Epidemiology of Ticks and Tick-Borne Pathogens in Domestic Ruminants across Southern African Development Community (SADC) Region from 1980 until 2021: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Literature Search and Eligible Studies
2.2. Characteristics of Eligible Studies
2.3. Pooling, Heterogeneity, and Sub-Group Analysis
2.3.1. Prevalence in Animals Based on Host, Study Years, Countries, Diagnostic Technique and Species of Tick-Borne Pathogens
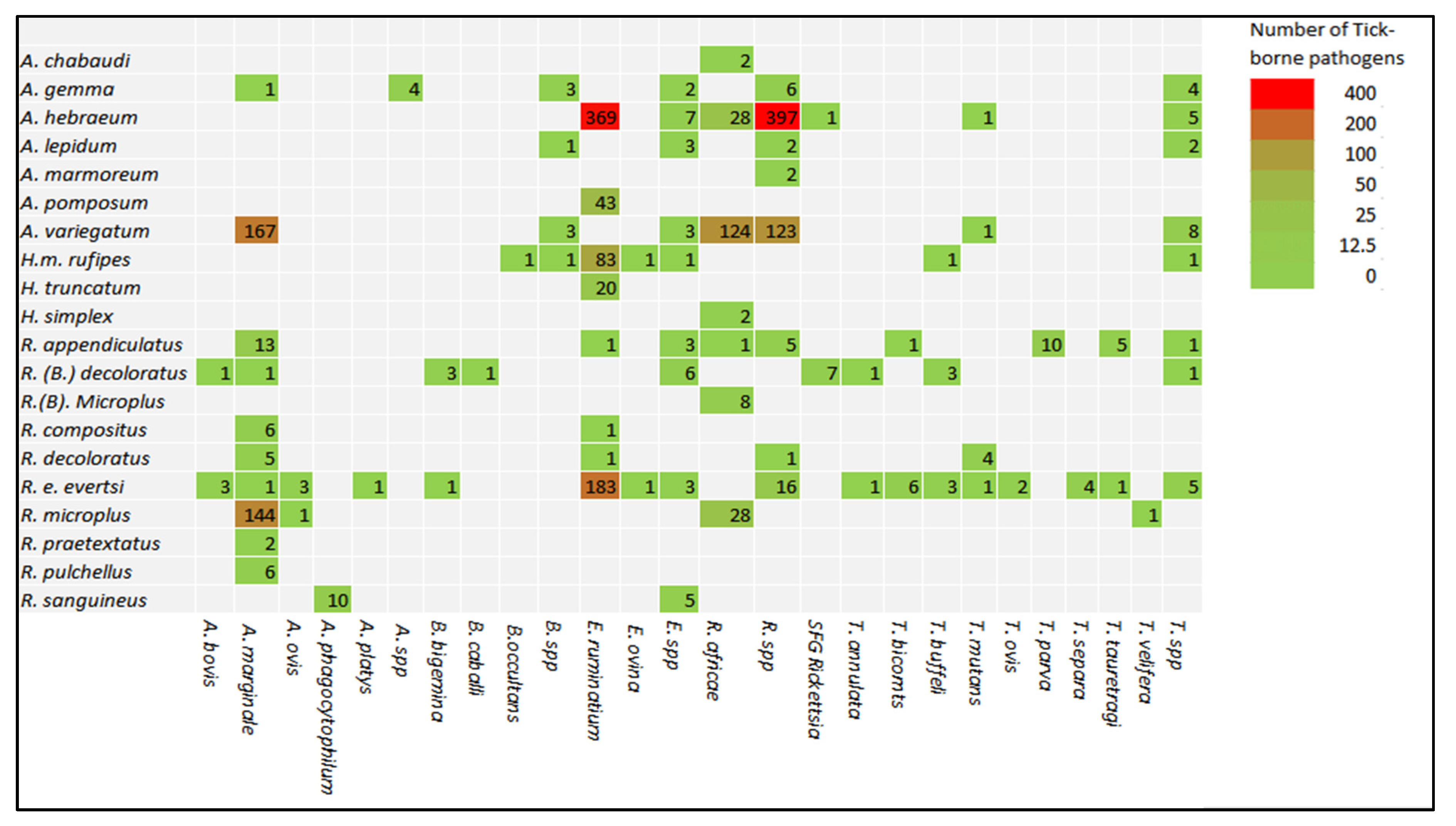
2.3.2. Prevalence of Tick-Borne Pathogens in Different Species of Ticks
2.3.3. Prevalence of Tick-Borne Pathogens in Different Species of Ticks
2.3.4. Assessment for Publication Bias in Studies Involving Domestic Ruminant Animals
3. Discussion
3.1. Ticks
3.2. Tick-Borne Pathogens in Different Animal Host
3.3. Limitations
4. Materials and Methods
4.1. Search Strategy and Criteria
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Meta-Analytic Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adenubi, O.T.; Ahmed, A.S.; Fasina, F.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crop. Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Wu, X.B.; Na, R.H.; Wei, S.S.; Zhu, J.S.; Peng, H.J. Distribution of tick-borne diseases in China. Parasites Vectors 2013, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. Int. J. Med. Microbiol. 2008, 298, 19–24. [Google Scholar] [CrossRef]
- Sirotkin, M.B.; Korenberg, E. Influence of abiotic factors on different developmental stages of the taiga (Ixodes persulcatus) and European forest (Ixodes ricinus) ticks. Zool. Zhurnal 2018, 97, 379–396. [Google Scholar] [CrossRef]
- Uspensky, I. Infections with Natural Focality Transmitted by Ixodid Ticks. Am. Entomol. 2016, 62, 260–262. [Google Scholar] [CrossRef][Green Version]
- Vorou, R.; Pierroutsakos, I.N.; Maltezou, H.C. Crimean-Congo hemorrhagic fever. Curr. Opin. Infect. Dis. 2007, 20, 495–500. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Horak, I.G.; Matthee, C.; Matthee, S. A new species of Ixodes (Acari: Ixodidae) from South African mammals. J. Parasitol. 2011, 97, 389–398. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Fernandes, É.K.; Bittencourt, V.R.; Roberts, D.W. Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp. Parasitol. 2012, 130, 300–305. [Google Scholar] [CrossRef]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2003, 28, 27–54. [Google Scholar] [CrossRef]
- Horak, I.G.; Nyangiwe, N.; De Matos, C.; Neves, L. Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and a subtropical coastal region of south-eastern Africa. Onderstepoort J. Vet. Res. 2009, 76, 263–278. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Pena, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the World: A List of Valid Species Names; Magnolia Press: Waco, TX, USA, 2010; Available online: http://hdl.handle.net/2263/17278 (accessed on 11 October 2020).
