The Current State of Knowledge on Baggio–Yoshinari Syndrome (Brazilian Lyme Disease-like Illness): Chronological Presentation of Historical and Scientific Events Observed over the Last 30 Years
Abstract
1. Introduction
2. Historical Background
2.1. The Beginning
2.2. The Research Schedule in Brazil
2.3. The Invitation from the Brazilian Ministry of Health
2.4. The First Lyme Disease Cases in Brazil
2.5. First Fieldwork Survey Conducted in Cotia County, São Paulo State
2.6. Second Fieldwork in Search of Borrelia sp. in Ticks from an Urban Forest Reserve in the State of Mato Grosso do Sul, Brazil
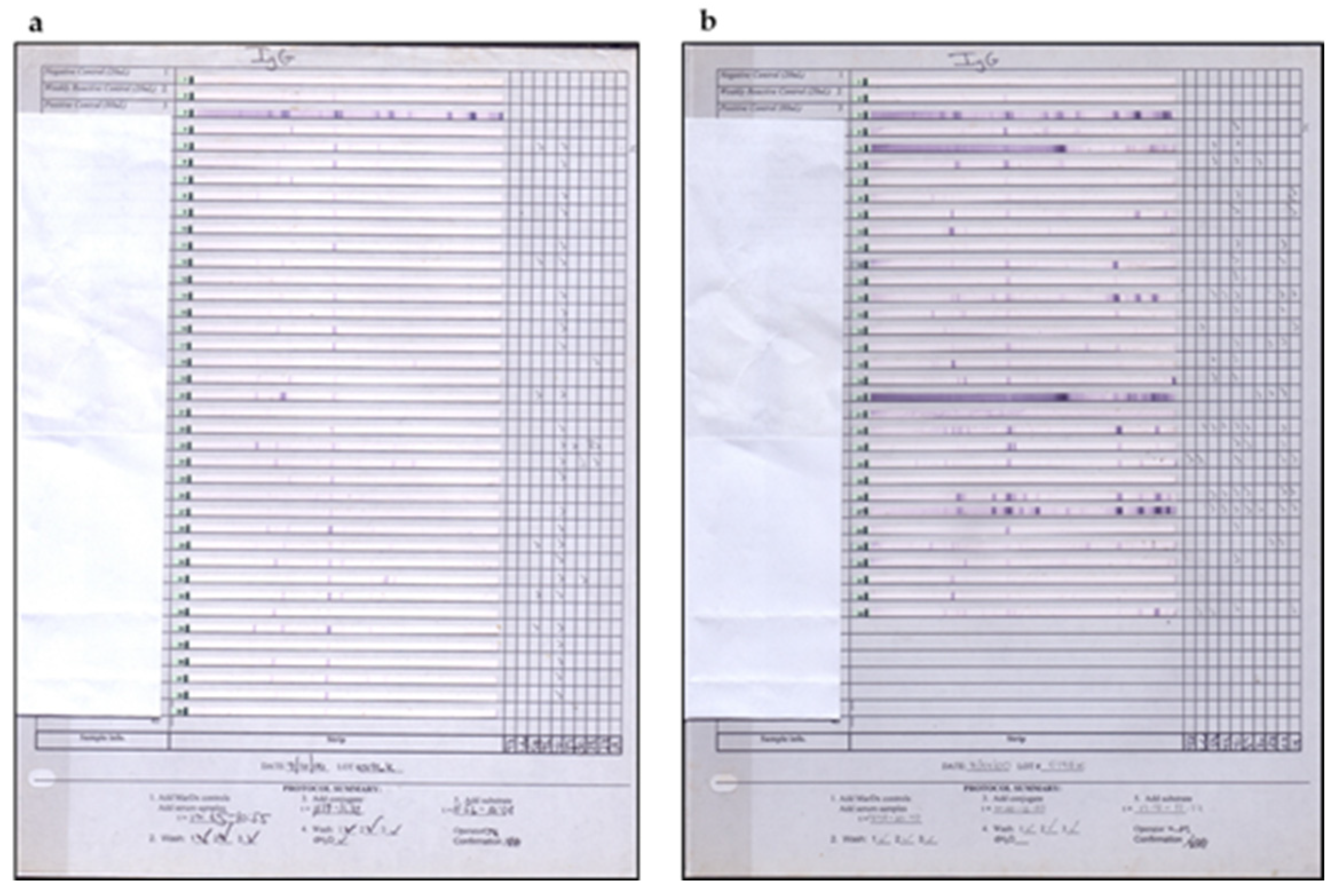
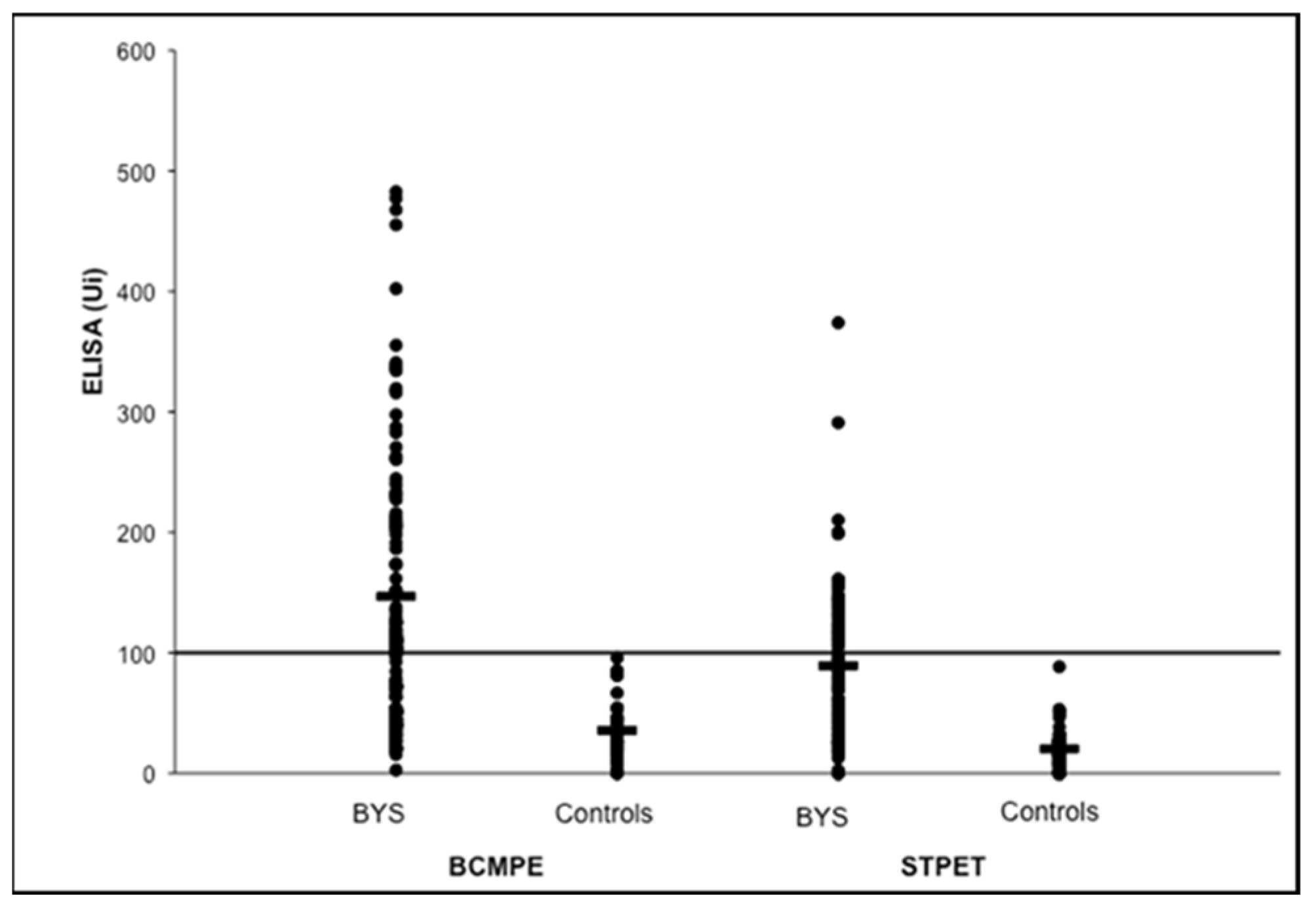
2.7. Brazilian Lyme Disease-like Patients Display Low Immune Response to B. burgdorferi
3. Reasons Why the Brazilian Lyme Disease-like Zoonosis Was Named Baggio–Yoshinari Syndrome
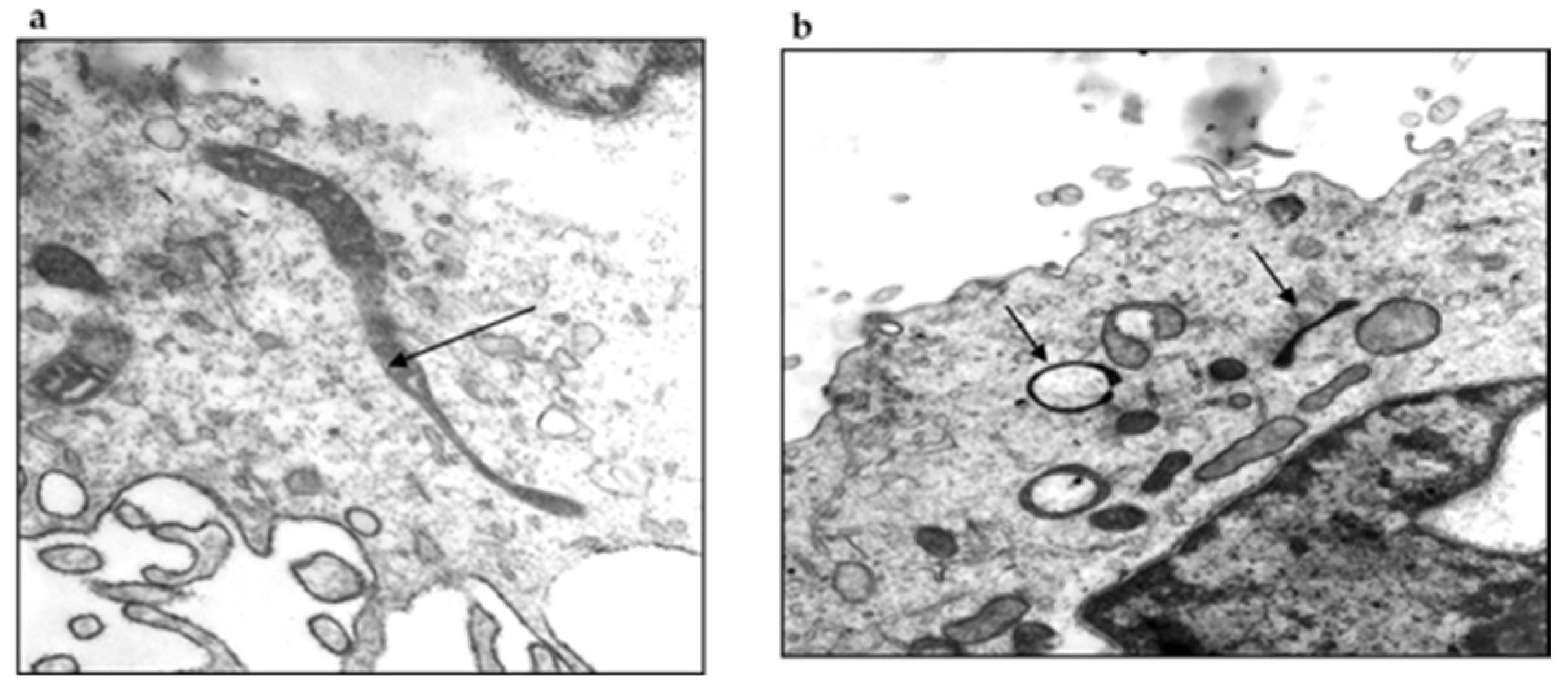
4. Searching for the Etiological Agent of Baggio–Yoshinari Syndrome
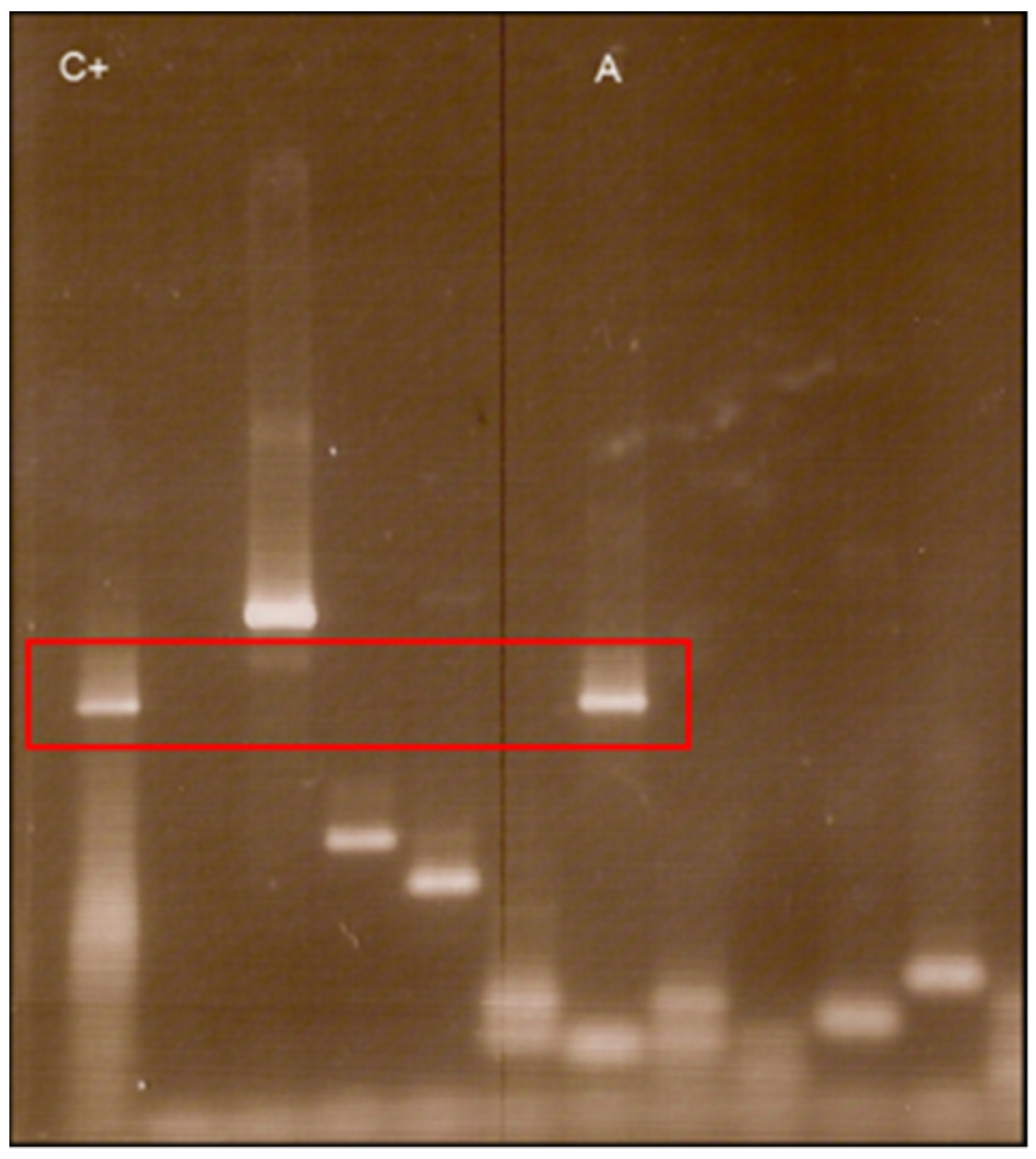
4.1. Molecular Biology Researches
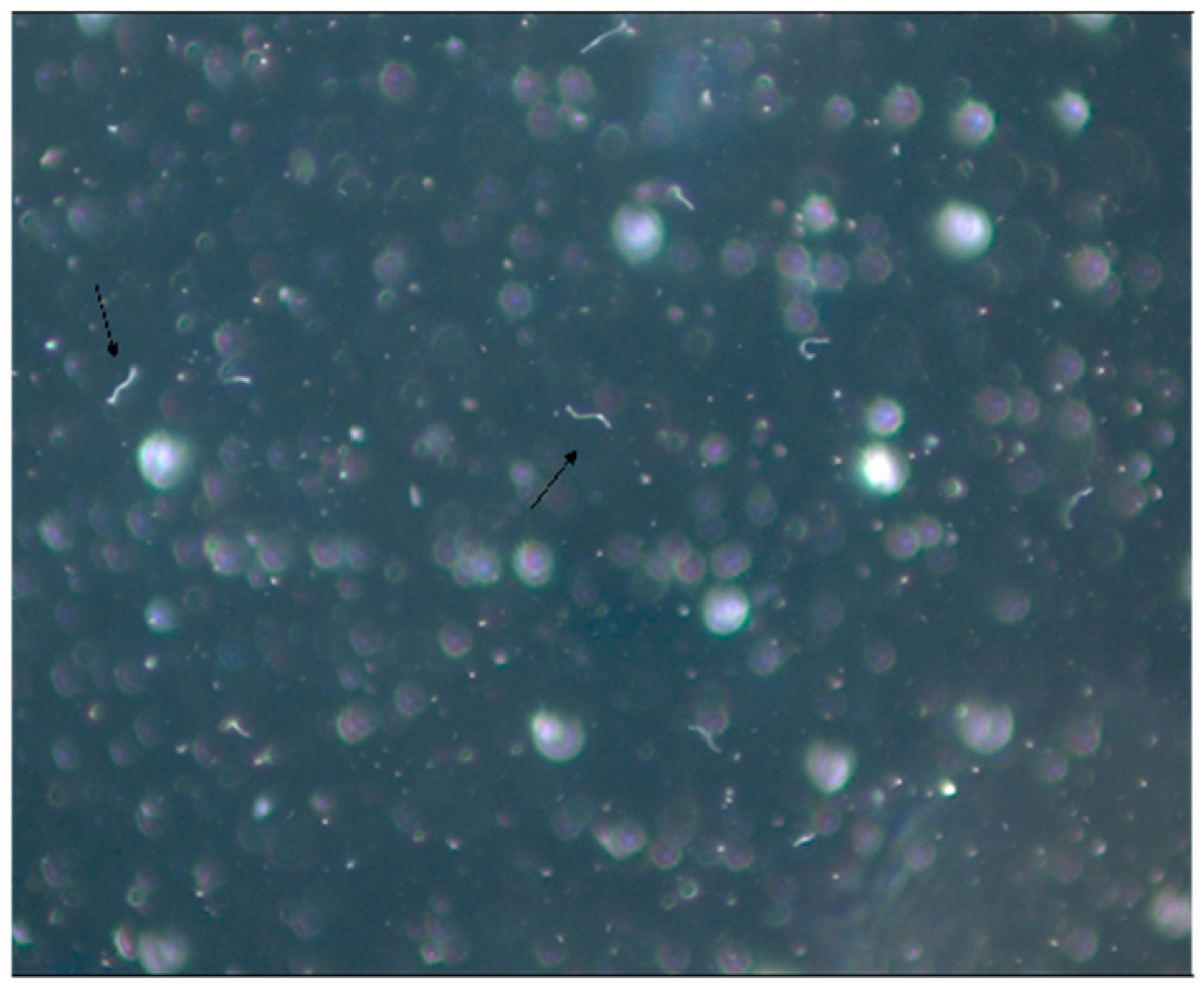
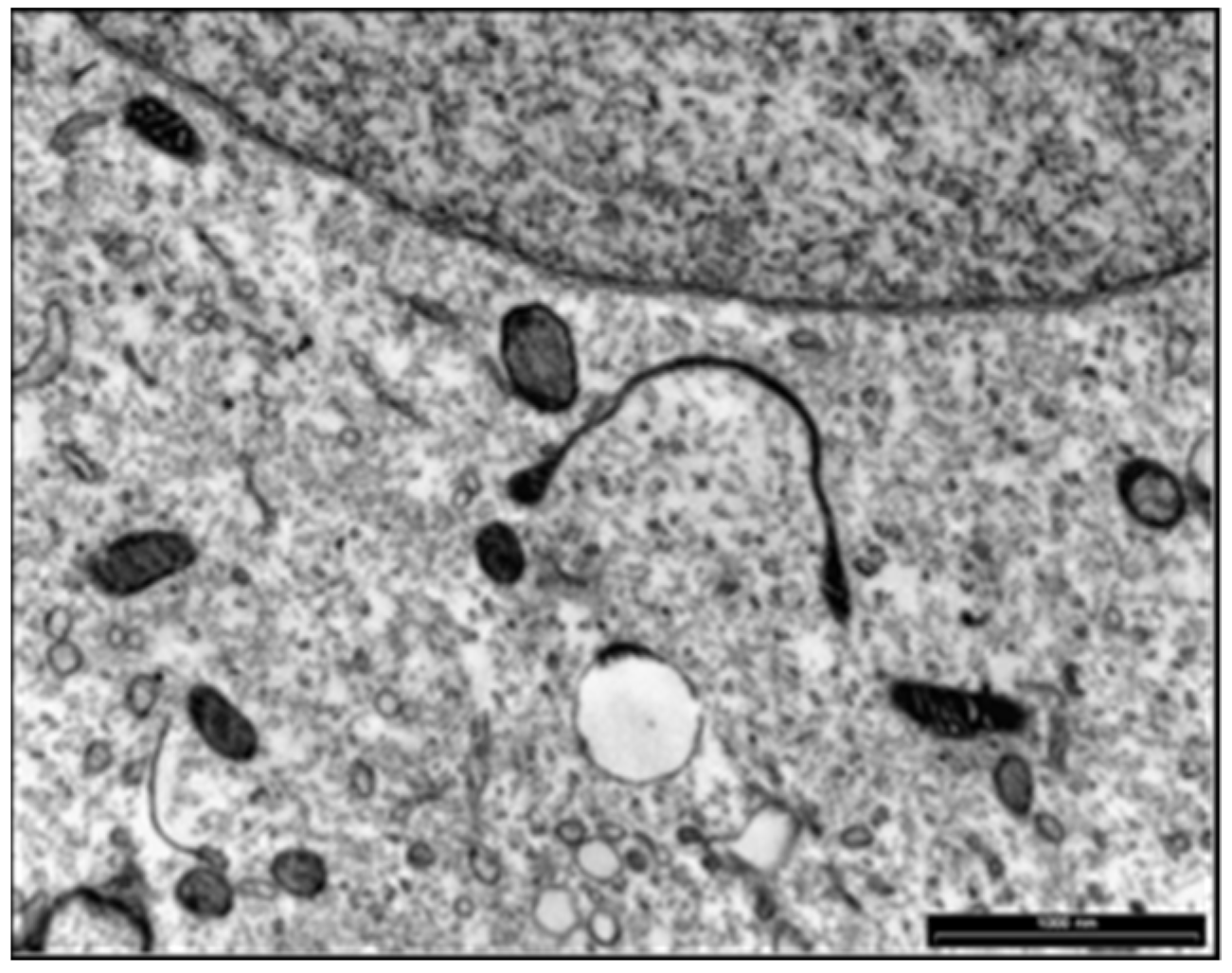
