Chicken Skin Decontamination of Thermotolerant Campylobacter spp. and Hygiene Indicator Escherichia coli Assessed by Viability Real-Time PCR
Abstract
:1. Introduction
2. Results
2.1. Design of a uidA-Targeting qPCR for Quantifying E. coli
2.2. Performance of the uidA-Targeting qPCR and Absence of False Positive Signals
2.3. The Novel uidA-Targeted qPCR Is Sensitive and Specific for E. coli/Shigella spp.
2.4. Combining the Novel qPCR with a Pre-Treatment Step Using PMA Allows the Differentiation of Viable and Dead E. coli
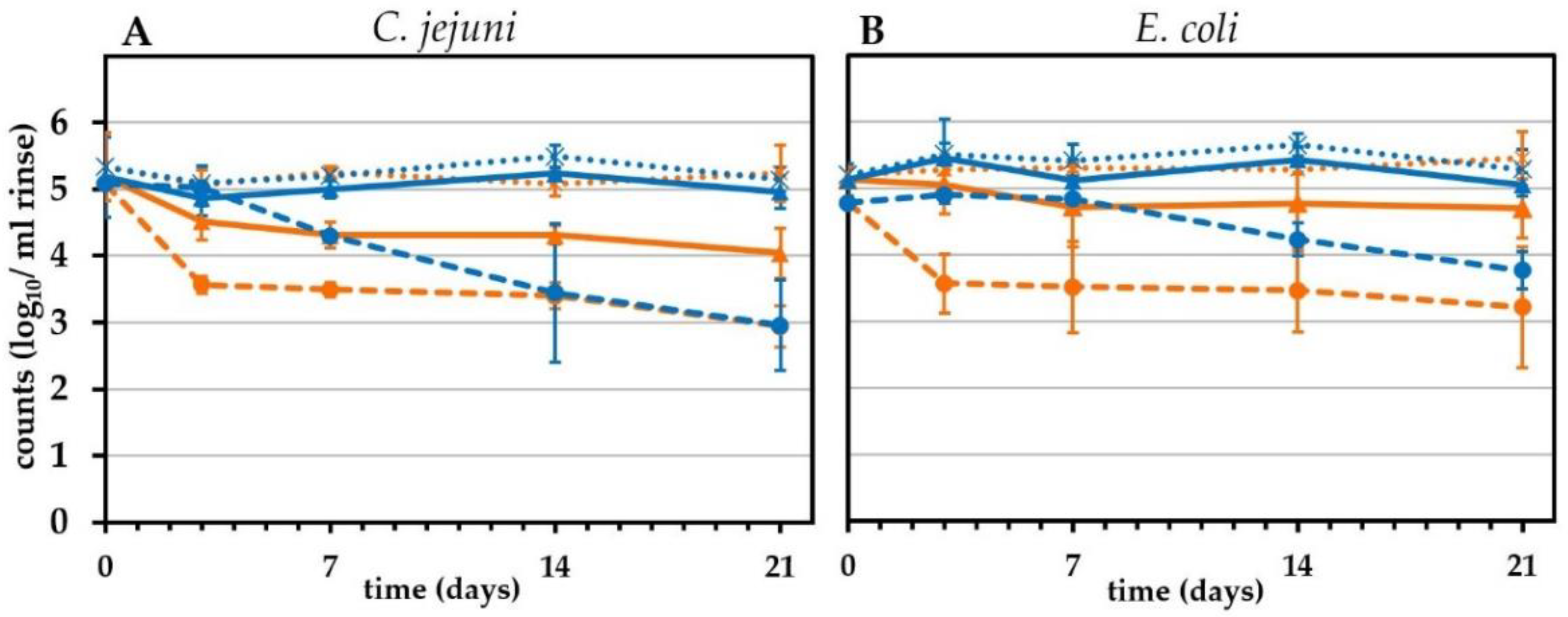
2.5. Storage of Chicken Skin in Refrigeration and Freezing Conditions
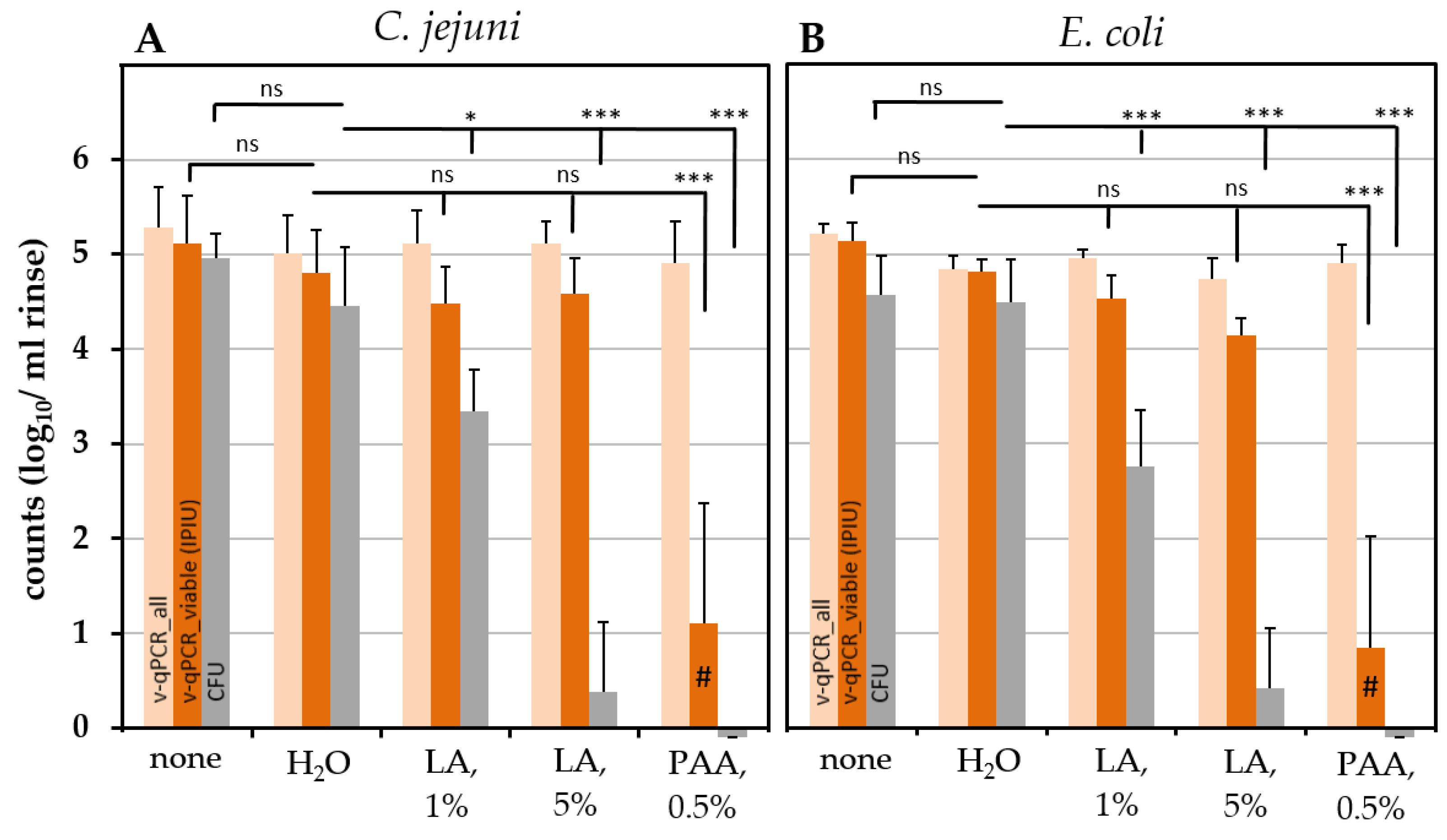
2.6. Effect of Chemical Treatment on the Survival of C. jejuni and ESBL-Producing E. coli on Chicken Skin
2.7. Long-Term Effects of Lactic Acid Treatment
3. Discussion
3.1. Establishing a Novel uidA-Based v-qPCR for the Quantification of Viable E. coli
3.2. Effect of Cooling and Freezing
3.3. PAA Treatment
3.4. LA Treatment
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. DNA Extraction and qPCR
4.3. Preparation of Quantification Standards for qPCR
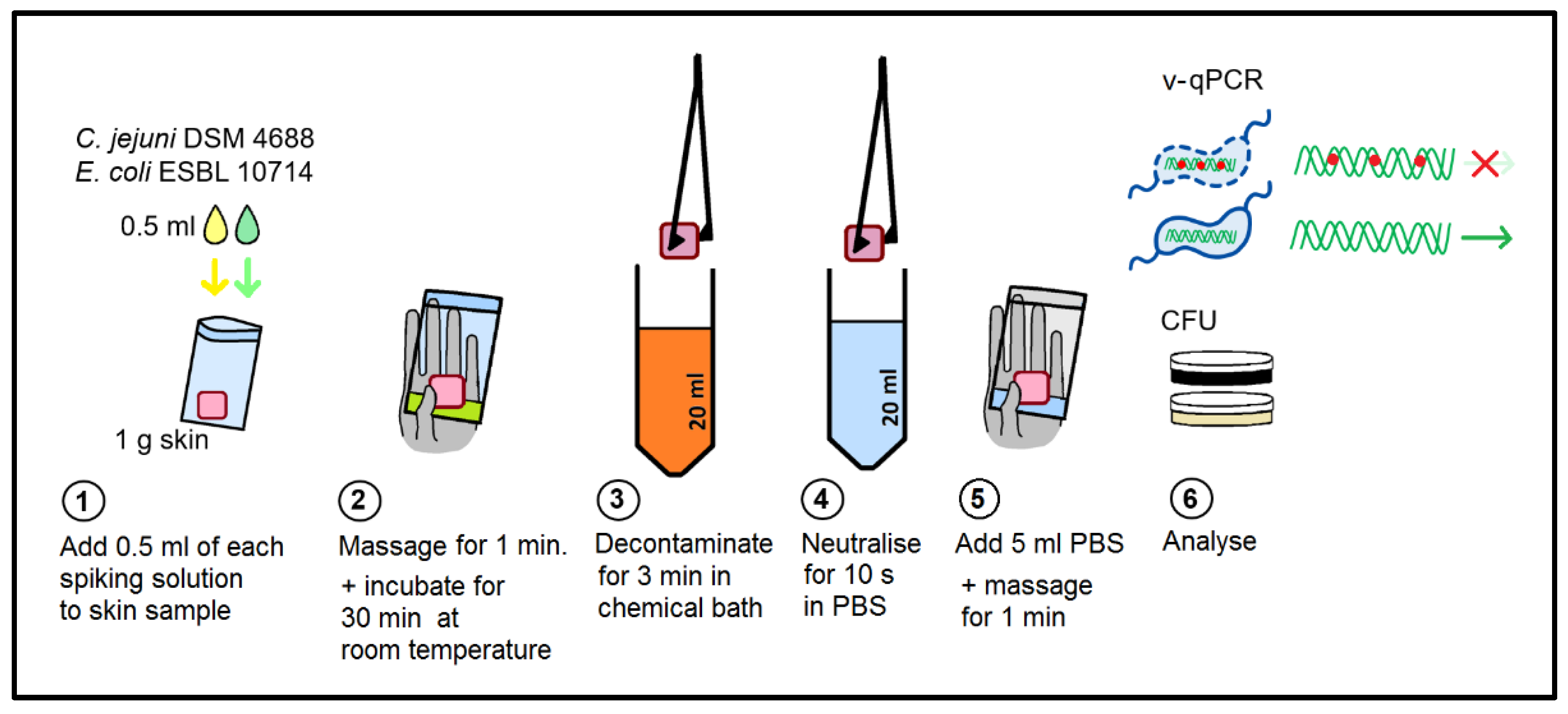
4.4. Survival Experiments with Chicken Skin
4.5. Viable/Dead Differentiation by v-qPCR
4.6. Data Handling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards; Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Guidance on the requirements for the development of microbiological criteria. EFSA J. 2017, 15, e05052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae