gulls Larus michahellis. J. Ornithol. 2014, 155, 399–409. [Google Scholar] [CrossRef]
- Lozano-León, A.; Rodríguez-Souto, R.; González-Escalona, N.; Llovo, J.; Iglesias-Canle, J.; Alvarez-Castro, A.; Garrido-Maestu, A. Detection, molecular characterization, and antimicrobial susceptibility, of Campylobacter spp. isolated from shellfish. Microb. Risk Anal. 2021, 18, 100–176. [Google Scholar] [CrossRef]
- Glünder, G. NaCl-tolerance of Campylobacter isolates from birds and Campylobacter type strains and variation of their serological behavior. J. Vet. Med. 1993, 40, 245–252. [Google Scholar] [CrossRef]
- Teunis, P.; Havelaar, A.; Vliegenthart, J.; Roessink, G. Risk assessment of Campylobacter species in shellfish: Identifying the unknown. Water Sci. Technol. 1997, 35, 29–34. [Google Scholar] [CrossRef]
- Adriatic Sea; Croatian Encyclopedia Online Edition; The Miroslav Krleža Institute of Lexicography: Zagreb, Croatia, 2021; Available online: http://www.enciklopedija.hr/Natuknica.aspx?ID=28478 (accessed on 1 July 2022).
- Brennhovd, O.; Kapperud, G.; Langeland, G. Survey of thermotolerant Campylobacter spp. and Yersinia spp. in three surface water sources in Norway. Int. J. Food Microbiol. 1992, 15, 327–338. [Google Scholar] [CrossRef]
- Rodriguez, S.; Araujo, R. Effect of environmental parameters on the inactivation of the waterborne pathogen Campylobacter in a Mediterranean river. J. Water Health 2012, 10, 100–107. [Google Scholar] [CrossRef][Green Version]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Welcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Republic of Croatia Ministry of Agriculture. Monitoring Plan for the Quality of the Sea, and Bivalve Molluscs in Production Areas and Areas for the Re-Laying of Live Bivalve Molluscs; Republic of Croatia Ministry of Agriculture: Zagreb, Croatia, 2020; Available online: http://www.veterinarstvo.hr/UserDocsImages/HranaZaZiv/Plan.pracenja.kakvoce.mora.i.skoljkasa.2020.pdf (accessed on 1 July 2022).