4.2. Microbiological Research
5. Differences in B. burgdorferi Antigens Expression in BYS and LD Patients
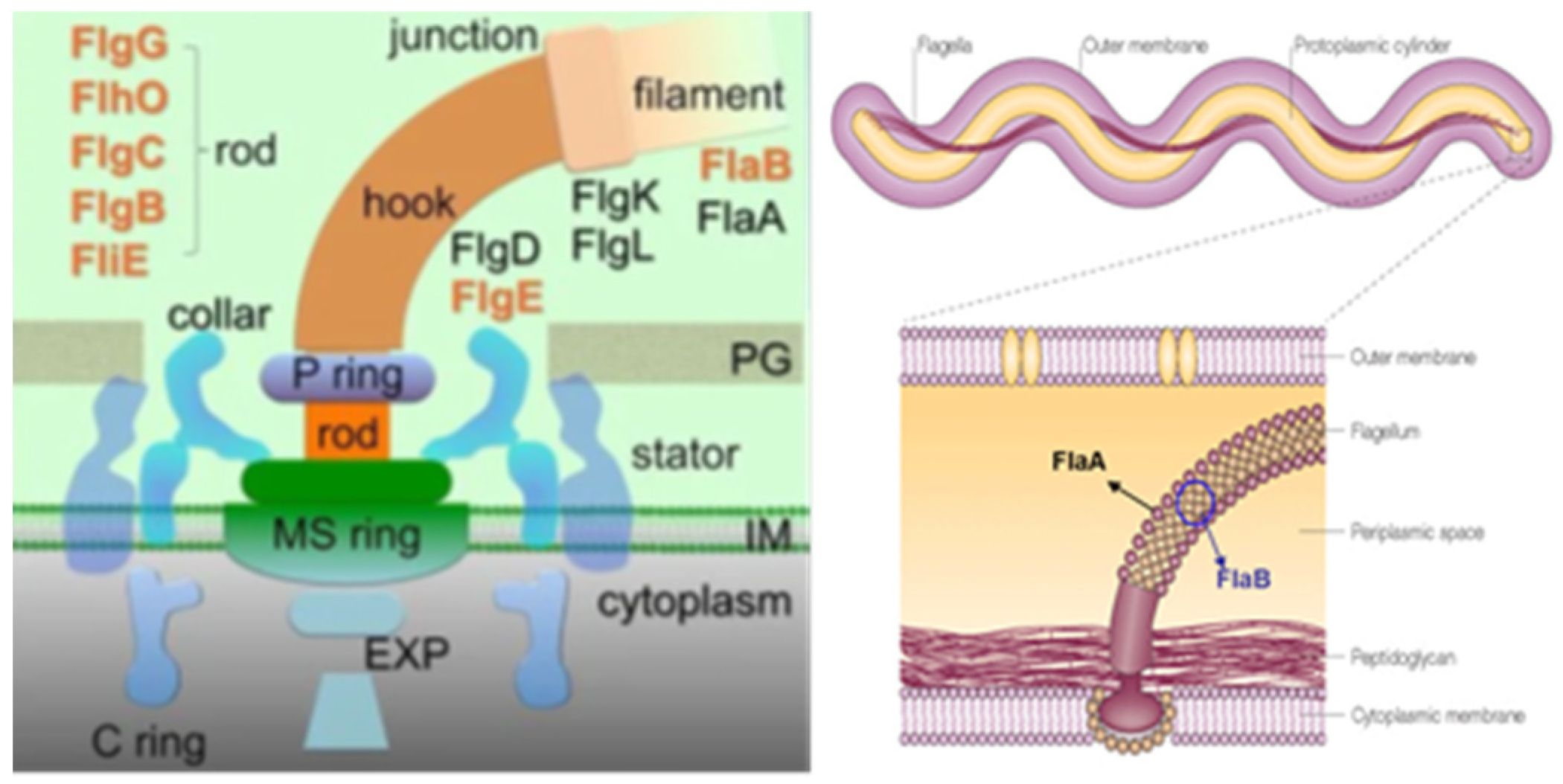
6. Borreliae Lyme Group
- ○
- Classical Organotropic form, with EM and transmitted by Ixodes sp.;
- ○
- Spirochaetemica Group (Borrelia mayonii) with EM transmitted by Ixodes sp.;
- ○
- Baggio–Yoshinari forms with EM, systemic manifestations, autoimmunity and chronic fatigue symptoms, with low immune response to B. burgdorferi and transmitted by ticks of the genera Amblyomma, Rhipicephalus (R. sanguineus, R. bovis) and Dermacentor.