counts on pig and ruminant carcasses along the slaughterline, factors influencing the counts and relationship between visual faecal contamination of carcasses and counts: A review. EFSA Supporting Publ. 2014, 11, 634e. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Bogaj, K.; Bortolaia, V.; Olsen, J.E.; Thomsen, L.E. Antibiotic-induced, increased conjugative transfer is common to diverse naturally occurring ESBL plasmids in Escherichia coli. Front. Microbiol. 2019, 10, 2119. [Google Scholar] [CrossRef]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, e06007. [Google Scholar] [CrossRef] [Green Version]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Federighi, M.; Tholozan, J.L.; Cappelier, J.M.; Tissier, J.P.; Jouve, J.L. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998, 15, 539–550. [Google Scholar] [CrossRef]
- Baffone, W.; Casaroli, A.; Citterio, B.; Pierfelici, L.; Campana, R.; Vittoria, E.; Guaglianone, E.; Donelli, G. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int. J. Food Microbiol. 2006, 107, 83–91. [Google Scholar] [CrossRef]
- Darcan, C.; Ozkanca, R.; Idil, O.; Flint, K.P. Viable but non-culturable state (VBNC) of Escherichia coli related to EnvZ under the effect of pH, starvation and osmotic stress in sea water. Pol. J. Microbiol. 2009, 58, 307–317. [Google Scholar]