- Latif, A.A.; Putterill, J.F.; De Klerk, D.G.; Pienaar, R.; Mans, B.J. Nuttalliella namaqua (Ixodoidea: Nuttalliellidae): First description of the male, immature stages and re-description of the female. PLoS ONE 2012, 7, e41651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mans, B.J.; de Klerk, D.; Pienaar, R.; de Castro, M.H.; Latif, A.A. The mitochondrial genomes of Nuttalliella namaqua (Ixodoidea: Nuttalliellidae) and Argas africolumbae (Ixodoidae: Argasidae): Estimation of divergence dates for the major tick lineages and reconstruction of ancestral blood-feeding characters. PLoS ONE 2012, 7, e49461. [Google Scholar] [CrossRef] [PubMed]
- Klemola, T.; Sormunen, J.J.; Mojzer, J.; Mäkelä, S.; Vesterinen, E.J. High tick abundance and diversity of tick-borne pathogens in a Finnish city. Urban Ecosyst. 2019, 22, 817–826. [Google Scholar] [CrossRef]
- Kernif, T.; Leulmi, H.; Raoult, D.; Parola, P. Emerging tick-borne bacterial pathogens. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Yusufmia, S.B.A.S.; Collins, N.E.; Nkuna, R.; Troskie, M.; Van den Bossche, P.; Penzhorn, B.L. Occurrence of Theileria parva and other haemoprotozoa in cattle at the edge of Hluhluwe-iMfolozi Park, KwaZulu-Natal, South Africa. J. S. Afri. Vet. Assoc. 2010, 81, 45–49. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Jensenius, M.; Parola, P.; Raoult, D. Threats to international travellers posed by tick-borne diseases. Travel Med. Infect. Dis. 2006, 4, 4–13. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E.; Strych, U.; Chang, L.Y.; Lim, Y.A.; Goodenow, M.M.; AbuBakar, S. Neglected tropical diseases among the Association of Southeast Asian Nations (ASEAN): Overview and update. PLoS Negl. Trop. Dis. 2015, 9, e0003575. [Google Scholar] [CrossRef]
- Boularias, G.; Azzag, N.; Gandoin, C.; Bouillin, C.; Chomel, B.; Haddad, N.; Boulouis, H.J. Bovines Harbor a Diverse Array of Vector-Borne Pathogens in Northeast Algeria. Pathogens 2020, 9, 883. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Andersson, T.; Aspi, J.; Bäck, J.; Cederberg, T.; Haavisto, N.; Halonen, H.; Hänninen, J.; Inkinen, J.; Kulha, N.; et al. Monitoring of ticks and tick-borne pathogens through a nationwide research station network in Finland. Ticks Tick-Borne Dis. 2020, 11, 101449. [Google Scholar] [CrossRef]
- Bilgic, H.B.; Bakırcı, S.; Kose, O.; Unlu, A.H.; Hacılarlıoglu, S.; Eren, H.; Weir, W.; Karagenc, T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasites Vectors 2017, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, G.; Santamaría-Espinosa, R.M.; Lira-Amaya, J.J.; Figueroa, J.V. Challenges in Tick-Borne Pathogen Detection: The Case for Babesia spp. Identification in the Tick Vector. Pathogens 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Gitau, G.K.; Perry, B.D.; Katende, J.M.; McDermott, J.J.; Morzaria, S.P.; Young, A.S. The prevalence of serum antibodies to tick-borne infections in cattle in smallholder dairy farms in Murang’a District, Kenya: A cross-sectional study. Prev. Vet. Med. 1997, 30, 95–107. [Google Scholar] [CrossRef]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef]
- Makala, L.H.; Mangani, P.; Fujisaki, K.; Nagasawa, H. The current status of major tick borne diseases in Zambia. Vet. Res. 2003, 34, 27–45. [Google Scholar] [CrossRef]
- Swai, E.S.; Karimuribo, E.D.; Kambarage, D.M.; Moshy, W.E.; Mbise, A.N. A comparison of seroprevalence and risk factors for Theileria parva and T. mutans in smallholder dairy cattle in the Tanga and Iringa regions of Tanzania. Vet. J. 2007, 174, 390–396. [Google Scholar] [CrossRef]
- Barradas, P.F.; Mesquita, J.R.; Ferreira, P.; Gärtner, F.; Carvalho, M.; Inácio, E.; Chivinda, E.; Katimba, A.; Amorim, I. Molecular identification and characterization of Rickettsia spp. and other tick-borne pathogens in cattle and their ticks from Huambo, Angola. Ticks Tick-Borne Dis. 2021, 12, 101583. [Google Scholar] [CrossRef]
- Kubelová, M.; Mazancová, J.; Široký, P. Theileria, Babesia, and Anaplasma detected by PCR in ruminant herds at Bié Province, Angola. Parasite 2012, 19, 417. [Google Scholar] [CrossRef]
- Sili, G.; Byaruhanga, C.; Horak, I.; Steyn, H.; Chaisi, M.; Oosthuizen, M.C.; Neves, L. Ticks and tick-borne pathogens infecting livestock and dogs in Tchicala-Tcholoanga, Huambo Province, Angola. Parasitol. Res. 2021, 120, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Stoltsz, H.; Byaruhanga, C.; Troskie, M.; Makgabo, M.; Oosthuizen, M.C.; Collins, N.E.; Neves, L. Improved detection of Babesia bigemina from various geographical areas in Africa using quantitative PCR and reverse line blot hybridisation. Ticks Tick-Borne Dis. 2020, 11, 101415. [Google Scholar] [CrossRef] [PubMed]
- Binta, M.G.; Losho, T.; Allsopp, B.A.; Mushi, E.Z. Isolation of Theileria taurotragi and Theileria mutans from cattle in Botswana. Vet. Parasitol. 1998, 77, 83–91. [Google Scholar] [CrossRef]
- Berthelsson, J.; Ramabu, S.S.; Lysholm, S.; Aspán, A.; Wensman, J.J. Anaplasma ovis infection in goat flocks around Gaborone, Botswana. Comp. Clin. Pathol. 2020, 29, 167–172. [Google Scholar] [CrossRef]
- Ramabu, S.S.; Kgwatalala, P.M.; Nsoso, S.J.; Gasebonwe, S.; Kgosiesele, E. Anaplasma infection prevalence in beef and dairy cattle in the southeast region of Botswana. Vet. Parasitol. Reg. Stud. Rep. 2017, 12, 4–8. [Google Scholar] [CrossRef]
- Chatanga, E.; Kainga, H.; Maganga, E.; Hayashida, K.; Katakura, K.; Sugimoto, C.; Nonaka, N.; Nakao, R. Molecular identification and genetic characterization of tick-borne pathogens in sheep and goats at two farms in the central and southern regions of Malawi. Ticks Tick-Borne Dis. 2021, 12, 101629. [Google Scholar] [CrossRef]
- De Jesus Fernandes, S.; Matos, C.A.; Freschi, C.R.; de Souza Ramos, I.A.; Machado, R.Z.; André, M.R. Diversity of Anaplasma species in cattle in Mozambique. Ticks Tick-Borne Dis. 2019, 10, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Neves, L.; Pedro, O.; Fafetine, J.; Do Rosa-rio, V.; Domingos, A. Molecular detection of Babesia spp. and other haemoparasitic infections of cattle in Maputo Province, Mozambique. Parasitology 2010, 137, 939–946. [Google Scholar] [CrossRef]
- Martins, T.M.; Pedro, O.C.; Caldeira, R.A.; do Rosário, V.E.; Neves, L.; Domingos, A. Detection of bovine babesiosis in Mozambique by a novel seminested hot-start PCR method. Vet. Parasitol. 2008, 153, 225–230. [Google Scholar] [CrossRef]
- Matos, C.A.; Gonçalves, L.R.; de Souza Ramos, I.A.; Mendes, N.S.; Zanatto, D.C.S.; André, M.R.; Machado, R.Z. Molecular detection and characterization of Ehrlichia ruminantium from cattle in Mozambique. Acta Trop. 2019, 191, 198–203. [Google Scholar] [CrossRef]
- Chaisi, M.E.; Baxter, J.R.; Hove, P.; Choopa, C.N.; Oosthuizen, M.C.; Brayton, K.A.; Khumalo, Z.T.; Mutshembele, A.M.; Mtshali, M.S.; Collins, N.E. Comparison of three nucleic acid-based tests for detecting Anaplasma marginale and Anaplasma centrale in cattle. Onderstepoort J. Vet. Res. 2017, 84, 1–9. [Google Scholar] [CrossRef]
- Hove, P.; Khumalo, Z.T.; Chaisi, M.E.; Oosthuizen, M.C.; Brayton, K.A.; Collins, N.E. Detection and Characterisation of Anaplasma marginale and A. centrale in South Africa. Vet. Sci. 2018, 5, 26. [Google Scholar] [CrossRef]
- Latif, A.A.; Troskie, P.C.; Peba, S.B.; Maboko, B.B.; Pienaar, R.; Mans, B.J. Corridor disease (buffalo-associated Theileria parva) outbreak in cattle introduced onto a game ranch and investigations into their carrier-state. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100331. [Google Scholar] [CrossRef] [PubMed]
- Marufu, M.C.; Chimonyo, M.; Mtshali, M.S.; Dzama, K.G. Molecular prevalence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in Nguni and local crossbred cattle under the low input production system in South Africa. In Proceedings of the 10th Annual Congress of the Southern African Society for Veterinary Epidemiology and Preventive Medicine, Farm Inn, South Africa, 1–3 August 2012; p. 57. [Google Scholar]
- Mbizeni, S.; Potgieter, F.T.; Troskie, C.; Mans, B.J.; Penzhorn, B.L.; Latif, A.A. Field and laboratory studies on Corridor disease (Theileria parva infection) in cattle population at the livestock/game interface of uPhongolo-Mkuze area, South Africa. Ticks Tick-Borne Dis. 2013, 4, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, L.S.; Taioe, O.M.; Smit, N.J.; Thekisoe, O.M. Parasites of veterinary importance from domestic animals in uMkhanyakude district of KwaZulu-Natal province. J. S. Afr. Vet. Assoc. 2020, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, M.S.; Mtshali, P.S. Molecular diagnosis and phylogenetic analysis of Babesia bigemina and Babesia bovis hemoparasites from cattle in South Africa. BMC Vet. Res. 2013, 9, 154. [Google Scholar] [CrossRef]