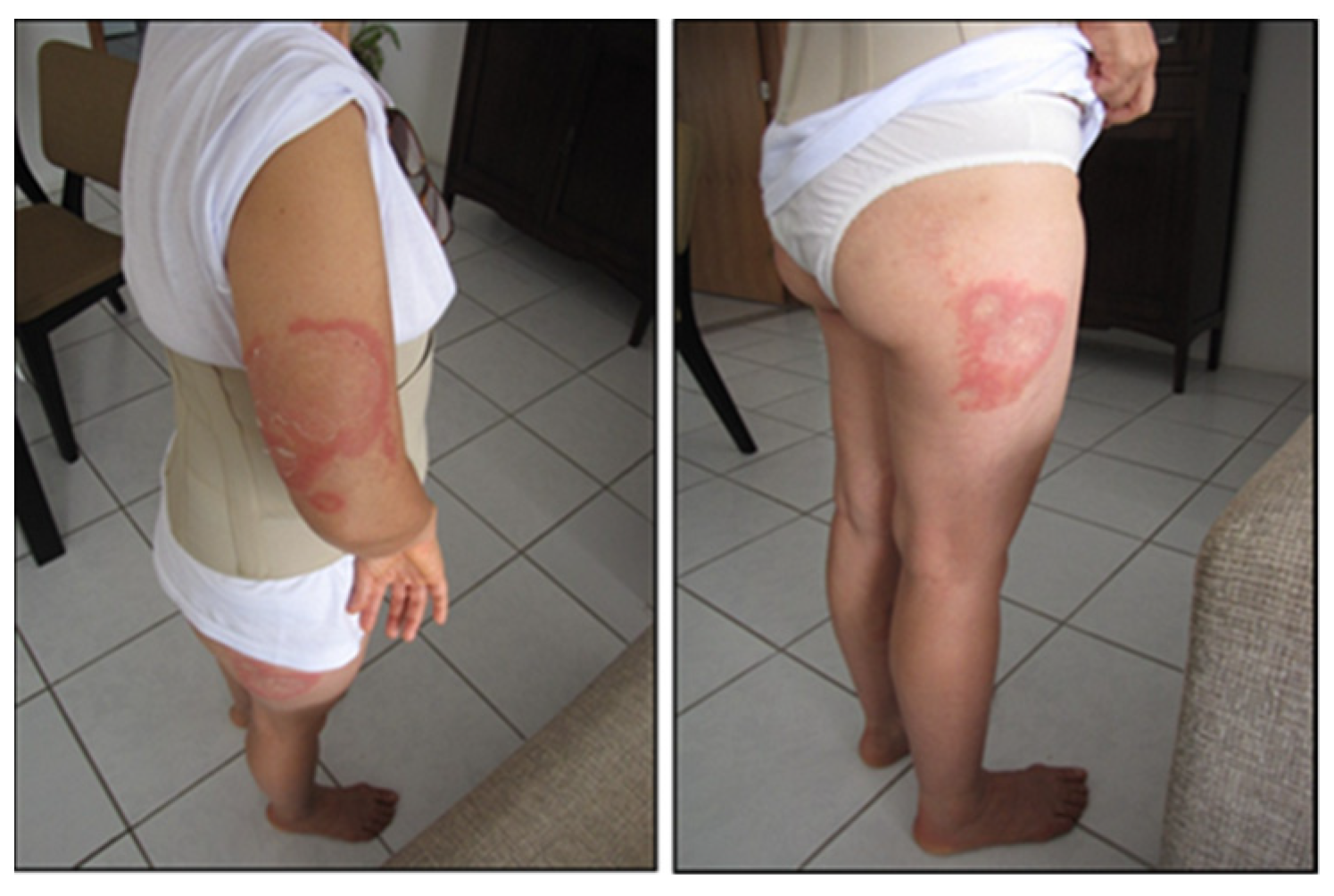
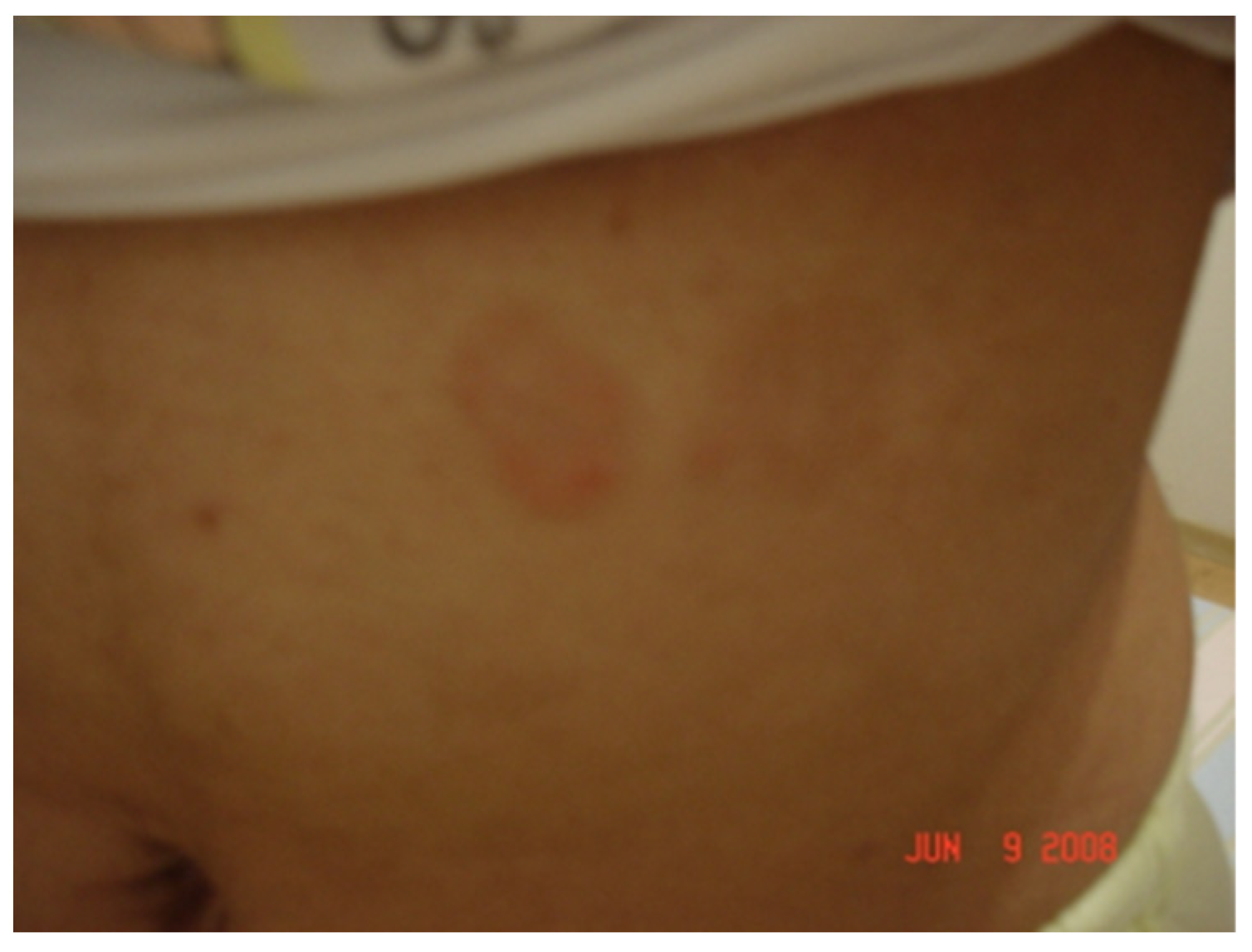
7. Clinical Involvement of Baggio–Yoshinari Syndrome
7.1. Clinical and Progression of BYS
- Localized acute infection;
- Early disseminated infection;
- Latent infection;
- Reactive disease expression resembling CFS/ME;
- Reactive disease expression resembling autoimmune or allergic diseases;
- Unclassified disease expression.