- Pienaar, J.A.; Singh, A.; Barnard, T.G. The viable but non-culturable state in pathogenic Escherichia coli: A general review. Afr. J. Lab. Med. 2016, 5, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Wang, Y.; An, H.; Hao, Y.; Hu, X.; Liao, X. New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, N.J.; Buhler, C.; Iwobi, A.N.; Huber, I.; Ellerbroek, L.; Appel, B.; Stingl, K. “Limits of control”—Crucial parameters for a reliable quantification of viable Campylobacter by real-time PCR. PLoS ONE 2014, 9, e88108. [Google Scholar] [CrossRef] [Green Version]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, M.H.; Lofstrom, C.; Hansen, T.B.; Christensen, L.S.; Olsen, J.E.; Hoorfar, J. Rapid quantification of viable Campylobacter bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl. Environ. Microbiol. 2010, 76, 5097–5104. [Google Scholar] [CrossRef] [Green Version]
- Bej, A.K.; DiCesare, J.L.; Haff, L.; Atlas, R.M. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl. Environ. Microbiol. 1991, 57, 1013–1017. [Google Scholar] [CrossRef] [Green Version]
- Chern, E.C.; Brenner, K.P.; Wymer, L.; Haugland, R.A. Comparison of fecal indicator bacteria densities in marine recreational waters by qPCR. Water Qual. Expo. Health 2009, 1, 203–214. [Google Scholar] [CrossRef]
- Frahm, E.; Obst, U. Application of the fluorogenic probe technique (TaqMan PCR) to the detection of Enterococcus spp. and Escherichia coli in water samples. J. Microbiol. Methods 2003, 52, 123–131. [Google Scholar] [CrossRef]