- EN ISO 10272-1:2017; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 1: Detection Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.A.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C.J. Multilocus Sequence Typing System for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Miller, W.G.; On, S.L.; Wang, G.; Fontanoz, S.; Lastovica, A.J.; Mandrell, R.E. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 2005, 43, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
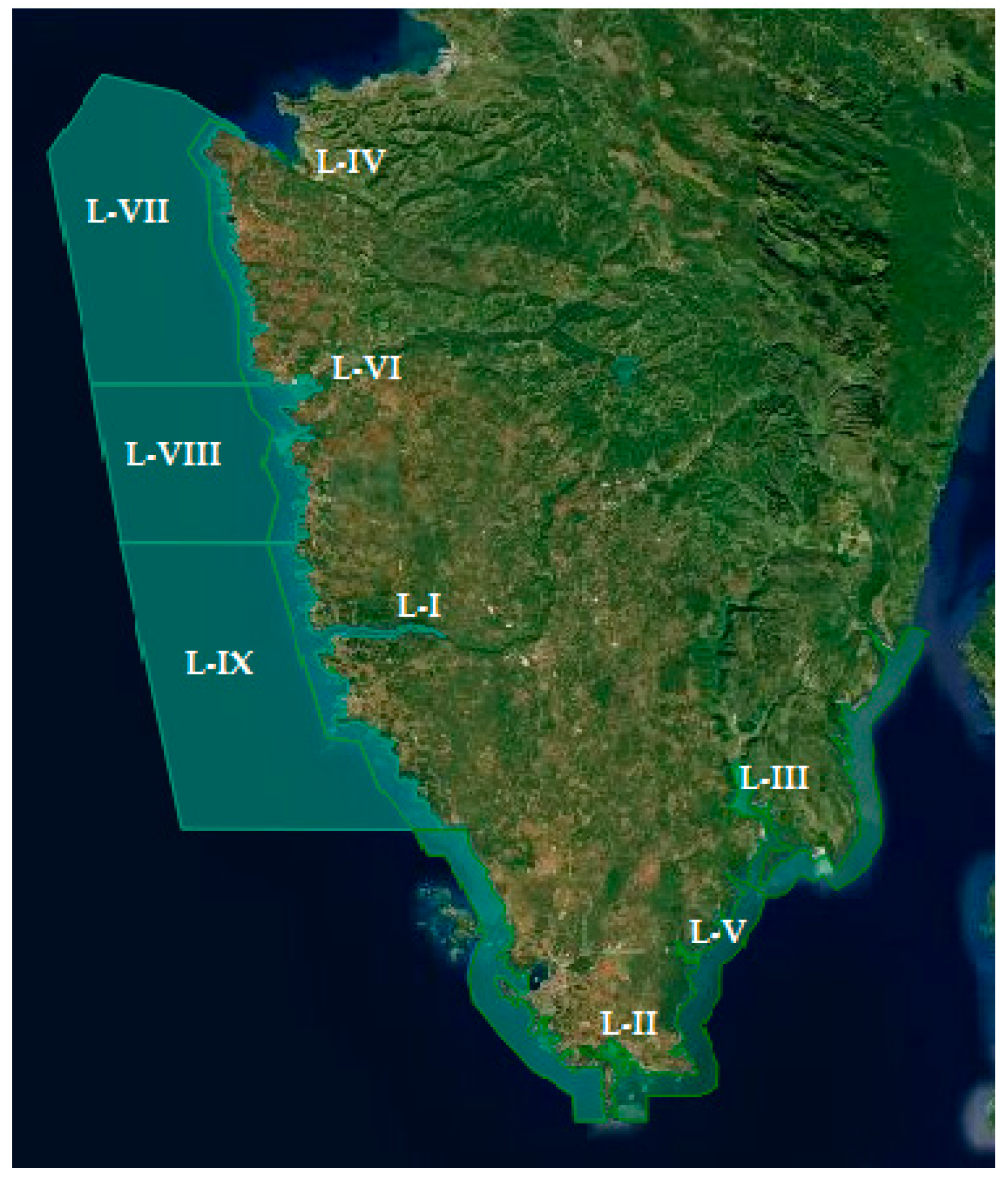
| Coast Location * | No. of Samples According Shelfish Species | Total of Samples | No. of Positive Samples | |||||
|---|---|---|---|---|---|---|---|---|
| Oysters | Scallops | Mussels | Total | with C. jejuni | with C. lari | |||
| East | L-III | - | - | 13 | 13 | 1 | 0 | 1 |
| L-V | - | - | 13 | 13 | 2 | 0 | 2 | |
| West | L-I | - | - | 13 | 13 | 7 | 0 | 7 |
| L-II | - | - | 13 | 13 | 1 | 0 | 1 | |
| L-IV | - | - | 13 | 13 | 5 | 0 | 5 | |
| L-VI | - | - | 13 | 13 | 4 | 1 | 3 | |
| L-VII | - | 10 | - | 10 | 0 | 0 | 0 | |
| L-VIII | - | 10 | - | 10 | 0 | 0 | 0 | |
| L-IX | 3 | 7 | - | 10 | 0 | 0 | 0 | |
| Total | 3 | 27 | 78 | 108 | 20 | 1 | 19 | |
| Month | Date of Sampling | Sea Temperature (°C) | Coast | Location | Campylobacter Species |
|---|---|---|---|---|---|
| August | 2 August. | 26 | West | L-I | C. lari |
| 9 August | 26 | L-IV | C. lari | ||
| 9 August. | 25 | L-VI | C. lari | ||
| 16 August | 25 | L-I | C. lari | ||
| 16 August | 27 | L-IV | C. lari | ||
| 23 August | 26 | L-IV | C. lari | ||
| September | 6 September | 25 | West | L-I | C. lari |
| 6 September | 24 | L-IV | C. lari | ||
| 13 September | 25 | L-I | C. lari | ||
| 13 September | 25 | L-VI | C. lari | ||
| 20 September | 23 | L-VI | C. jejuni | ||
| 27 September | 23 | L-VI | C. lari | ||
| October | 4 October | 23 | West | L-I | C. lari |
| 4 October | 21 | L-II | C. lari | ||
| 13 October | 20 | L-IV | C. lari | ||
| 11 October | 22 | L-I | C. lari | ||
| 25 October | 18 | L-I | C. lari | ||
| 4 October | 21 | East | L-V | C. lari | |
| 4 October | 19 | L-III | C. lari | ||
| 11 October | 19 | L-V | C. lari |
| Isolate | ST | adk | atpA | glnA | glyA | pgi | pgm | tkt |