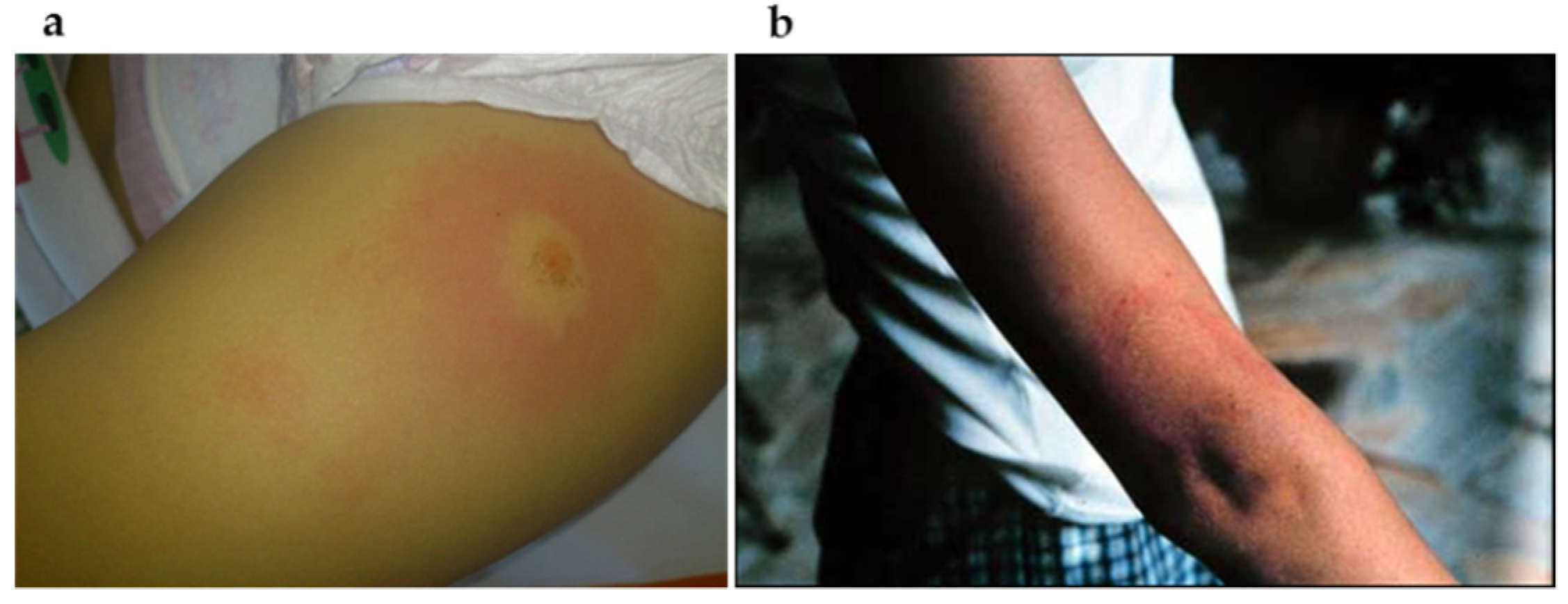
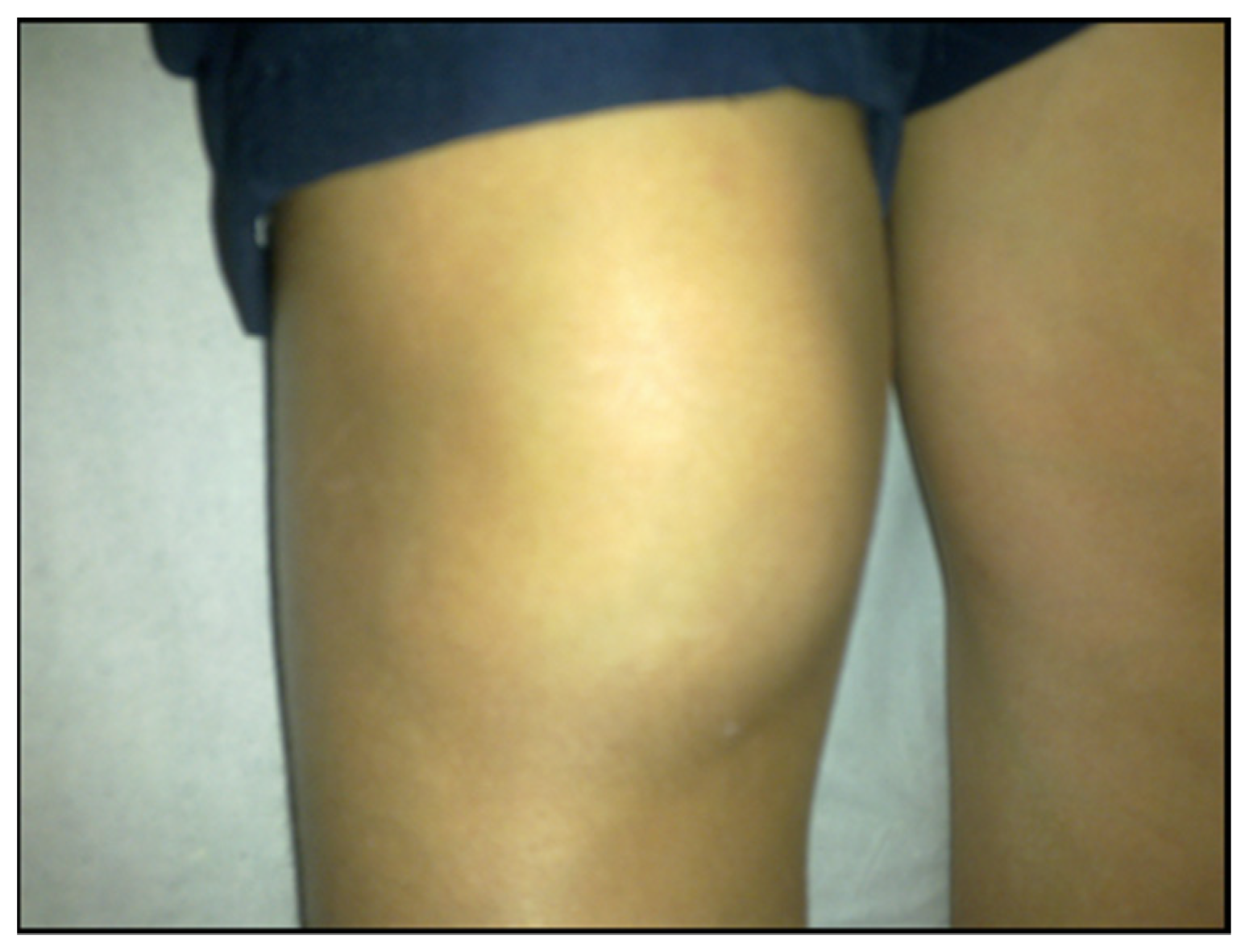
7.1.1. Localized Acute Infection
7.1.2. Early Disseminated Infection
7.1.3. Latent Infection
7.1.4. Reactive Disease Expression Resembling CFS/ME
- Severe fatigue that persists for longer than 6 months;
- Post-exertional malaise;
- Unrefreshing sleep.