- Mtshali, P.S.; Mtshali, M.S. In silico and phylogenetic analyses of partial BbRAP-1, BbCP2, BbSBP-4 and BbβTUB gene sequences of Babesia bovis isolates from cattle in South Africa. BMC Vet. Res. 2017, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, M.S.; De la Fuente, J.; Ruybal, P.; Kocan, K.M.; Vicente, J.; Mbati, P.A.; Shkap, V.; Blouin, E.F.; Mohale, N.E.; Moloi, T.P.; et al. Prevalence and genetic diversity of Anaplasma marginale strains in cattle in South Africa. Zoonoses Public Health 2007, 54, 23–30. [Google Scholar] [CrossRef]
- Mtshali, M.S.; Steyn, H.C.; Mtshali, P.S.; Mbati, P.A.; Kocan, K.M.; Latif, A.; Shkap, V. The detection and characterization of multiple tick-borne pathogens in cattle at Ficksburg and Reitz (Free State Province, South Africa) using reverse line blot hybridization. Afri. J. Microbiol. Res. 2013, 7, 646–651. [Google Scholar] [CrossRef]
- Mtshali, P.S.; Tsotetsi, A.M.; Thekisoe, M.M.O.; Mtshali, M.S. Nested PCR detection and phylogenetic analysis of Babesia bovis and Babesia bigemina in cattle from peri-urban localities in Gauteng province, South Africa. J. Vet. Med. Sci. 2013, 76, 145–150. [Google Scholar] [CrossRef]
- Mutshembele, A.M.; Cabezas-Cruz, A.; Mtshali, M.S.; Thekisoe, O.M.; Galindo, R.C.; de la Fuente, J. Epidemiology and evolution of the genetic variability of Anaplasma marginale in South Africa. Ticks Tick-Borne Dis. 2014, 5, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, R.; Troskie, P.C.; Josemans, A.I.; Potgieter, F.T.; Maboko, B.B.; Latif, A.A.; Mans, B.J. Investigations into the carrier-state of Theileria spp.(buffalo) in cattle. Int. J. Parasitol. Parasites Wildl. 2002, 11, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Moumouni, P.F.A.; Taioe, M.; Jirapattharasate, C.; Liu, M.; Wang, G.; Gao, Y.; Guo, H.; Lee, S.H.; Zheng, W.; et al. Molecular analysis of tick-borne protozoan and rickettsial pathogens in small ruminants from two South African provinces. Parasitol. Int. 2018, 67, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Steyn, H.C.; Pretorius, A. Genetic diversity of Ehrlichia ruminantium field strains from selected farms in South Africa. Onderstepoort J. Vet. Res. 2020, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.E.; Latif, A.A.; Oosthuizen, M.C.; Troskie, M.; Penzhorn, B.L. Occurrence of Theileria parva infection in cattle on a farm in the Ladysmith district, KwaZulu-Natal, South Africa. J. S. Afri. Vet. Assoc. 2008, 79, 31–35. [Google Scholar] [CrossRef]
- Laisser, E.L.K.; Kipanyula, M.J.; Msalya, G.; Mdegela, R.H.; Karimuribo, E.D.; Mwilawa, A.J.; Mwega, E.D.; Kusiluka, L.; Chenyambuga, S.W. Tick burden and prevalence of Theileria parva infection in Tarime zebu cattle in the lake zone of Tanzania. Trop. Anim. Health Prod. 2014, 46, 1391–1396. [Google Scholar] [CrossRef]
- Kazungu, Y.E.; Mwega, E.; Neselle, M.O.; Sallu, R.; Kimera, S.I.; Gwakisa, P. Incremental effect of natural tick challenge on the infection and treatment method-induced immunity against T. parva in cattle under agro-pastoral systems in Northern Tanzania. Ticks Tick-Borne Dis. 2015, 6, 587–591. [Google Scholar] [CrossRef]
- Kerario, I.I.; Chenyambuga, S.W.; Mwega, E.D.; Rukambile, E.; Simulundu, E.; Simuunza, M.C. Diversity of two Theileria parva CD8+ antigens in cattle and buffalo-derived parasites in Tanzania. Ticks Tick-Borne Dis. 2019, 10, 1003–1017. [Google Scholar] [CrossRef]
- Kimaro, E.G.; Mor, S.M.; Gwakisa, P.; Toribio, J.A. Seasonal occurrence of Theileria parva infection and management practices amongst Maasai pastoralist communities in Monduli District, Northern Tanzania. Vet. Parasitol. 2017, 246, 43–52. [Google Scholar] [CrossRef]
- Magulu, E.; Kindoro, F.; Mwega, E.; Kimera, S.; Shirima, G.; Gwakisa, P. Detection of carrier state and genetic diversity of Theileria parva in ECF-vaccinated and naturally exposed cattle in Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100312. [Google Scholar] [CrossRef]
- Mwamuye, M.M.; Odongo, D.; Kazungu, Y.; Kindoro, F.; Gwakisa, P.; Bishop, R.P.; Nijhof, A.M.; Obara, I. Variant analysis of the sporozoite surface antigen gene reveals that asymptomatic cattle from wildlife-livestock interface areas in northern Tanzania harbour buffalo-derived T. parva. Parasitol. Res. 2020, 119, 3817–3828. [Google Scholar] [CrossRef]
- Ringo, A.E.; Moumouni, P.F.A.; Lee, S.H.; Liu, M.; Khamis, Y.H.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; Galon, E.M.; et al. Molecular detection and characterization of tick-borne protozoan and rickettsial pathogens isolated from cattle on Pemba Island, Tanzania. Ticks Tick-Borne Dis. 2018, 9, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Rizk, M.A.; Moumouni, P.F.A.; Liu, M.; Galon, E.M.; Li, Y.; Ji, S.; Tumwebaze, M.; Byamukama, B.; Thekisoe, O.; et al. Molecular detection and characterization of tick-borne haemoparasites among cattle on Zanzibar Island, Tanzania. Acta Trop. 2020, 211, 105598. [Google Scholar] [CrossRef]
- Rukambile, E.; Machuka, E.; Njahira, M.; Kyalo, M.; Skilton, R.; Mwega, E.; Chota, A.; Mathias, M.; Sallu, R.; Salih, D. Population genetic analysis of Theileria parva isolated in cattle and buffaloes in Tanzania using minisatellite and microsatellite markers. Vet. Parasitol. 2016, 224, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Thekisoe, O.M.M.; Omolo, J.D.; Swai, E.S.; Hayashida, K.; Zhang, J.; Sugimoto, C.; Inoue, N. Preliminary application and evaluation of loop-mediated isothermal amplification (LAMP) for detection of bovine theileriosis and trypanosomosis in Tanzania: Research communication. Onderstepoort J. Vet. Res. 2007, 74, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Iseki, H.; Alhassan, A.; Ohta, N.; Thekisoe, O.M.; Yokoyama, N.; Inoue, N.; Nambota, A.; Yasuda, J.; Igarashi, I. Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J. Microbial. Methods 2007, 71, 281–287. [Google Scholar] [CrossRef]
- Muleya, W.; Namangala, B.; Simuunza, M.; Nakao, R.; Inoue, N.; Kimura, T.; Ito, K.; Sugimoto, C.; Sawa, H. Population genetic analysis and sub-structuring of Theileria parva in the northern and eastern parts of Zambia. Parasites Vectors 2012, 5, 255. [Google Scholar] [CrossRef] [PubMed]
- Musinguzi, S.P.; Suganuma, K.; Asada, M.; Laohasinnarong, D.; Sivakumar, T.; Yokoyama, N.; Namangala, B.; Sugimoto, C.; Suzuki, Y.; Xuan, X.; et al. A PCR-based survey of animal African trypanosomosis and selected piroplasm parasites of cattle and goats in Zambia. J. Vet. Med. Sci. 2016, 78, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Simuunza, M.; Weir, W.; Courcier, E.; Tait, A.; Shiels, B. Epidemiological analysis of tick-borne diseases in Zambia. Vet. Parasitol. 2011, 175, 331–342. [Google Scholar] [CrossRef]
- Squarre, D.; Nakamura, Y.; Hayashida, K.; Kawai, N.; Chambaro, H.; Namangala, B.; Sugimoto, C.; Yamagishi, J. Investigation of the piroplasm diversity circulating in wildlife and cattle of the greater Kafue ecosystem, Zambia. Parasites Vectors 2020, 13, 599. [Google Scholar] [CrossRef]
- Tembo, S.; Collins, N.E.; Sibeko-Matjila, K.P.; Troskie, M.; Vorster, I.; Byaruhanga, C.; Oosthuizen, M.C. Occurrence of tick-borne haemoparasites in cattle in the Mungwi District, Northern Province, Zambia. Ticks Tick-Borne Dis. 2018, 9, 707–717. [Google Scholar] [CrossRef]
- Yamada, S.; Konnai, S.; Imamura, S.; Simuunza, M.; Chembensofu, M.; Chota, A.; Nambota, A.; Onuma, M.; Ohashi, K. PCR-based detection of blood parasites in cattle and adult Rhipicephalus appendiculatus ticks. Vet. J. 2009, 182, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, I.; Kelly, P.J.; Wray, K.; Musuka, G.; Trees, A.J.; Jongejan, F. Babesia bovis and B. bigemina DNA detected in cattle and ticks from Zimbabwe by polymerase chain reaction. J. S. Afri. Vet. Assoc. 2000, 71, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Socolovschi, C.; Kernif, T.; Temmam, S.; Lagadec, E.; Tortosa, P.; Parola, P. First molecular detection of Rickettsia africae in ticks from the Union of the Comoros. Parasites Vectors 2014, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Krüger, A.; Rakotondranary, S.J.; Ratovonamana, R.Y.; Poppert, S.; Ganzhorn, J.U.; Tappe, D. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020, 205, 105339. [Google Scholar] [CrossRef] [PubMed]
- Pothmann, D.; Poppert, S.; Rakotozandrindrainy, R.; Hogan, B.; Mastropaolo, M.; Thiel, C.; Silaghi, C. Prevalence and genetic characterization of Anaplasma marginale in zebu cattle (Bos indicus) and their ticks (Amblyomma variegatum, Rhipicephalus microplus) from Madagascar. Ticks Tick-Borne Dis. 2016, 7, 1116–1123. [Google Scholar] [CrossRef]