|---|---|---|---|---|---|---|---|---|
| sh01 | 300 | 7 | 1 | 1 | 53 | 1 | 84 | 2 |
| sh03 | 223 | 128 | 6 | 1 | 1 | 1 | 1 | 36 |
| sh04 | 223 | 128 | 6 | 1 | 1 | 1 | 1 | 36 |
| sh05 | 301 | 147 | 150 | 31 | 88 | 129 | 173 | 149 |
| sh06 | 301 | 147 | 150 | 31 | 88 | 129 | 173 | 149 |
| sh09 | 302 | 109 | 113 | 94 | 27 | 188 | 141 | 146 |
| sh11 | 303 | 103 | 4 | 1 | 1 | 1 | 3 | 36 |
| sh12 | 76 | 7 | 1 | 1 | 1 | 1 | 1 | 2 |
| sh13 | 77 | 7 | 1 | 1 | 53 | 1 | 3 | 2 |
| sh14 | 301 | 147 | 150 | 31 | 88 | 129 | 173 | 149 |
| sh17 | 77 | 7 | 1 | 1 | 53 | 1 | 3 | 2 |
| sh18 | 82 | 8 | 6 | 1 | 1 | 1 | 1 | 86 |
| sh19 | 307 | 8 | 2 | 1 | 2 | 186 | 1 | 2 |
| sh20 | 307 | 8 | 2 | 1 | 2 | 186 | 1 | 2 |
| sh21 | 304 | 20 | 39 | 14 | 49 | 41 | 35 | 20 |
| sh22 | 308 | 20 | 18 | 122 | 49 | 187 | 14 | 164 |
| sh23 | 308 | 20 | 18 | 122 | 49 | 187 | 14 | 164 |
| sh25 | 305 | 17 | 114 | 93 | 27 | 73 | 136 | 101 |
| sh26 | 311 | 30 | 153 | 95 | 104 | 189 | 174 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurinović, L.; Ječmenica, B.; Džafić, N.; Brlek Gorski, D.; Šimpraga, B.; Krstulović, F.; Amšel Zelenika, T.; Humski, A. First Data on Campylobacter spp. Presence in Shellfish in Croatia. Pathogens 2022, 11, 943. https://doi.org/10.3390/pathogens11080943
Jurinović L, Ječmenica B, Džafić N, Brlek Gorski D, Šimpraga B, Krstulović F, Amšel Zelenika T, Humski A. First Data on Campylobacter spp. Presence in Shellfish in Croatia. Pathogens. 2022; 11(8):943. https://doi.org/10.3390/pathogens11080943
Chicago/Turabian StyleJurinović, Luka, Biljana Ječmenica, Natalija Džafić, Diana Brlek Gorski, Borka Šimpraga, Fani Krstulović, Tajana Amšel Zelenika, and Andrea Humski. 2022. "First Data on Campylobacter spp. Presence in Shellfish in Croatia" Pathogens 11, no. 8: 943. https://doi.org/10.3390/pathogens11080943
APA StyleJurinović, L., Ječmenica, B., Džafić, N., Brlek Gorski, D., Šimpraga, B., Krstulović, F., Amšel Zelenika, T., & Humski, A. (2022). First Data on Campylobacter spp. Presence in Shellfish in Croatia. Pathogens, 11(8), 943. https://doi.org/10.3390/pathogens11080943