- Heijnen, L.; Medema, G. Quantitative detection of E. coli, E. coli O157 and other shiga toxin producing E. coli in water samples using a culture method combined with real-time PCR. J. Water Health 2006, 4, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Silkie, S.S.; Tolcher, M.P.; Nelson, K.L. Reagent decontamination to eliminate false-positives in Escherichia coli qPCR. J. Microbiol. Methods 2008, 72, 275–282. [Google Scholar] [CrossRef]
- Liu, Y.; Mustapha, A. Detection of viable Escherichia coli O157:H7 in ground beef by propidium monoazide real-time PCR. Int. J. Food Microbiol. 2014, 170, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Miszczycha, S.D.; Ganet, S.; Duniere, L.; Rozand, C.; Loukiadis, E.; Thevenot-Sergentet, D. Novel real-time PCR method to detect Escherichia coli O157:H7 in raw milk cheese and raw ground meat. J. Food Prot. 2012, 75, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, J.Q. Real-time PCR methodology for selective detection of viable Escherichia coli O157:H7 cells by targeting Z3276 as a genetic marker. Appl. Environ. Microbiol. 2012, 78, 5297–5304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnetzinger, F.; Pan, Y.; Nocker, A. Use of propidium monoazide and increased amplicon length reduce false-positive signals in quantitative PCR for bioburden analysis. Appl. Microbiol. Biotechnol. 2013, 97, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- European Union: European Commission. Commission Regulation (EU) 2017/1495 of 23 August 2017 amending Regulation (EC) No 2073/2005 as regards Campylobacter in broiler carcasses. Off. J. Eur. Union 2017, OJ L 218, 1–6. Available online: http://data.europa.eu/eli/reg/2017/1495/oj (accessed on 9 June 2022).