- Matsimbe, A.M.; Magaia, V.; Sanches, G.S.; Neves, L.; Noormahomed, E.; Antunes, S.; Domingos, A. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91–102. [Google Scholar] [CrossRef]
- Adelabu, O.A.; Iweriebor, B.C.; Okoh, A.I.; Obi, L.C. Phylogenetic profiling for zoonotic Ehrlichia spp. from ixodid ticks in the Eastern Cape, South Africa. Transbound. Emerg. Dis. 2020, 67, 1247–1256. [Google Scholar] [CrossRef]
- Berggoetz, M.; Schmid, M.; Ston, D.; Wyss, V.; Chevillon, C.; Pretorius, A.M.; Gern, L. Protozoan and bacterial pathogens in tick salivary glands in wild and domestic animal environments in South Africa. Ticks Tick-Borne Dis. 2014, 5, 176–185. [Google Scholar] [CrossRef]
- Guo, H.; Moumouni, P.F.A.; Thekisoe, O.; Gao, Y.; Liu, M.; Li, J.; Galon, E.M.; Efstratiou, A.; Wang, G.; Jirapattharasate, C.; et al. Genetic characterization of tick-borne pathogens in ticks infesting cattle and sheep from three South African provinces. Ticks Tick-Borne Dis. 2019, 10, 875–882. [Google Scholar] [CrossRef]
- Iweriebor, B.C.; Mmbaga, E.J.; Adegborioye, A.; Igwaran, A.; Obi, L.C.; Okoh, A.I. Genetic profiling for Anaplasma and Ehrlichia species in ticks collected in the Eastern Cape Province of South Africa. BMC Microbial. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Iweriebor, B.C.; Nqoro, A.; Obi, C.L. Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa. Pathogens 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Collins, N.E.; Brayton, K.A.; Chaisi, M.; Blumberg, L.; Frean, J.; Gall, C.A.; MWentzel, J.; Wills-Berriman, S.; Boni, L.D.; et al. Anaplasma phagocytophilum and Other Anaplasma spp. in Various Hosts in the Mnisi Community, Mpumalanga Province, South Africa. Microorganisms 2020, 8, 1812. [Google Scholar] [CrossRef]
- Jongejan, F.; Berger, L.; Busser, S.; Deetman, I.; Jochems, M.; Leenders, T.; De Sitter, B.; Van der Steen, F.; Wentzel, J.; Stoltsz, H. Amblyomma hebraeum is the predominant tick species on goats in the Mnisi Community Area of Mpumalanga Province South Africa and is co-infected with Ehrlichia ruminantium and Rickettsia africae. Parasites Vectors 2020, 13, 172. [Google Scholar] [CrossRef]
- Damian, D.; Damas, M.; Wensman, J.J.; Berg, M. Molecular Diversity of Hard Tick Species from Selected Areas of a Wildlife-Livestock Interface Ecosystem at Mikumi National Park, Morogoro Region, Tanzania. Vet. Sci. 2021, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Fyumagwa, R.D.; Simmler, P.; Meli, M.L.; Hoare, R.; Hofmann-Lehmann, R.; Lutz, H. Prevalence of Anaplasma marginale in different tick species from Ngorongoro Crater, Tanzania. Vet. Parasitol. 2009, 161, 154–157. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kwak, Y.S.; Kim, J.Y.; Nam, S.H.; Lee, I.Y.; Mduma, S.; Keyyu, J.; Fyumagwa, R.; Yong, T.S. Prevalence of tick-borne pathogens from ticks collected from cattle and wild animals in Tanzania in 2012. Korean J. Parasitol. 2018, 56, 305. [Google Scholar] [CrossRef] [PubMed]
- Savadye, D.T.; Kelly, P.J.; Mahan, S.M. Evidence to show that an agent that cross-reacts serologically with Cowdria ruminantium in Zimbabwe is transmitted by ticks. Exp. Appl. Acarol. 1998, 22, 111–122. [Google Scholar] [CrossRef]
- Gilbert, L. Louping ill virus in the UK: A review of the hosts, transmission and ecological consequences of control. Exp. Appl. Acarol. 2016, 68, 363–374. [Google Scholar] [CrossRef]
- Randolph, S.E. Tick ecology: Processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 2004, 129, S37–S65. [Google Scholar] [CrossRef]
- Njiiri, E.N. The Occurrence of Ehrlichia ruminantium and Other Haemoparasites in Calves in Western Kenya Determined by Reverse Line Blot Hybridization Assay, Real-Time PCR and Nested PCR. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2013. Available online: http://hdl.handle.net/2263/26197 (accessed on 22 January 2021).
- Basu, A.K.; Charles, R. Chapter 1—A general account of ticks. In Ticks of Trinidad and Tobago—An Overview; Academic Press: Cambridge, MA, USA, 2017; pp. 1–33. [Google Scholar]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.U.D.; Cabezas-Cruz, A.; Jabbar, A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Davis, J.O.N.; Alam, U.; Korman, A.M.Y.; Rutherford, J.S.; Rosenberg, R.; Azad, A.F. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Diatta, G.; Zolia, Y.; Balde, M.C.; Kohar, H.; Trape, J.F.; Raoult, D. Tick-borne rickettsiae in Guinea and Liberia. Ticks Tick-Borne Dis. 2012, 3, 43–48. [Google Scholar] [CrossRef]
- Hornok, S.; Abichu, G.; Meli, M.L.; Tánczos, B.; Sulyok, K.M.; Gyuranecz, M.; Gönczi, E.; Farkas, R.; Hofmann-Lehmann, R. Influence of the biotope on the tick infestation of cattle and on the tick-borne pathogen repertoire of cattle ticks in Ethiopia. PLoS ONE 2014, 9, e106452. [Google Scholar] [CrossRef] [PubMed]
- Kumsa, B.; Socolovschi, C.; Almeras, L.; Raoult, D.; Parola, P. Occurrence and genotyping of Coxiella burnetii in ixodid ticks in Oromia, Ethiopia. Am. J. Trop. Med. Hyg. 2015, 93, 1074. [Google Scholar] [CrossRef]
- Gondard, M.; Cabezas-Cruz, A.; Charles, R.A.; Vayssier-Taussat, M.; Albina, E.; Moutailler, S. Ticks and tick-borne pathogens of the Caribbean: Current understanding and future directions for more comprehensive surveillance. Front. Cell. Infect. Microbial. 2017, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Ozubek, S.; Dumanli, N. A survey of ixodid ticks feeding on cattle and prevalence of tick-borne pathogens in the Black Sea region of Turkey. Vet. Parasitol. 2012, 187, 567–571. [Google Scholar] [CrossRef]
- Sivakumar, T.; Tattiyapong, M.; Okubo, K.; Suganuma, K.; Hayashida, K.; Igarashi, I.; Zakimi, S.; Matsumoto, K.; Inokuma, H.; Yokoyama, N. PCR detection of Babesia ovata from questing ticks in Japan. Ticks Tick-Borne Dis. 2014, 5, 305–310. [Google Scholar] [CrossRef]
- Chaligiannis, Ι.; de Mera, I.G.F.; Papa, A.; Sotiraki, S.; de la Fuente, J. Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Exp. Appl. Acarol. 2018, 74, 443–453. [Google Scholar] [CrossRef]
- Mossaad, E.; Gaithuma, A.; Mohamed, Y.O.; Suganuma, K.; Umemiya-Shirafuji, R.; Ohari, Y.; Salim, B.; Liu, M.; Xuan, X. Molecular Characterization of Ticks and Tick-Borne Pathogens in Cattle from Khartoum State and East Darfur State, Sudan. Pathogens 2021, 10, 580. [Google Scholar] [CrossRef]
- Rehman, A.; Conraths, F.J.; Sauter-Louis, C.; Krücken, J.; Nijhof, A.M. Epidemiology of tick-borne pathogens in the semi-arid and the arid agro-ecological zones of Punjab province, Pakistan. Transbound. Emerg. Dis. 2019, 66, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Magaia, V.; Taviani, E.; Cangi, N.; Neves, L. Molecular detection of Rickettsia africae in Amblyomma ticks collected in cattle from Southern and Central Mozambique. J. Infect. Dev. Ctries. 2020, 14, 614–622. [Google Scholar] [CrossRef]
- Althaus, F.; Greub, G.; Raoult, D.; Genton, B. African tick-bite fever: A new entity in the differential diagnosis of multiple eschars in travelers. Description of five cases imported from South Africa to Switzerland. Int. J. Infect. Dis. 2010, 14, e274–e276. [Google Scholar] [CrossRef]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick-Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef]
- Byamukama, B.; Vudriko, P.; Tumwebaze, M.A.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; LI, J.; Galon, E.M.; Ringo, A.; Liu, M.; et al. Molecular detection of selected tick-borne pathogens infecting cattle at the wildlife–livestock interface of Queen Elizabeth National Park in Kasese District, Uganda. Ticks Tick-Borne Dis. 2021, 12, 101772. [Google Scholar] [CrossRef]
- Belkahia, H.; Said, M.B.; El Hamdi, S.; Yahiaoui, M.; Gharbi, M.; Daaloul-Jedidi, M.; Mhadhbi, M.; Jedidi, M.; Darghouth, M.A.; Klabi, I.; et al. First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rumin. Res. 2014, 121, 404–410. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Santos-Silva, M.M. The distribution of ticks (Acari: Ixodidae) of domestic livestock in Portugal. Exp. Appl. Acarol. 2005, 36, 233–246. [Google Scholar] [CrossRef]
- Zhang, J.; Kelly, P.; Guo, W.; Xu, C.; Wei, L.; Jongejan, F.; Loftis, A.; Wang, C. Development of a generic Ehrlichia FRET-qPCR and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasites Vectors 2015, 8, 506. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Koney, E.B.M.; Dogbey, O.; Walker, A.R. Incidence and prevalence of tick-borne haemoparasites in domestic ruminants in Ghana. Vet. Parasitol. 2004, 124, 25–42. [Google Scholar] [CrossRef]
- Southern African Development Community (SADC). Livestock Production. 2012. Available online: https://www.sadc.int/themes/agriculture-food-security/livestock-production/ (accessed on 5 July 2021).