- Cognitive impairment;
- Orthostatic intolerance which can be induced by stress, infections, endocrine disorders, sleep problems and medications.
7.1.5. Reactive Disease Expression Resembling Autoimmune or Allergic Diseases
7.1.6. Unclassified Disease Expression
8. Treatment of Baggio–Yoshinari Syndrome
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshinari, N.H. A long journey to understand Borrelia burgdorferi in Brazil. Rev. Bras. Reumatol. 2009, 49, 483–486. [Google Scholar] [CrossRef][Green Version]
- Yoshinari, N.H.; Steere, A.C.; Cossermelli, W. A review of Lyme disease. AMB Rev. Assoc. Med. Bras. 1989, 35, 34–38. [Google Scholar] [PubMed]
- Yoshinari, N.H.; Barros, P.J.L.; Cruz, F.C.M. Clinica e sorologia da doença de Lyme no Brasil. Rev. Bras. Reumatol. 1992, 32, 57–61. [Google Scholar]
- Barros-Battesti, D.M. Estudos de Carrapatos e Pequenos Mamíferos Silvestres Naturalmente Infectados Com Espiroquetas Semelhantes à Borrelia no Município de Itapevi. Ph.D. Thesis, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, Brazil, 1998; 142p. [Google Scholar]
- Barros-Battesti, D.M.; Martins, R.; Bertim, C.R.; Yoshinari, N.H.; Bonoldi, V.L.; Leon, E.P.; Miretzki, M.; Schumaker, T.T. Land fauna composition of small mammals of a fragment of Atlantic Forest in the State of São Paulo, Brazil. Rev. Bras. Zool. 2000, 17, 241–249. [Google Scholar] [CrossRef][Green Version]
- Barros-Battesti, D.M.; Yoshinari, N.H.; Bonoldi, V.L.N.; de Castro Gomes, A. Parasitism by Ixodes didelphidis and I. loricatus (Acari: Ixodidae) on small wild mammals from an Atlatic Forest in the State of Sao Paulo, Brazil. J. Med. Entomol. 2000, 37, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Abel, I.; Marzagão, G.; Yoshinari, N.; Schumaker, T. Borrelia-like spirochetes recovered from ticks and small mammals collected in the Atlantic Forest Reserve, Cotia county, State of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 2000, 95, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.O. Principios, padronização e validação das propostas sorológicas—Técnicas imunoenzimáticas. In Imunodiagnóstico em Medicina Veterinária; Madruga, C.R., Araújo, F.R., Soares, C.O., Eds.; Embrapa Gado de Corte: Campo Grande, Brazil, 2001; pp. 145–175. [Google Scholar]
- Yoshinari, N.H.; Abrão, M.G.; Bonoldi, V.L.N.; Soares, C.O.; Madruga, C.R.; Scofield, A.; Massard, C.L.; Da Fonseca, A.H. Coexistence of antibodies to tick-borne agents of babesiosis and Lyme borreliosis in patients from Cotia county, State of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 2003, 98, 311–318. [Google Scholar] [CrossRef]
- Naka, E.N.; da Costa, I.P.; Arão, C.A.B.; Soares, C.O.; Yoshinari, N.H. Detection of anti- Borrelia and anti-Babesia antibodies in the serum of children with clinical manifestations and compatible epidemiology with Lyme-like disease in the State of Mato Grosso do Sul. Rev. Bras. Reumatol. 2008, 48, 74–85. [Google Scholar] [CrossRef][Green Version]
- Costa, I.P.; Yoshinari, N.H.; Barros, P.J.; Bonoldi, V.L.; Leon, E.P.; Zeitune, A.D.; Cossermelli, W. Lyme disease in Mato Grosso do Sul State, Brazil: Report of three clinical cases, including the first of Lyme meningitis in Brazil. Rev. Hosp. Clin. 1996, 51, 253–257. [Google Scholar]
- Costa, I.P.; Bonoldi, V.L.N.; Yoshinari, N.H. Perfil clínico e laboratorial da Doença de Lyme símile no Estado de Mato Grosso do Sul: Análise de 16 pacientes. Rev. Bras. Reumatol. 2001, 41, 142–150. [Google Scholar]
- Costa, I.P.; Bonoldi, V.L.N.; Yoshinari, N.H. Search for Borrelia sp. in ticks collected from potential reservoirs in an urban forest reserve in the State of Mato Grosso do Sul, Brazil: A short report. Mem. Inst. Oswaldo Cruz 2002, 97, 631–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbour, A.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996, 173, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.P. Pesquisa de Anticorpos Anti-Borrelia e do Agente etiológico Em Soro e Liquor de Pacientes Com Manifestações Clínicas Compatíveis Com a Doença de Lyme, no Estado de Mato Grosso do Sul. Ph.D. Thesis, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil, 1998. [Google Scholar]
- Costa, I.P.; Yoshinari, N.H. Doença de Lyme símile brasileira (Síndrome de Baggio-Yoshinari). In Livro da Sociedade Brasileira de Reumatologia; Vasconcelos, J.T.S., Marques Neto, A.J.F., Eds.; Samuel Katsuki Shinjo: Sebastião Cezar Radominski, Manole, 2019; pp. 438–452. [Google Scholar]
- Dressler, F.; Yoshinari, N.H.; Steere, A.C. The T-cell prolierative assay in the diagnosis of Lyme disease. Ann. Intern. Med. 1991, 115, 533–539. [Google Scholar] [CrossRef]
- Yoshinari, N.H.; Barros, P.J.L.; Yasuda, P.H.; Baggio, D.; Steere, A.C.; Pagliarine, R.C. Estudo epidemiológico da doença de Lyme no Brasil. Rev. Hosp. Clin. Fac Med. Univ. Sao Paulo 1992, 47, 71–75. [Google Scholar]
- Barros, P.J.; Bonoldi, V.; Yoshinari, N.H. Características clínicas da Doença de Lyme no Brasil. Rev. Bras. Reumatol. 1996, 36, 67–74. [Google Scholar]
- Steere, A.C. Lyme disease. N. Engl. J. Med. 2001, 345, 115–125. [Google Scholar] [CrossRef]
- Dressler, F.; Whalen, J.A.; Reinhardt, B.N.; Steere, A.C. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 1993, 167, 392–400. [Google Scholar] [CrossRef]
- Mandell, H.; Steere, A.C.; Reinhardt, B.N.; Yoshinari, N.; Munsat, T.L.; A Brod, S.; A Clapshaw, P. Lack of antibodies to Borrelia burgdorferi in patients with amyotrophic lateral sclerosis. N. Engl. J. Med. 1989, 320, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.H.; De Barros, P.J.; Bonoldi, V.L.; Ishikawa, M.; Battesti, D.M.; Pirana, S.; Da Fonseca, A.H.; Schumaker, T.T. Outline of Lyme borreliosis in Brazil. Rev. Hosp. Clin. Fac. Med. Sao Paulo 1997, 52, 111–117. [Google Scholar] [PubMed]
- Pirana, S.; Yoshinari, N.H.; Silveira, A.M.; Bento, R.F.; Bonoldi, V.L.N. Serum reactivity for Borrelia burgdorferi, Borrelia afzellii and Borrelia garinii antigens in patients with peripheral facial paralysis in Brazil. Braz. J. Rheumatol. 2000, 40, 55–59. [Google Scholar]
- Barros, P.J.L. Clinical and Laboratory Characterization of Lyme Disease in Brazil, Using Immunological Methods and Polymerase Chain Reaction. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2000. [Google Scholar]
- Mantovani, E.; Costa, I.; Gauditano, G.; Bonoldi, V.; Higuchi, M.; Yoshinari, N. Lyme disease-like syndrome in Brazil. Is it a new tick borne disease or Lyme disease variation? Braz. J. Med. Biol. Res. 2007, 40, 443–456. [Google Scholar] [CrossRef]
- Mantovani, E.; Gauditano, G.; Bonoldi, V.L.N.; Yoshinari, N.H. Análise clínica e sorológica de pacientes com Síndrome Infecto-Reacional Lyme-Símile. Rev. Paul Reumatol. 2007, 6, 29. [Google Scholar]
- Yoshinari, N.H.; Bonoldi, V.L.N.; Battesti, D.M.B.; Schumaker, T.S. Doença de Lyme-símile no Brasil. (editorial). Rev. Bras. Reumatol. 1999, 39, 57–58. [Google Scholar]
- Gauditano, G.; Bonoldi, V.L.N.; Costa, I.P.; Battesti, D.M.B.; Barros, P.J.L.; Fonseca, A.H.; Yoshinari, N.H. Síndrome de Lyme-símile ou complexo infecto-reacional do carrapato-Síndrome de Baggio-Yoshinari. Rev. Paul Reumatol. 2005, 4, 16–17. [Google Scholar]