- Loretz, M.; Stephan, R.; Zweifel, C. Antimicrobial activity of decontamination treatments for poultry carcasses: A literature survey. Food Control 2010, 21, 791–804. [Google Scholar] [CrossRef]
- Musavian, H.S.; Krebs, N.H.; Nonboe, U.; Corry, J.E.; Purnell, G. Combined steam and ultrasound treatment of broilers at slaughter: A promising intervention to significantly reduce numbers of naturally occurring campylobacters on carcasses. Int. J. Food Microbiol. 2014, 176, 23–28. [Google Scholar] [CrossRef]
- Anang, D.M.; Rusul, G.; Bakar, J.; Ling, F.H. Effects of lactic acid and lauricidin on the survival of Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157:H7 in chicken breast stored at 4 °C. Food Control 2007, 18, 961–969. [Google Scholar] [CrossRef]
- Boysen, L.; Wechter, N.S.; Rosenquist, H. Effects of decontamination at varying contamination levels of Campylobacter jejuni on broiler meat. Poult. Sci. 2013, 92, 1425–1429. [Google Scholar] [CrossRef]
- Rajkovic, A.; Tomic, N.; Smigic, N.; Uyttendaele, M.; Ragaert, P.; Devlieghere, F. Survival of Campylobacter jejuni on raw chicken legs packed in high-oxygen or high-carbon dioxide atmosphere after the decontamination with lactic acid/sodium lactate buffer. Int. J. Food Microbiol. 2010, 140, 201–206. [Google Scholar] [CrossRef]
- Harrison, D.; Corry, J.E.; Tchórzewska, M.A.; Morris, V.K.; Hutchison, M.L. Freezing as an intervention to reduce the numbers of campylobacters isolated from chicken livers. Lett. Appl. Microbiol. 2013, 57, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, L.J.; Bowers, J.W.; Townsend, J.C.; McKee, S.R. The microbial and quality properties of poultry carcasses treated with peracetic acid as an antimicrobial treatment. Poult. Sci. 2008, 87, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Isohanni, P.M.; Lyhs, U. Use of ultraviolet irradiation to reduce Campylobacter jejuni on broiler meat. Poult. Sci. 2009, 88, 661–668. [Google Scholar] [CrossRef] [PubMed]
- James, C.; James, S.J.; Hannay, N.; Purnell, G.; Barbedo-Pinto, C.; Yaman, H.; Araujo, M.; Gonzalez, M.L.; Calvo, J.; Howell, M.; et al. Decontamination of poultry carcasses using steam or hot water in combination with rapid cooling, chilling or freezing of carcass surfaces. Int. J. Food Microbiol. 2007, 114, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.D.; Han, I.Y.; Dawson, P.L.; Northcutt, J.K. Survival of artificially inoculated Escherichia coli and Salmonella Typhimurium on the surface of raw poultry products subjected to crust freezing. Poult. Sci. 2011, 90, 2874–2878. [Google Scholar] [CrossRef]
- Stefani, L.M.; Backes, R.G.; Faria, G.A.; Biffi, C.P.; de Almeida, J.M.; da Silva, H.K.; das Neves, G.B.; Langaro, A. Trimming and washing poultry carcass to reduce microbial contamination: A comparative study. Poult. Sci. 2014, 93, 3119–3122. [Google Scholar] [CrossRef]
- European Union: European Commission. Commission Regulation (EU) No 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcases. Off. J. Eur. Union 2013, OJ L 34, 1–3. Available online: http://data.europa.eu/eli/reg/2013/101/oj (accessed on 9 June 2022).