- Muhanguzi, D.; Ikwap, K.; Picozzi, K.; Waiswa, C. Molecular characterization of Anaplasma and Ehrlichia species in different cattle breeds and age groups in Mbarara district (Western Uganda). Int. J. Anim. Vet. Adv. 2010, 2, 76–88. [Google Scholar]
- De la Fuente, J.; Torina, A.; Caracappa, S.; Tumino, G.; Furlá, R.; Almazán, C.; Kocan, K.M. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet. Parasitol. 2005, 133, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Noaman, V.; Shayan, P.; Amininia, N. Molecular diagnostic of Anaplasma marginale in carrier cattle. Iran. J. Parasitol. 2009, 4, 26–33. [Google Scholar]
- Aktas, M.; Altay, K.; Dumanli, N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick-Borne Dis. 2011, 2, 62–65. [Google Scholar] [CrossRef]
- Soosaraei, M.; Haghi, M.M.; Etemadifar, F.; Fakhar, M.; Teshnizi, S.H.; Asfaram, S.; Esboei, B.R. Status of Anaplasma spp. infection in domestic ruminants from Iran: A systematic review with meta-analysis. Parasite Epidemiol. Control 2020, 11, e00173. [Google Scholar] [CrossRef]
- Mohammadian, B.; Noaman, V.; Emami, S.J. Molecular survey on prevalence and risk factors of Anaplasma spp. infection in cattle and sheep in West of Iran. Trop. Anim. Health Prod. 2021, 53, 266. [Google Scholar] [CrossRef]
- Salehi-Guilandeh, S.; Sadeghi-Dehkordi, Z.; Sadeghi-Nasab, A. Molecular detection of Anaplasma spp. in cattle of Talesh County, North of Iran. Bulg. J. Vet. Med. 2019, 22, 457–465. [Google Scholar] [CrossRef]
- Liu, X.; Yan, B.; Wang, Q.; Jiang, M.; Tu, C.; Chen, C.; Hornok, S.; Wang, Y. Babesia vesperuginis in common pipistrelle (Pipistrellus pipistrellus) and the bat soft tick Argas vespertilionis in the People’s Republic of China. J. Wildl. Dis. 2018, 54, 419–421. [Google Scholar] [CrossRef]
- Amorim, L.S.; Wenceslau, A.A.; Carvalho, F.S.; Carneiro, P.L.S.; Albuquerque, G.R. Bovine babesiosis and anaplasmosis complex: Diagnosis and evaluation of the risk factors from Bahia, Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 328–336. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Triana-Chávez, O.; Holguín-Rocha, A.; Tobon-Castaño, A.; Mejía-Jaramillo, A.M. Molecular surveillance and phylogenetic traits of Babesia bigemina and Babesia bovis in cattle (Bos taurus) and water buffaloes (Bubalus bubalis) from Colombia. Parasites Vectors 2018, 11, 510. [Google Scholar] [CrossRef]
- Kocan, K.M. Targeting ticks for control of selected hemoparasitic diseases of cattle. Vet. Parasitol. 1995, 57, 121–151. [Google Scholar] [CrossRef]
- Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Detection of Ehrlichia ruminantium infection in cattle in Cameroon. BMC Res. Notes 2018, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, Z.; Krücken, J.; Baumann, M.; Ahmed, J.S.; Clausen, P.H.; Nijhof, A.M. Molecular detection of tick-borne pathogens in cattle from Southwestern Ethiopia. PLoS ONE 2017, 12, e0188248. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites Vectors 2016, 9, 388. [Google Scholar] [CrossRef]
- Deem, S.L.; Noval, R.A.I.; Yonow, T.; Peter, T.F.; Mahan, S.M.; Burridge, M.J. The epidemiology of heartwater: Establishment and maintenance of endemic stability. Parasitol. Today 1996, 12, 402–405. [Google Scholar] [CrossRef]
- . Leeflang, P.; Ilemobade, A.A. 1977. Tick-borne diseases of domestic animals in Northern Nigeria. Trop. Anim. Health Prod. 1977, 9, 211–218. [Google Scholar] [CrossRef]
- Moumouni, P.F.A.; Aboge, G.O.; Terkawi, M.A.; Masatani, T.; Cao, S.; Kamyingkird, K.; Jirapattharasate, C.; Zhou, M.; Wang, G.; Liu, M.; et al. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasites Vectors 2015, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Oura, C.A.; Tait, A.; Asiimwe, B.; Lubega, G.W.; Weir, W. Theileria parva genetic diversity and haemoparasite prevalence in cattle and wildlife in and around Lake Mburo National Park in Uganda. Parasitol. Res. 2011, 108, 1365–1374. [Google Scholar] [CrossRef]
- Salih, D.A.; El Hussein, A.M.; Kyule, M.N.; Zessin, K.H.; Ahmed, J.S.; Seitzer, U. Determination of potential risk factors associated with Theileria annulata and Theileria parva infections of cattle in the Sudan. Parasitol. Res. 2007, 101, 1285–1288. [Google Scholar] [CrossRef]
- Tomassone, L.; Grego, E.; Callà, G.; Rodighiero, P.; Pressi, G.; Gebre, S.; Zeleke, B.; De Meneghi, D. Ticks and tick-borne pathogens in livestock from nomadic herds in the Somali Region, Ethiopia. Exp. Appl. Acarol. 2012, 56, 391–401. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.E.; Knobel, D.; Chaisi, M.E.; Vorster, I.; Steyn, H.C.; Oosthuizen, M.C. Molecular investigation of tick-borne haemoparasite infections among transhumant zebu cattle in Karamoja Region, Uganda. Vet. Parasitoi. Reg. Stud. Rep. 2016, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Okal, M.N.; Odhiambo, B.K.; Otieno, P.; Bargul, J.L.; Masiga, D.; Villinger, J.; Kalayou, S. Anaplasma and Theileria pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface. Microorganisms 2020, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Oura, C.A.L.; Bishop, R.P.; Wampande, E.M.; Lubega, G.W.; Tait, A. Application of a reverse line blot assay to the study of haemoparasites in cattle in Uganda. Int. J. Parasitol. 2004, 34, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Yin, H.; Qi, B.; Gubbels, M.J.; Beyer, D.; Niemann, S.; Jongejan, F.; Ahmed, J.S. Simultaneous detection and differentiation of Theileria and Babesia parasites infecting small ruminants by reverse line blotting. Parasitol. Res. 2004, 92, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Rothstein, H.; Cohen, J. Comprehensive Meta-Analysis: A Computer Program for Research Synthesis; Biostat: Englewood, NJ, USA, 1999. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
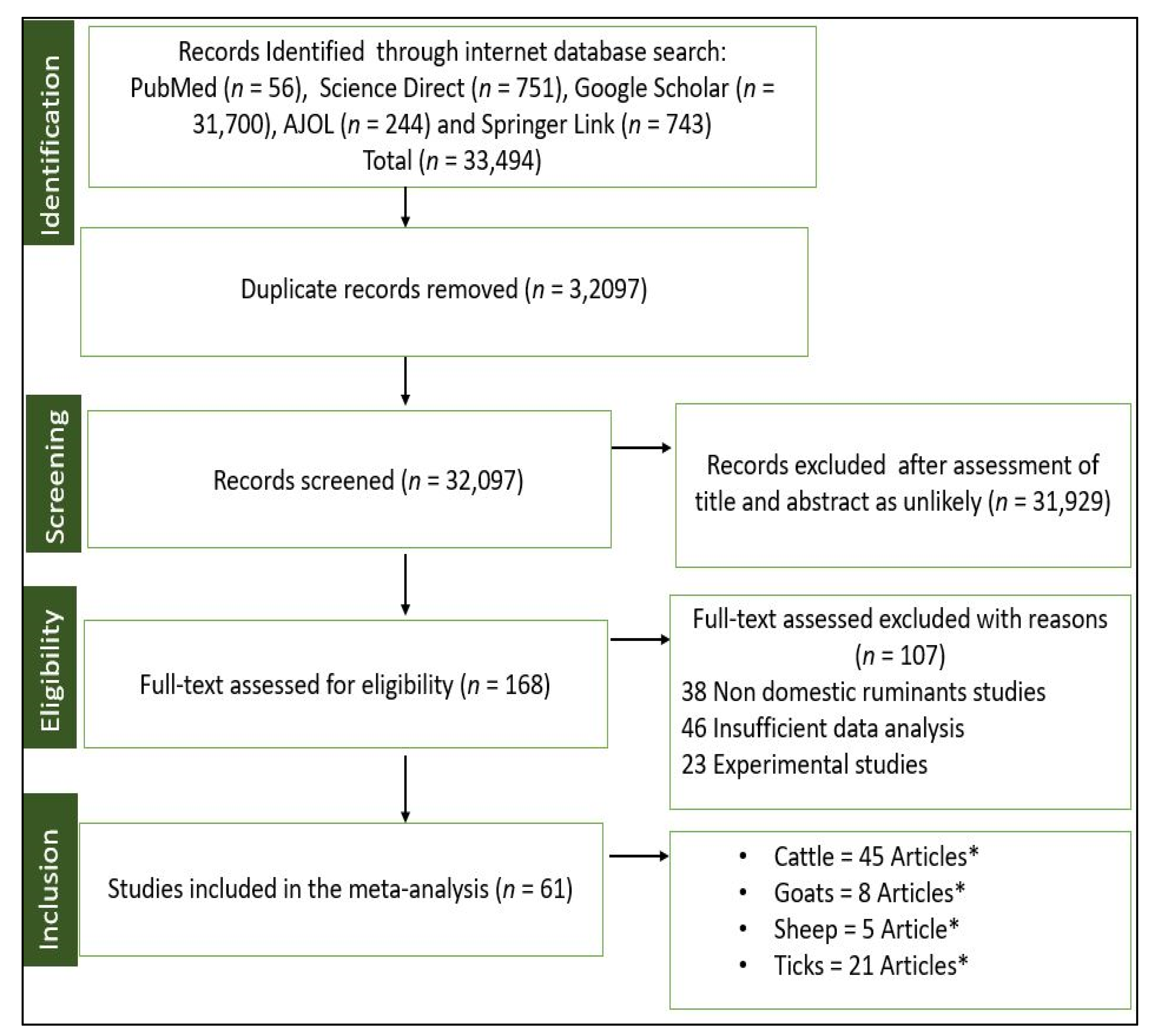


| Countries | Hosts | Sample Size | Total No. of Pathogens Detected | Pathogens Detected (No. of Positives, Prevalence (%) | Reference |
|---|---|---|---|---|---|
| Angola | Cattle | 98 | 11 | A. platys (n = 3; 3.06%), A. capra (n = 6; 6.12%), A. phagocytophilum (n = 2; 2.04%) | [30] |