- Masters, E.; Granter, S.; Duray, P.; Cordes, P. Physician-diagnosed erythema migrans-like rashes following Lone Star tick bites. Arch. Dermatol. 1998, 134, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.S.; Luttrell, M.P.; Howerth, E.W.; Moore, V.A.; Davidson, W.R.; Stallknecht, D.E.; Little, S.E. First culture isolation of Borrelia lonestari, putative agente of Southern tick-associated rash illness. J. Clin. Microbiol. 2004, 42, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Liebling, M.R.; Nishio, M.J.; Rodriguez, A.; Sigal, L.H.; Jin, T.; Louie, J.S. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1993, 36, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Tashibu, H.; Yamada, K.; Masuzawa, T.; Yanagihara, Y. Polymerase chain reaction analysis of Borrelia species in Japan. Microbiol. Immunol. 1994, 38, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, E. Identificação do Agente Etiológico da Doença de Lyme-Símile Brasileira (Síndrome de Baggio-Yoshinari). Ph.D. Thesis, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Stromdahl, E.Y.; Williamson, P.C.; Kollars, T.M.; Evans, S.R.; Barry, R.K.; Vince, M.A.; Dobbs, N.A. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J. Clin. Microbiol. 2003, 41, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.M.; Armstrong, P.M.; Smith, R.D.; Telford, S.R., 3rd. Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J. Clin. Microbiol. 2001, 39, 494–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iyer, R.; Kalu, O.; Purser, J.; Norris, S.; Stevenson, B.; Schwartz, I. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 2003, 71, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Motaleb; Liu, J.; Hu, B.; Caimano, M.J.; Miller, M.R.; Charon, N.W. Initial characterization of the FlgE hook high molecular weight complex of Borrelia burgdorferi. PLoS ONE 2014, 9, e98338. [Google Scholar] [CrossRef]
- Mantovani, E.; Marangoni, R.G.; Gauditano, G.; Bonoldi, V.L.; Yoshinari, N.H. Amplification of the flgE gene provides evidence for the existence of a Brazilian borreliosis. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.A.; De Rezende, J.; Silva, D.B.D.S.; Alves, F.D.C.G.; De Oliveira, C.E.; Da Costa, I.P. Molecular evidence of Borrelia burgdorferi sensu lato in patients in Brazilian central-western region. Rev. Bras. Reumatol. 2017, 57, 641–645. [Google Scholar] [CrossRef]
- Sal, M.S.; Li, C.; Motalab, M.A.; Shibata, S.; Aizawa, S.-I.; Charon, N.W. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook basal body structure. J. Bacteriol. 2008, 190, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.A.; Tilly, K.; Stewart, P.E. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 2005, 3, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, K.; Boquoi, T.; Hu, B.; Motaleb, M.A.; Miller, K.A.; James, M.E.; Charon, N.W.; Manson, M.D.; Norris, S.J.; et al. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2013, 110, 14390–14395. [Google Scholar] [CrossRef]
- Gonçalves, D.D.; Carreira, T.; Nunes, M.; Benitez, A.; Lopes-Mori, F.M.R.; Vidotto, O.; De Freitas, J.C.; Vieira, M.L. First record of Borrelia burgdorferi B31 strain in Dermacentor nitens ticks in northern region of Paraná (Brazil). Braz. J. Microb. 2013, 44, 883–887. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, D.D.; Moura, R.A.; Nunes, M.; Carreira, T.; Vidotto, O.; Freitas, J.C.; Vieira, M.L. Borrelia burgdorferi sensu lato in humans in a rural area of Paraná State, Brazil. Braz. J. Microbiol. 2015, 46, 571–575. [Google Scholar] [CrossRef]
- Talhari, S.; Santos, M.N.D.S.; Talhari, C.; Ferreira, L.C.D.L.; Silva, R.M.; Zelger, B.; Massone, C.; Ribeiro-Rodrigues, R. Borrelia burgdorferi ¨sensu lato¨ in Brazil: Occurrence confirmed by Immunohistochemistry and focus floating microscopy. Acta Trop. 2010, 115, 200–204. [Google Scholar] [CrossRef]
- Tully, J.G. Culture medium formulation for primary isolation and maintenance of Mollicutes. In Molecular and Diagnostic Procedures in Mycoplasmology; Joseph, T., Schmuel, R., Eds.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Messmer, T.O.; Skelton, S.K.; Moroney, J.F.; Daugharty, H.; Fields, B.S. Application of a Nested, Multiplex PCR to Psittacosis Outbreaks. J. Clin. Microbiol. 1997, 35, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Baltch, A.L.; Ritz, W.J.; Carpenter, A.N.; Halse, T.A.; Bopp, L.H. In Vitro Activities of Garenoxacin and Levofloxacin against Chlamydia pneumoniae are not affected by presence of Mycoplasma DNA. Antimicrob. Agents Chemother. 2004, 48, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia Burgd. Apmis 2004, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Onwuamaegbu, M.; Belcher, R.; Soare, C. Cell wall-deficient bactéria as cause of infections: A review of the clinical significance. J. Int. Med. Res. 2005, 33, 1–20. [Google Scholar] [CrossRef]
- Kersten, A.; Poitschek, C.; Rauch, S.; Aberer, E. Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob. Agents Chemother. 1995, 39, 1127–1133. [Google Scholar] [CrossRef][Green Version]
- Yoshinari, N.H.; Vasconcelos, A.S.; Tiriba, A.C.; Gauditano, G.; Mantovani, E.; Bonoldi, V.L.N. Report of unusual presence of latent microorganisms in animals: A risk to research and health of employees? Braz. J. Rheumatol. 2009, 49, 517–528. [Google Scholar]
- Meriläinen, L.; Schwarzbach, A.; Herranen, A.; Gilbert, L. Morphological and biochemichemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161 Pt 3, 516–527. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Kybicova, K.; Vancova, M. Metamorphoses of Lyme disease spirochetes: Phenomenon of Borrelia persisters. Parasit Vectors 2019, 12, 237. [Google Scholar] [CrossRef]
- Karvonen, K.; Nykky, J.; Marjomäki, V.; Gilbert, L. Distinctive Evasion Mechanisms to Allow Persistence of Borrrelia burgdorferi in Different Human Cell Lines. Front. Microbiol. 2021, 3004. [Google Scholar] [CrossRef]
- Palmer, G.H.; Bankhead, T.; Seifert, H.S. Antigenic Variation in Bacterial Pathogens. Microbiol. Spectr. 2016, 4, 1–4. [Google Scholar] [CrossRef]
- Chaconas, G.; Castellanos, M.; Verhey, T.B. Changing of the guard: How the Lyme disease spirochete subverts the host immune response. J. Biol. Chem. 2020, 295, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Barros, P.J.; Levy, L.H.; Monteiro, F.G.; Yoshinari, N.H. Lyme disease: Cutaneous involvement and treatment of the initial phases. Rev. Assoc. Med. Bras. 1993, 39, 170–172. [Google Scholar] [PubMed]
- Da Fonseca, A.H.; Salles, R.D.S.; Salles, S.D.A.N.; Madureira, R.C.; Yoshinari, N.H. Borreliose de Lyme-símile: Uma doença emergente e relevante para a Dermatologia no Brasil. An. Bras. Dermatol. 2005, 80, 171–178. [Google Scholar] [CrossRef]
- Kowacs, P.A.; Martins, R.T.; Piovesan, E.J.; Pinto, M.C.A.; Yoshinari, N.H. Chronic unremitting headache associated with Lyme disease-like illness. Arq. Neuro-Psiquiatr 2013, 71, 470–473. [Google Scholar] [CrossRef][Green Version]