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the evaluation of the safety and efficacy of peroxyacetic acid solutions for reduction of pathogens on poultry carcasses and meat. EFSA J. 2014, 12, 3599. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Kibbee, R.; Linklater, N.; Ormeci, B. Eliminating false positives in a qPCR assay for the detection of the uidA gene in Escherichia coli. J. Water Health 2013, 11, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Kibbee, R.J.; Örmeci, B. Development of a sensitive and false-positive free PMA-qPCR viability assay to quantify VBNC Escherichia coli and evaluate disinfection performance in wastewater effluent. J. Microbiol. Methods 2017, 132, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; Heise, J.; Thieck, M.; Wulsten, I.F.; Pacholewicz, E.; Iwobi, A.N.; Govindaswamy, J.; Zeller-Péronnet, V.; Scheuring, S.; Luu, H.Q.; et al. Challenging the “gold standard” of colony-forming units—Validation of a multiplex real-time PCR for quantification of viable Campylobacter spp. in meat rinses. Int. J. Food Microbiol. 2021, 359, 109417. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Pietsch, K.; Zucker, R.; Mayr, A.; Müller-Hohe, E.; Messelhäusser, U.; Sing, A.; Busch, U.; Huber, I. Validation of a duplex real-time PCR for the detection of Salmonella spp. in different food products. Food Anal. Methods 2011, 4, 259–267. [Google Scholar] [CrossRef]
- Damaré, J.M.; Campbell, D.F.; Johnston, R.W. Simplified direct plating method for enhanced recovery of Escherichia coli in food. J. Food Sci. 1985, 50, 1736–1737. [Google Scholar] [CrossRef]
- Feng, P.C.; Hartman, P.A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl. Environ. Microbiol. 1982, 43, 1320–1329. [Google Scholar] [CrossRef] [Green Version]
- Frampton, E.W.; Restaino, L.; Blaszko, N. Evaluation of the β-glucuronidase substrate 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-GLUC) in a 24-h direct plating method for Escherichia coli. J. Food Prot. 1988, 51, 402–404. [Google Scholar] [CrossRef]
- Hansen, W.; Yourassowsky, E. Detection of beta-glucuronidase in lactose-fermenting members of the family Enterobacteriaceae and its presence in bacterial urine cultures. J. Clin. Microbiol. 1984, 20, 1177–1179. [Google Scholar] [CrossRef] [Green Version]
- Ratnam, S.; March, S.B.; Ahmed, R.; Bezanson, G.S.; Kasatiya, S. Characterization of Escherichia coli serotype O157:H7. J. Clin. Microbiol. 1988, 26, 2006–2012. [Google Scholar] [CrossRef] [Green Version]
- Pacholewicz, E.; Buhler, C.; Wulsten, I.F.; Kraushaar, B.; Luu, H.Q.; Iwobi, A.N.; Huber, I.; Stingl, K. Internal sample process control improves cultivation-independent quantification of thermotolerant Campylobacter. Food Microbiol. 2019, 78, 53–61. [Google Scholar] [CrossRef]
- Wulsten, I.F.; Galeev, A.; Stingl, K. Underestimated survival of Campylobacter in raw milk highlighted by viability real-time PCR and growth recovery. Front. Microbiol. 2020, 11, 1107. [Google Scholar] [CrossRef]