| Angola | Cattle | 76 | 51 | A. marginale (n = 29; 38.15%), Anaplasma spp. (n = 6; 7.89%), B. bigemina (n = 2; 2.63%), T. velifera (n = 22; 28.95%), Theileria spp. (n = 6; 7.89%) | [31] |
| Angola | Goats | 13 | 13 | A. ovis (n = 13; 100.00%) | [30] |
| Angola | Cattle | 88 | 78 | A. bovis (n = 1; 1.14%), A. centrale (n = 11; 12.50%), A. marginale (n = 25; 28.41%), Anaplasma spp. (n = 22; 25.00%), Anaplasma spp (n = 22; 25.00%), A. platys (n = 16; 18.18%), B. bigemina (n = 35; 39.77%), B. rossi (n = 1; 1.14%), E. ruminatium (n = 3; 3.41%), T. velifera (n = 69; 78.41%), T. mutans (n = 65; 73.86), Theileria spp. (n = 63; 71.59%) | [32] |
| Angola | Goats | 82 | 2 | A. centrale (n = 2; 2.44%) | [32] |
| Angola | Sheep | 85 | 68 | A. centrale (n = 2; 2.35%), A. marginale (n = 1; 1.18%), Anaplasma spp. (n = 4; 4.71%), A. platys (n = 5; 5.88%), B. bovis (n = 1; 1.18%), T. ovis (n = 68; 80.00%), Theileria spp. (n = 46; 54.12%) | [32] |
| Angola | Cattle | 76 | 38 | B. bigemina (n = 38; 50.00%) | [33] |
| Botswana | Cattle | 276 | 2 | T. mutans (n = 1; 0.36%), T. taurotragi (n = 1; 0.36%) | [34] |
| Botswana | Goats | 100 | 76 | A. ovis (n = 76; 76.00%) | [35] |
| Botswana | Cattle | 429 | 135 | Anaplasma spp. (n = 135; 31.47%) | [36] |
| Malawi | Goats | 99 | 74 | A. ovis (n = 61; 61.62%), Anaplasma spp. (n = 74; 74.75%) | [37] |
| Malawi | Sheep | 8 | 8 | A. ovis (n = 8; 100%), Anaplasma spp. (n = 8; 100%) | [37] |
| Mozambique | Cattle | 219 | 213 | A. marginale (n = 213; 97.26%), A. phagocytophilum (n = 6; 2.74%), Anaplasma spp. (n = 191; 87.21%) | [38] |
| Mozambique | Cattle | 477 | 323 | A. centrale (n = 20; 4.19%), A. bovis (n = 4; 0.84%), A. marginale (n = 42; 8.80%), B. bigemina (n = 267; 55.97%), B. bovis (n = 201; 42.14%), Ehrlichia spp. (n = 29; 6.08%), T. mutans (n = 250; 52.41%), T. taurotragi (n = 5; 1.05%), T. velifera (n = 255; 53.46%), Theileria spp. (n = 41; 8.59%) | [39] |
| Mozambique | Cattle | 117 | 104 | B. bigemina (n = 104; 88.89%), B. bovis (n = 97; 82.91%) | [40] |
| Mozambique | Cattle | 210 | 31 | E. ruminatium (n = 31; 14.76%) | [41] |
| Mozambique | Cattle | 49 | 6 | B. bigemina (n = 6; 12.24%) | [33] |
| South Africa | Cattle | 66 | 51 | A. centrale (n = 27; 40.91%), A. marginale (n = 51; 77.27%) | [42] |
| South Africa | Cattle | 517 | 295 | A. centrale (n = 88; 17.02%), A. marginale (n = 295; 57.06%) | [43] |
| South Africa | Cattle | 200 | 54 | T. parva (n = 54; 27.00%) | [44] |
| South Africa | Cattle | 149 | 88 | A. marginale (n = 88; 59.06%) | [45] |
| South Africa | Cattle | 846 | 140 | T. parva (n = 140; 16.55%) | [46] |
| South Africa | Cattle | 109 | 57 | B. bigemina (n = 24; 22.02%) B. bovis (n = 33; 30.27%) | [47] |
| South Africa | Goats | 31 | 0 | 0.00 | [47] |
| South Africa | Sheep | 10 | 3 | T. ovis (n = 3; 30.00%) | [47] |
| South Africa | Cattle | 430 | 278 | B. bigemina (n = 278; 64.65%), B. bovis (n = 151; 35.12%) | [48] |
| South Africa | Cattle | 50 | 32 | B. bovis (n = 32; 64.00%) | [49] |
| South Africa | Cattle | 215 755 | 129 | A. marginale (n = 129; 60.00%) Anaplasma spp. (n = 648; 85.83%) | [50] |
| South Africa | Cattle | 74 | 39 | A. marginale (n = 39; 52.70%), B. bigemina (n = 2; 2.70%), Ehrlichia spp. (n = 14; 18.92%), T. taurotragi (n = 26; 35.14%) | [51] |
| South Africa | Cattle | 268 | 210 | B. bigemina (n = 204; 76.12%), B. bovis (n = 95; 35.45%) | [52] |
| South Africa | Cattle | 250 | 182 | A. marginale (n = 182; 72.80%) | [53] |
| South Africa | Cattle | 265 | 78 | T. parva (n = 78; 29.43%) | [54] |
| South Africa | Cattle | 70 | 55 | Anaplasma spp. (n = 55; 78.57%) | [36] |
| South Africa | Goats | 61 | 54 | A. ovis (n = 28; 45.90%), E. ruminatium (n = 12; 19.67%), T. ovis (n = 14; 22.95%) | [55] |
| South Africa | Sheep | 30 | 10 | A. ovis (n = 5; 16.67%), E. ruminatium (n = 1; 3.33%), T. ovis (n = 4; 13.33%) | [56] |
| South Africa | Cattle | 1723 | 48 | E. ruminatium (n = 48; 2.79%) | [57] |
| South Africa | Goats | 308 | 17 | E. ruminatium (n = 17; 5.52%) | [57] |
| South Africa | Sheep | 350 | 20 | E. ruminatium (n = 20; 5.71%) | [57] |
| South Africa | Cattle | 81 | 30 | B. bigemina (n = 30; 37.04%) | [33] |
| South Africa | Cattle | 170 | 106 | B. bigemina (n = 6; 3.53%), B. bovis (n = 9; 5.29%), T. parva (n = 8; 4.71%), T. taurotragi (n = 89; 52.35%) | [57] |
| South Africa | Cattle | 60 | 50 | B. rossi (n = 1; 1.67%)T. mutans (n = 49; 81.67%), T. parva (n = 4; 6.67%), T. taurotragi (n = 1; 1.67%), T. velifera (n = 42; 70.00%) | [17] |
| Tanzania | Cattle | 354 | 98 | T. parva (n = 98; 27.68%) | [58] |
| Tanzania | Cattle | 381 | 374 | T. parva (n = 374; 98.16%) | [59] |
| Tanzania | Cattle | 130 | 124 | T. parva (n = 124; 95.38%) | [60] |
| Tanzania | Cattle | 960 | 303 | T. parva (n = 303; 31.56%) | [61] |
| Tanzania | Cattle | 336 | 116 | T. parva (n = 116; 34.52%) | [62] |
| Tanzania | Cattle | 160 | 39 | T. parva (n = 39; 24.38%) | [63] |
| Tanzania | Cattle | 245 | 153 | A. marginale (n = 39; 15.92%), B. bigemina (n = 43; 17.55%), B. bovis (n = 11; 4.49%), T. mutans (n = 105; 42.86%), T. ovis (n = 3; 1.22%), T. parva (n = 63; 25.71%), T. taurotragi (n = 70; 30.20%) | [64] |
| Tanzania | Cattle | 236 | 152 | A. marginale (n = 24; 10.17%), B. bigemina (n = 12; 5.08%), B. bovis (n = 5; 2.12%), T. mutans (n = 90; 38.14%), T. parva (n = 81; 34.32%), T. taurotragi (n = 73; 30.93%), T. velifera (n = 8; 3.39%) | [65] |
| Tanzania | Cattle | 150 | 105 | T. parva (n = 105; 70.00%) | [66] |
| Tanzania | Cattle | 64 | 9 | Theileria spp. (n = 9; 14.06%) | [67] |
| Zambia | Cattle | 130 | 21 | B. bigemina (n = 19; 21.11%) B. bovis (n = 2; 2.22%) | [68] |
| Zambia | Cattle | 142 | 78 | T. parva (n = 78; 54.93%) | [69] |
| Zambia | Cattle | 472 | 79 | B. bigemina (n = 76; 16.10%), T. parva (n = 3; 0.64%) | [70] |
| Zambia | Goats | 53 | 0 | 0 | [70] |
| Zambia | Cattle | 579 | 181 | Anaplasma spp. (n = 69; 11.92%), E. ruminatium (n = 5; 0.86%), T. mutans (n = 94; 16.23%), T. parva (n = 4; 0.69%), T. taurotragi (n = 4; 0.69%) | [71] |