- Lorenzi, M.C.; Bittar, R.S.M.; Pedalini, M.E.B.; Zerati, F.; Yoshinari, N.H.; Bento, R. Sudden deafness and Lyme disease. Laryngoscope 2003, 113, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Miziara, C.S.M.G.; Serrano, V.A.G.; Yoshinari, N. Passage of Borrelia burgdorferi through diverse Ixodid hard ticks causes distinct diseases: Lyme borreliosis and Baggio-Yoshinari syndrome. Clinics 2018, 73, e394. [Google Scholar] [CrossRef] [PubMed]
- Passos, S.; Gazeta, R.E.; Latorre, M.D.R.; Durigon, E.; Gauditano, G.; Yoshinari, N.H. Epidemiological characteristics of Lyme-like disease in children. Rev. Assoc. Med. Bras. 2009, 55, 139–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pirana, S.; Bento, R.F.; Bogar, P.; Silveira, J.A.M.; Yoshinari, N.H. Paralisia facial e surdez súbita bilateral na doença de Lyme. Rev. Bras. Otorrinol. 1992, 62, 500–502. [Google Scholar]
- Rosa Neto, N.S.; Gauditano, G.; Yoshinari, N.H. Chronic lymphomonocytic meningoencephalitis, oligoarthritis and erythema nodosum: Report of Baggio-Yoshinari syndrome of long and relapsing evolution. Rev. Bras. Reumatol. 2014, 54, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.T.; Schmitt, A.; Greboge, P.; Arana, J.; Moreira, A.T.R.; Yoshinari, N.H. Neurorretinite associada à ceratite intersticial: Relato do primeiro caso de doença de Lyme no Estado do Paraná. Rev. Bras. Oftalmol. 2003, 62, 275–283. [Google Scholar]
- Shinjo, S.K.; Gauditano, G.; Marchiori, P.E.; Bonoldi, V.L.N.; Costa, I.P.; Mantovani, E.; Yoshinari, N.H. Manifestação neurológica na síndrome de Baggio-Yoshinari (Síndrome brasileira semelhante à doença de Lyme). Rev. Bras. Reumatol. 2009, 49, 492–505. [Google Scholar] [CrossRef]
- Bonoldi, V.L.N. Estudo Laboratorial de Agentes Infecciosos Transmitidos Por Carrapatos Em Pacientes Com a Doença de Lyme-símile Brasileira (Síndrome de Baggio-Yoshinari). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2009; 114p. [Google Scholar]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The Chronic Fatigue Syndrome: A comprehensive Approach to its Definition and Study. Ann. Int. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Shapiro, E.D.; Soffer, G.K. A Review of Post-Treatment Lyme Disease Syndrome and Chronic Lyme Disease for the Practicing Immunologist. Clin. Rev. Allergy Immunol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2021, 19, 162. [Google Scholar] [CrossRef]
- Eskandari, F.; I Webster, J.; Sternberg, E.M. Neural imune pathway and their connection to inflammatory diseases. Arthritis Res. Ther. 2003, 5, 251–265. [Google Scholar] [CrossRef]
- Arvikar, S.L.; Crowley, J.T.; Sulka, K.B.; Steere, A.C. Autoimmune Arthritides, Rheumatoid Arthritis, Psoriatic Arthritis, or Peripheral Spondyloarthropathy, Following Lyme Disease. Arthritis Rheumatol. 2017, 69, 194–202. [Google Scholar] [CrossRef]
- Armangue, T.; Leypoldt, F.; Dalmau, J. Auto-immune encephalitis as differential diagnosis of infectious encephalitis. Curr. Opin. Neurol. 2014, 27, 361–368. [Google Scholar] [CrossRef]
- Dalmau, J.; Tüzün, E.; Wu, H.Y.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplasic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Jammoul, A.; Li, Y.; Era-Grant, A. Autoantibody-mediated encephalitis: Not just paraneoplastic, nor just limbic, and not untreatable. Clevel. Clin. J. Med. 2016, 83, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.H.; Oyafuso, L.K.; Monteiro, F.G.; de Barros, P.J.; da Cruz, F.C.; Ferreira, L.G.; Bonasser, F.; Baggio, D.; Cossermelli, W. Lyme disease. Report of a case observed in Brazil]. Rev. Hosp. Clin. Fac Med. Sao Paulo 1993, 48, 170–174. [Google Scholar] [PubMed]
- Yoshinari, N.H.; Barros, P.J.L.; Gauditano, G.; Fonseca, A.H. Report of 57 cases of Lyme-like disease (LLD) in Brazil. Arthritis Rheum. 1999, 43, S188. [Google Scholar]
- Yoshinari, N.H.; Gauditano, G.; Bonoldi, V.L.N.; Barros, P.J.L.; Novaes, G.S.; Simis, D.R.C.; Martins, D.D.; Poles, M.M. Lyme disease-like syndrome and skin manifestations. Rev. Bras. Reumatol. 1999, 39, 65–69. [Google Scholar]
- Yoshinari, N.; Spolidorio, M.; Bonoldi, V.L.; Sotto, M. Lyme disease like syndrome associated lymphocytoma: First case report in Brazil. Clinics 2007, 62, 525–526. [Google Scholar] [CrossRef][Green Version]
- Yoshinari, N.H.; Mantovani, E.; Bonoldi, V.L.; Marangoni, R.G.; Gauditano, G. Brazilian lyme-like disease or Baggio-Yoshinari syndrome: Exotic and emerging Brazilian tick-borne zoonosis. Rev. Assoc. Med. Bras. 2010, 56, 363–369. [Google Scholar] [CrossRef]
- Yoshinari, N.H.; Gouveia, E.A. Doença de Lyme e Síndrome de Baggio-Yoshinari. In Rotinas de Diagnóstico e Tratamento das Doenças Infecciosas e Parasitárias; Tavares, W., Marinho, L.A.C., Eds.; Editora Atheneu: São Paulo, Brazil, 2015; pp. 294–303. [Google Scholar]
- Vien, V.P.; Bassi, R.; Maxim, T.; Bogochi, I.I. Lyme disease vs Baggio-Yoshinari syndrome in a returned traveler from Brazil. J. Travel Med. 2017, 24, 1–2. [Google Scholar] [CrossRef]







| SBY (n = 68) | Normal (n = 50) | |
|---|---|---|
| ELISA | 23 (33.8%) * | 6 (12%) |
| Western Blotting (WB) | 40 (58.8%) * | 6 (12%) |
| ELISA and WB | 19 (27.9%) | 4 (8%) |
| ELISA plus WB | 44 (64.7%) * | 8 (16%) |
| Positives (n) | Frequencies (%) | |
|---|---|---|
| Ticks (n = 47) * | 2 | 4.25 |
| Buffalos (n = 27) | 1 | 3.70 |
| Horses (n = 26) | 1 | 3.85 |
| Groups | Sub-Groups | Humans | Clinical Aspect | Hosts Reservoirs | Vectors/Ticks | |
|---|---|---|---|---|---|---|
| Erythema migrans | Febbre | Hard-Ticks | ||||
| Lyme Group | Organotropism | Yes | Yes | No | Rodents | Ixodes sp. |
| High Spirochaetemia | Yes | Yes | Yes | Rodents | Ixodes sp. | |
| Baggio–Yoshinari | Yes | Yes | Yes (78%) | Amblyomma sp., Rhipicephalus sp. | ||
| Stage | BCMPE | STPET |
|---|---|---|
| Early (n–20) | 8 (40.0%) | 6 (30.0%) |
| Late (n–78) | 50 (64.1%) * | 30 (38.4%) |
| Total (n = 98) | 58 (59.2%) | 36 (36.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshinari, N.H.; Bonoldi, V.L.N.; Bonin, S.; Falkingham, E.; Trevisan, G. The Current State of Knowledge on Baggio–Yoshinari Syndrome (Brazilian Lyme Disease-like Illness): Chronological Presentation of Historical and Scientific Events Observed over the Last 30 Years. Pathogens 2022, 11, 889. https://doi.org/10.3390/pathogens11080889
Yoshinari NH, Bonoldi VLN, Bonin S, Falkingham E, Trevisan G. The Current State of Knowledge on Baggio–Yoshinari Syndrome (Brazilian Lyme Disease-like Illness): Chronological Presentation of Historical and Scientific Events Observed over the Last 30 Years. Pathogens. 2022; 11(8):889. https://doi.org/10.3390/pathogens11080889
Chicago/Turabian StyleYoshinari, Natalino Hajime, Virginia Lucia Nazario Bonoldi, Serena Bonin, Erica Falkingham, and Giusto Trevisan. 2022. "The Current State of Knowledge on Baggio–Yoshinari Syndrome (Brazilian Lyme Disease-like Illness): Chronological Presentation of Historical and Scientific Events Observed over the Last 30 Years" Pathogens 11, no. 8: 889. https://doi.org/10.3390/pathogens11080889
APA StyleYoshinari, N. H., Bonoldi, V. L. N., Bonin, S., Falkingham, E., & Trevisan, G. (2022). The Current State of Knowledge on Baggio–Yoshinari Syndrome (Brazilian Lyme Disease-like Illness): Chronological Presentation of Historical and Scientific Events Observed over the Last 30 Years. Pathogens, 11(8), 889. https://doi.org/10.3390/pathogens11080889