- Gill, C.O.; Harris, L.M. Survival and growth of Campylobacter fetus subsp. jejuni on meat and in cooked foods. Appl. Environ. Microbiol. 1982, 44, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garénaux, A.; Ritz, M.; Jugiau, F.; Rama, F.; Federighi, M.; de Jonge, R. Role of oxidative stress in C. jejuni inactivation during freeze-thaw treatment. Curr. Microbiol. 2009, 58, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Georgsson, F.; Thornorkelsson, A.E.; Geirsdóttir, M.; Reiersen, J.; Stern, N.J. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 2006, 23, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Botteldoorn, N.; Coucke, W.; Denayer, S.; Dierick, K.; Uyttendaele, M. Effect of exposure to stress conditions on propidium monoazide (PMA)-qPCR based Campylobacter enumeration in broiler carcass rinses. Food Microbiol. 2015, 48, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lazou, T.P.; Gelasakis, A.I.; Chaintoutis, S.C.; Iossifidou, E.G.; Dovas, C.I. Method-dependent implications in foodborne pathogen quantification: The case of Campylobacter coli survival on meat as comparatively assessed by colony count and viability PCR. Front. Microbiol. 2021, 12, 604933. [Google Scholar] [CrossRef]
- OECD Existing Chemicals Database, Peroxyacetic acid, SIDS Initial Assessment Profile, File: 79210_SIAP_final.pdf of 16 April 2008. Available online: https://hpvchemicals.oecd.org/UI/SIDS_Details.aspx?key=1f07490d-8c31-44e8-a2d6-7b261e310720&idx=0 (accessed on 11 May 2022).
- Zhang, L.; Garner, L.J.; Mckee, S.R.; Bilgili, S.F. Effectiveness of several antimicrobials used in a postchill decontamination tank against Salmonella and Campylobacter on broiler carcass parts. J. Food Prot. 2018, 81, 1134–1141. [Google Scholar] [CrossRef]
- Nagel, G.M.; Bauermeister, L.J.; Bratcher, C.L.; Singh, M.; McKee, S.R. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int. J. Food Microbiol. 2013, 165, 281–286. [Google Scholar] [CrossRef]
- Thomas, C.; Schönknecht, A.; Püning, C.; Alter, T.; Martin, A.; Bandick, N. Effect of peracetic acid solutions and lactic acid on microorganisms in on-line reprocessing systems for chicken slaughter plants. J. Food Prot. 2020, 83, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Rasschaert, G.; Piessens, V.; Scheldeman, P.; Leleu, S.; Stals, A.; Herman, L.; Heyndrickx, M.; Messens, W. Efficacy of electrolyzed oxidizing water and lactic acid on the reduction of Campylobacter on naturally contaminated broiler carcasses during processing. Poult. Sci. 2013, 92, 1077–1084. [Google Scholar] [CrossRef]
- Coşansu, S.; Ayhan, K. Effects of lactic and acetic acid treatments on Campylobacter jejuni inoculated onto chicken leg and breast meat during storage at 4 °C and −18 °C. J. Food Processing Preserv. 2010, 34, 98–113. [Google Scholar] [CrossRef]
- Riedel, C.T.; Brøndsted, L.; Rosenquist, H.; Haxgart, S.N.; Christensen, B.B. Chemical decontamination of Campylobacter jejuni on chicken skin and meat. J. Food Prot. 2009, 72, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- OECD Existing Chemicals Database, Lactic Acid, SIDS Initial Assessment Profile, File: 50215_SIAP_final_Oct10.pdf of 20 October 2009. Available online: https://hpvchemicals.oecd.org/UI/SIDS_Details.aspx?key=7828dbad-7405-4619-afa1-9250df698ad7&idx=0 (accessed on 11 May 2022).