| Zambia | Cattle | 232 | 99 | B. bigemina (n = 24; 10.34%), T. mutans (n = 11; 4.74%), T. parva (n = 23; 9.91%), T. taurotragi (n = 41; 17.67%) | [72] |
| Zambia | Cattle | 299 | 259 | A. marginale (n = 77; 25.75%), B. bigemina (n = 10; 3.34%), B. bovis (n = 23; 7.69%), T. mutans (n = 163; 54.52%), T. parva (n = 1; 0.33%), T. velifera (n = 153; 51.17%) | [73] |
| Zambia | Cattle | 71 | 34 | A. marginale (n = 34; 47.89%), B. bigemina (n = 16; 22.54%), T. parva (n = 16; 22.54%) | [74] |
| Zimbabwe | Cattle | 94 | 33 | B. bigemina (n = 33; 35.11%), B. bovis (n = 27; 28.72%) | [75] |
| Risk Factors | Number of Studies | Pooled Prevalence Estimates | Measure of Heterogeneity | Q-p | Publication Bias | |||
|---|---|---|---|---|---|---|---|---|
| Sample Size | No. of Positive | Prevalence 95% CI | Q | I2 | Begg and Mazumdar Rank p-Value | |||
| Overall Animals | 48 | 12693 | 5172 | 52.2 (43.9–60.3) | 2820.792 | 98.33 | 0.609 | 0.065 |
| Animal hosts | ||||||||
| Cattle | 45 | 12693 | 5172 | 51.2 (42.9–59.4) | 2491.04 | 98.23 | 0.779 | 0.056 |
| Goats | 8 | 663 | 236 | 29.9 (7.3–69.9) | 252.68 | 97.23 | 0.325 | 0.310 |
| Sheep | 5 | 483 | 109 | 45.4 (9.4–87.0) | 146.22 | 97.26 | 0.861 | 0.312 |
| Genus Anaplasma | ||||||||
| A. bovis | 2 | 565 | 5 | 0.88 | - | - | - | - |
| A. centrale | 4 | 1148 | 146 | 14.7 (5.9–32.0) | 69.01 | 95.65 | 0.001 | 0.500 |
| A. marginale | 14 | 2982 | 1264 | 45.9 (31.3–61.3) | 618.20 | 97.90 | 0.605 | 0.351 |
| A. phagocytophilum | 2 | 317 | 8 | 2.52 | - | - | - | - |
| Anaplasma spp. | 7 | 2216 | 1126 | 45.6 (17.9–76.3) | 760.30 | 99.21 | 0.797 | 0.440 |
| Genus Babesia | ||||||||
| B. bigemina | 22 | 4393 | 1280 | 20.8 (12.4–32.6) | 1007.80 | 97.92 | 0.000 | 0.068 |
| B. bovis | 14 | 2733 | 723 | 20.3 (12.7–30.9) | 373.29 | 96.52 | 0.000 | 0.070 |
| Genus Ehrlichia | ||||||||
| E. ruminantium | 5 | 2936 | 118 | 4.2 (1.6–10.2) | 74.03 | 94.60 | 0.000 | 0.500 |
| Ehrlichia spp. | 2 | 551 | 43 | 7.80 | - | - | - | - |
| Genus Theileria | ||||||||
| Theileria spp. | 1 | 64 | 9 | 14.06 | - | - | - | - |
| T. mutans | 10 | 2591 | 831 | 29.1 (17.5–44.4) | 369.35 | 97.56 | 0.009 | 0.210 |
| T. parva | 20 | 6288 | 1712 | 25.0 (17.6–34.1) | 687.51 | 97.24 | 0.000 | 0.097 |
| T. velifera | 6 | 1236 | 549 | 43.0 (26.4–61.4) | 135.20 | 96.30 | 0.459 | 0.286 |
| Diagnostic technique | ||||||||
| nPCR | 14 | 3815 | 2006 | 61.5 (45.6–75.2) | 799.92 | 98.38 | 0.155 | 0.104 |
| PCR | 28 | 5432 | 2291 | 43.6 (34.8–52.8) | 863.936 | 96.88 | 0.172 | 0.376 |
| qPCR | 4 | 2534 | 475 | 31.0 (6.7–73.8) | 537.17 | 99.44 | 0.393 | 0.248 |
| RLB | 7 | 1428 | 863 | 63.0 (42.0–80.0) | 201.38 | 97.02 | 0.222 | 0.440 |
| RT-PCR | 2 | 1046 | 194 | 18.55 | - | - | - | - |
| htPCR | 1 | 117 | 86 | 73.50 | - | - | - | - |
| Study year | ||||||||
| 1990–2000 | 1 | 276 | 2 | 0.72 | - | - | - | - |
| 2001–2010 | 9 | 2023 | 1170 | 63.6 (49.1–75.9) | 273.97 | 97.08 | 0.066 | 0.267 |
| 2011–2020 | 21 | 5085 | 2586 | 57.3 (46.4–67.6) | 844.80 | 97.63 | 0.187 | 0.040 |
| Study countries | ||||||||
| Angola | 4 | 338 | 178 | 54.3 (21.9–83.5) | 85.86 | 96.51 | 0.814 | 0.248 |
| Botswana | 2 | 705 | 137 | 19.43 | - | - | - | - |
| Mozambique | 5 | 1072 | 677 | 62.9 (25.3–89.5) | 255.31 | 98.43 | 0.521 | 0.312 |
| South Africa | 18 | 5543 | 1922 | 52.2 (37.6–66.4) | 1212.40 | 98.60 | 0.772 | 0.367 |
| Tanzania | 10 | 3016 | 1474 | 57.8 (42.2–72.0) | 432.85 | 97.92 | 0.326 | 0.020 |
| Zambia | 7 | 1925 | 751 | 41.7 (24.1–61.7) | 330.51 | 98.18 | 0.417 | 0.226 |
| Zimbabwe | 1 | 94 | 33 | 35.11 | - | - | - | - |
| Countries | Hosts | Tick Species | Molecular Technique | Sample Size | Counts of Detected Pathogens in Ticks | Pathogens Detected (No. of Positives, Prevalence (%) | Reference |
|---|---|---|---|---|---|---|---|
| Angola | Cattle | A. variegatum, R. decoloratus | PCR | 116 | 6 | R. africae (n = 5; 4.31%), T. mutans (n = 1; 0.86%) | [30] |
| Angola | Cattle, goats, sheep | R. compositus | PCR, RLB | 2963 | 43 | E. ruminatium (n = 43; 1.45%) | [32] |
| Comoros | Cattle, Goats | A. variegatum, R. appendiculatus, R.(B). microplus | PCR | 512 | 94 | R. africae (n = 94; 18.36%) | [76] |
| Madagascar | Cattle, Goats | H. simplex, R. microplus | PCR | 235 | 60 | R. africae (n = 60; 26.67%) | [77] |
| Madagascar | Cattle | A. variegatum, R. microplus | PCR | 499 | 312 | A. marginale (n = 311; 62.32%), A. ovis (n = 1; 0.15%) | [78] |
| Mozambique | Cattle | A. variegatum, R. microplus | PCR | 646 | 5 | R. africae (n = 4; 0.62%) T. velifera (n = 1; 0.15%) | [79] |
| South Africa | Cattle, goats, sheep | R. appendiculatus, R. decoloratus, R. e. evertsi | PCR | 1200 | 26 | E. ruminatium (n = 19; 1.58%), A. bovis (n = 1; 0.25%), A. marginale (n = 2; 0.15%), A. ovis (n = 3; 0.33%), B. caballi (n = 1; 0.25%) | [80] |
| South Africa | Cattle, sheep | A. hebraeum, H.m. rufipes, R. appendiculatus, R. (B.) decoloratus, R. e. evertsi | PCR | 7364 | 58 | B. bigemina (n = 4; 0.31%), Babesia spp. (n = 1; 0.38%), E. ruminatium (n = 5; 2.15%), E. ovina (n = 2; 0.17%), Ehrlichia spp. (n = 8; 0.61%), T. bicornis (n = 7; 0.75%), T. buffeli (n = 7; 0.45%), T. mutans (n = 2; 0.18%), T. ovis (n = 2; 0.22%), T. separata (n = 4; 0.44%), T. taurotragi (n = 3; 0.32%), Theileria spp. (n = 13; 0.71%) | [81] |
| South Africa | Cattle, sheep | A. hebraeum, R. appendiculatus, R. decoloratus, R. e. evertsi | PCR | 130 | 24 | A. marginale (n = 5; 3.85%), E. ruminatium (n = 2; 1.54%), Rickettsia spp. (n = 10; 7.69%), T. mutans (n = 4; 3.08%), T. taurotragi (n = 3; 2.31%) | [82] |
| South Africa | Cattle, goats, sheep | A. hebraeum, R. appendiculatus, R. decoloratus, R. e. evertsi, R. sanguineus | PCR | 760 | 16 | Ehrlichia spp. (n = 16; 2.10%) | [83] |
| South Africa | Cattle, goats, sheep | A. hebraeum, H. truncatum, R. appendiculatus, R. e. evertsi, R. microplus, R. simus | PCR | 903 | 60 | Rickettsia spp. (n = 60; 6.64%) | [84] |
| South Africa | Cattle | R. sanguineus | PCR | 100 | 10 | A. phagocytophilum (n = 10; 10%) | [85] |
| South Africa | Cattle, goats, sheep | A. hebraeum | PCR | 1403 | 344 | E. ruminatium (n = 344; 24.52%) | [57] |
| South Africa | Goats | A. hebraeum | PCR | 630 | 47 | E. ruminatium (n = 19; 3.02%) R. africae (n = 28; 4.44%) | [86] |
| Tanzania | Cattle, Goats | - | PCR | 819 | 0 | - | [87] |
| Tanzania | Cattle | A. gemma, R. appendiculatus, R. praetextatus, R. pulchellus | PCR | 527 | 28 | A. marginale (n = 28; 5.31%) | [88] |
| Tanzania | Cattle | A. gemma, A. lepidum, A. marmoreum, A. variegatum, H. impeltatum, R. pulchellus | PCR | 263 | 160 | Babesia spp. (n = 7; 2.66%), Ehrlichia spp. (n = 6; 2.28%), Rickettsia spp. (n = 133; 50.57%), Theileria spp. (n = 14; 5.32%) | [89] |
| Zambia | Cattle | A. variegatum | RLB | 5288 | 1 | E. ruminatium (n = 1; 0.02%) | [73] |
| Zambia | Cattle | R. appendiculatus | PCR | 74 | 10 | T. parva (n = 10; 13.51%) | [74] |
| Zimbabwe | Cattle | H. truncatum, R. e. evertsi | PCR | 1141 | 288 | E. ruminatium (n = 288; 25.24%) | [90] |
| Zimbabwe | Cattle | R. appendiculatus | PCR | 36 | 18 | B. bigemina (n = 12; 33.33%), B. bovis (n = 6; 16.67%) | [75] |
| Risk Factors | Number of Studies | Pooled Prevalence Estimates | Measure of Heterogeneity | Q-p | Publication Bias | |||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Number of Positive | Prevalence 95% CI (%) | Q | I2 | Begg and Mazumdar Rank p-Value | |||
| Overall ticks | 20 | 18355 | 1601 | 7.7 (4.0–14.3) | 2310.69 | 99.18 | 0.000 | 0.060 |
| Genus Anaplasma | ||||||||
| A. marginale | 4 | 2428 | 348 | 6.8 (0.6–45.2) | 333.05 | 99.10 | 0.034 | 0.248 |
| Genus Ehrlichia | ||||||||
| E. ruminantium | 8 | 3719 | 701 | 4.6 (2.2–9.1) | 347.46 | 97.98 | 0.000 | 0.161 |
| Ehrlichia spp. | 3 | 1543 | 31 | 2.1 (1.4–3.3) | 3.02 | 33.72 | 0.000 | 0.301 |
| Genus Rickettsia | ||||||||
| R. africae | 5 | 978 | 185 | 18.0 (7.4–37.5) | 104.23 | 96.16 | 0.003 | 0.164 |
| Rickettsia spp. | 3 | 859 | 203 | 39.0 (4.0–90.8) | 136.03 | 98.53 | 0.749 | 0.059 |
| Genus Theileria | ||||||||
| T. mutans | 3 | 1193 | 7 | 2.6 (0.2–31.2) | 23.58 | 91.52 | 0.012 | 0.301 |
| Risk Factors | Number of Studies | Pooled Prevalence Estimates | Measure of Heterogeneity | Q-p | Publication Bias | |||
|---|---|---|---|---|---|---|---|---|
| Sample Size | No. of Positive | Prevalence95% CI (%) | Q | I2 | Begg and Mazumdar Rank p-Value | |||
| Genus Amblyomma | 15 | 3987 | 959 | 25.0 (14.7–39.1) | 598.25 | 97.66 | 0.001 | 0.200 |
| A. chabaudi | 1 | 2 | 2 | 100.00 | - | - | - | - |
| A. gemma | 2 | 79 | 20 | 25.32 | ||||
| A. hebraeum | 7 | 2344 | 456 | 14.2 (8.9–21.8) | 64.23 | 90.66 | 0.000 | 0.440 |
| A. lepidum | 1 | 42 | 8 | 19.05 | - | - | - | - |
| A. marmoreum | 1 | 11 | 2 | 18.18 | - | - | - | - |
| A. pomposum | 1 | 617 | 43 | 6.97 | - | - | - | - |
| A. variegatum | 7 | 3713 | 431 | 43.9 (10.1–84.4) | 250.42 | 97.60 | 0.804 | 0.440 |
| Genus Haemaphysalis | 1 | 19 | 2 | 10.53 | - | - | - | - |
| H. simplex | 1 | 19 | 2 | 10.53 | - | - | - | - |
| Genus Hyalomma | 2 | 909 | 119 | 13.1 | - | - | - | - |
| H.m. rufipes | 2 | 582 | 89 | 15.29 | - | - | - | - |
| H. truncatum | 1 | 327 | 20 | 6.12 | - | - | - | - |
| Genus Rhipicephalus | 14 | 8730 | 522 | 8.0 (3.2–18.6) | 841.80 | 98.46 | 0.000 | 0.162 |
| R. appendiculatus | 9 | 899 | 40 | 3.7 (1.6–8.3) | 46.25 | 82.70 | 0.000 | 0.266 |
| R. (B.) decoloratus | 3 | 424 | 36 | 36.9 (3.1–91.5) | 63.91 | 96.87 | 0.000 | 0.30 |
| R. compositus | 2 | 181 | 7 | 3.87 | - | - | - | - |
| R. decoloratus | 2 | 42 | 12 | 28.57 | - | - | - | - |
| R.(B). microplus | 2 | 312 | 14 | 4.49 | - | - | - | - |
| R. e. evertsi | 5 | 1718 | 234 | 7.4 (1.1–35.8) | 317.76 | 98.74 | 0.011 | 0.312 |
| R. microplus | 3 | 693 | 174 | 15.4 (1.1–75.5) | 173.07 | 98.84 | 0.238 | 0.301 |
| R. praetextatus | 1 | 23 | 2 | 8.70 | - | - | - | - |
| R. pulchellus | 1 | 22 | 6 | 27.27 | - | - | - | - |
| R. sanguineus | 2 | 280 | 15 | 5.36 | - | - | - | - |
| S/No. | Source | Query/Search String | Results |
|---|---|---|---|
| 1 | PubMed | Ticks and tick-borne pathogens in Southern Africa; Prevalence of “Anaplasma” “Babesia” “Ehrlichia” and/or “Theileria” | 56 |
| 2 | Science direct | Ticks and tick-borne pathogens in Southern Africa; Prevalence of “Anaplasma” “Babesia” “Ehrlichia” and/or “Theileria” | 751 |
| 3 | Google scholar | Ticks and tick-borne pathogens in Southern Africa; Prevalence of “Anaplasma” “Babesia” “Ehrlichia” and/or “Theileria” | 31,700 |
| 4 | AJOL | Ticks and tick-borne pathogens in Southern Africa; Prevalence of “Anaplasma” “Babesia” “Ehrlichia” and/or “Theileria” | 244 |
| 5 | Springer Link | Ticks and tick-borne pathogens in Southern Africa; Prevalence of “Anaplasma” “Babesia” “Ehrlichia” and/or “Theileria” | 743 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawana, M.; Onyiche, T.E.; Ramatla, T.; Mtshali, S.; Thekisoe, O. Epidemiology of Ticks and Tick-Borne Pathogens in Domestic Ruminants across Southern African Development Community (SADC) Region from 1980 until 2021: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 929. https://doi.org/10.3390/pathogens11080929
Tawana M, Onyiche TE, Ramatla T, Mtshali S, Thekisoe O. Epidemiology of Ticks and Tick-Borne Pathogens in Domestic Ruminants across Southern African Development Community (SADC) Region from 1980 until 2021: A Systematic Review and Meta-Analysis. Pathogens. 2022; 11(8):929. https://doi.org/10.3390/pathogens11080929
Chicago/Turabian StyleTawana, Mpho, ThankGod E. Onyiche, Tsepo Ramatla, Sibusiso Mtshali, and Oriel Thekisoe. 2022. "Epidemiology of Ticks and Tick-Borne Pathogens in Domestic Ruminants across Southern African Development Community (SADC) Region from 1980 until 2021: A Systematic Review and Meta-Analysis" Pathogens 11, no. 8: 929. https://doi.org/10.3390/pathogens11080929
APA StyleTawana, M., Onyiche, T. E., Ramatla, T., Mtshali, S., & Thekisoe, O. (2022). Epidemiology of Ticks and Tick-Borne Pathogens in Domestic Ruminants across Southern African Development Community (SADC) Region from 1980 until 2021: A Systematic Review and Meta-Analysis. Pathogens, 11(8), 929. https://doi.org/10.3390/pathogens11080929