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Smigic, N.; Rajkovic, A.; Nielsen, D.S.; Siegumfeldt, H.; Uyttendaele, M.; Devlieghere, F.; Arneborg, N. Intracellular pH as an indicator of viability and resuscitation of Campylobacter jejuni after decontamination with lactic acid. Int. J. Food Microbiol. 2009, 135, 136–143. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Chen, Z.; Chen, D.; Kan, J. Sublethal injury and recovery of Escherichia coli O157:H7 and K-12 after exposure to lactic acid. Food Control 2017, 82, 190–195. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhang, R.; Shi, H. The effect of manganese and iron on mediating resuscitation of lactic acid-injured Escherichia coli. Lett. Appl. Microbiol. 2022. [Google Scholar] [CrossRef]
- Mayr, A.M.; Lick, S.; Bauer, J.; Thärigen, D.; Busch, U.; Huber, I. Rapid detection and differentiation of Campylobacter jejuni, Campylobacter coli, and Campylobacter lari in food, using multiplex real-time PCR. J. Food Prot. 2010, 73, 241–250. [Google Scholar] [CrossRef]




| Reference Strains | Results | Field Strains | Number of Isolates | Results |
|---|---|---|---|---|
| Escherichia coli DSM 1103 | positive | Escherichia coli, MUG+ | 108 | positive |
| Escherichia coli EDL 933 | positive | Escherichia coli, MUG(+) | 12 | positive |
| Escherichia coli DSM 498 | positive | Escherichia coli, MUG- | 22 | positive |
| Shigella sonnei DSM 5570 | positive | Enterobacter cloacae complex | 1 | negative |
| Escherichia albertii DSM 17582 | negative | Escherichia albertii | 2 | negative |
| Escherichiafergusonii DSM 13698 | negative | Aeromonas jandaei | 1 | negative |
| Escherichia hermannii DSM 4560 | negative | Citrobacter koseri | 1 | negative |
| Enterococcus faecalis DSM 20478 | negative | Hafnia alvei | 3 | negative |
| Klebsiella pneumoniae DSM 30104 | negative | |||
| Proteus mirabilis DSM 4479 | negative | |||
| Pseudomonas aeruginosa DSM 1117 | negative | |||
| Staphylococcus aureus DSM 1104 | negative | |||
| Salmonella enterica DSM 11320 | negative | |||
| IPC-ntb2 (plasmid) | negative | |||
| Campylobacter jejuni DSM 4688 | negative | |||
| Campylobacter jejuni NCTC 11168 | negative | |||
| Campylobacter sputorum DSM 5363/ISPC | negative | |||
| Campylobacter coli DSM 4689 | negative | |||
| Campylobacter lari DSM 11375 | negative | |||
| Treatment | Decontamination Solution (Before Use) | Neutralization Bath (PBS, after Use) | Chicken Skin Rinse |
|---|---|---|---|
| LA, 1% | 2 | 7 | 6 (5.5–7) |
| LA, 5% | 1.5 | 5 (4.5–5.5) | 4 (4–5) |
| PAA, 0.5% | 5 | 7 | 6.5 |
| MilliQ water | 5 (4.5–5) | 7.5 | 7–7.5 |
| tap water | 7.5 | 7.5 | 7–7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulsten, I.F.; Thieck, M.; Göhler, A.; Schuh, E.; Stingl, K. Chicken Skin Decontamination of Thermotolerant Campylobacter spp. and Hygiene Indicator Escherichia coli Assessed by Viability Real-Time PCR. Pathogens 2022, 11, 706. https://doi.org/10.3390/pathogens11060706
Wulsten IF, Thieck M, Göhler A, Schuh E, Stingl K. Chicken Skin Decontamination of Thermotolerant Campylobacter spp. and Hygiene Indicator Escherichia coli Assessed by Viability Real-Time PCR. Pathogens. 2022; 11(6):706. https://doi.org/10.3390/pathogens11060706
Chicago/Turabian StyleWulsten, Imke F., Maja Thieck, André Göhler, Elisabeth Schuh, and Kerstin Stingl. 2022. "Chicken Skin Decontamination of Thermotolerant Campylobacter spp. and Hygiene Indicator Escherichia coli Assessed by Viability Real-Time PCR" Pathogens 11, no. 6: 706. https://doi.org/10.3390/pathogens11060706
APA StyleWulsten, I. F., Thieck, M., Göhler, A., Schuh, E., & Stingl, K. (2022). Chicken Skin Decontamination of Thermotolerant Campylobacter spp. and Hygiene Indicator Escherichia coli Assessed by Viability Real-Time PCR. Pathogens, 11(6), 706. https://doi.org/10.3390/pathogens11060706






