Microbiological, Clinical and Radiological Aspects of Diabetic Foot Ulcers Infected with Methicillin-Resistant and -Sensitive Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Study
2.2. Isolated Bacteria
2.3. Antimicrobial Susceptibility of S. aureus Strains
2.4. MRSA versus MSSA DFU Infections
2.5. Diabetic Foot Ulcers Complicated or Not with Osteitis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Bacterial Strains
4.3. Antimicrobial Susceptibility
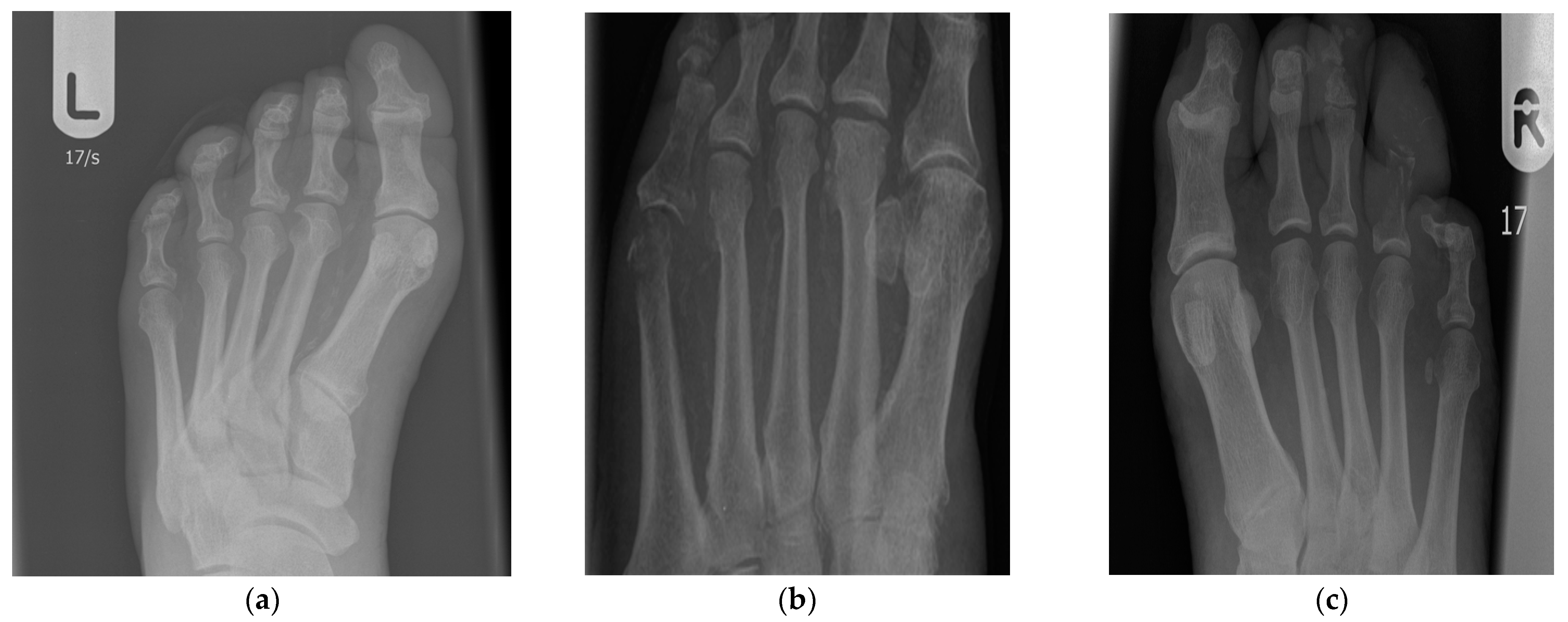
4.4. Osteitis and X-ray Evaluation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro-Soares, M.; Russell, D.; Boyko, E.J.; Jeffcoate, W.; Mills, J.L.; Morbach, S.; Game, F.; on behalf of the International Working Group on the Diabetic Foot(IWGDF). Guidelines on the classification of diabetic foot ulcers (IWGDF 2019). Diabetes Metab. Res. Rev. 2020, 36, e3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemann, A.H.; Hofmann, G.O. Principles of the therapy of bone infections in adult extremities: Are there any new developments? Strateg. Trauma Limb Reconstr. 2009, 4, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonomini, A.R.; Riva, E.; Di Bonaventura, G.; Gherardi, G. Rapid detection of methicillin-resistant Staphylococcus aureus directly from blood for the diagnosis of bloodstream infections: A mini-review. Diagnostics 2020, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, E.; Garbacz, K.; Kosecka-Strojek, M.; Schubert, J.; Bania, J.; Międzobrodzki, J. Presence of egc-positive major clones ST 45, 30 and 22 among methicillin-resistant and methicillin-susceptible oral Staphylococcus aureus strains. Sci. Rep. 2020, 10, 18889. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; LeFrock, J.L.; Lew, D.P.; Mader, J.T.; Norden, C.; et al. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 2006, 117, 212S–238S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; Pathrose, E.; Bal, A.; Bhalekar, P.; Sekar, P.; Krishnan, P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef]
- Banu, A.; Noorul Hassan, M.M.; Rajkumar, J.; Srinivasa, S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015, 8, 280–285. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Tomasz, A.; de Lencastre, H. Secrets of success of a human pathogen: Molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 2002, 2, 180–189. [Google Scholar] [CrossRef]
- Eleftheriadou, I.; Tentolouris, N.; Argiana, V.; Jude, E.; Boulton, A.J. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs 2010, 70, 1785–1797. [Google Scholar] [CrossRef]
- Stacey, H.J.; Clements, C.S.; Welburn, S.C.; Jones, J.D. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: A meta-analysis. Acta Diabetol. 2019, 56, 907–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhar, S.; Vyas, N.; Unnikrishnan, M.; Rodrigues, G.; Mukhopadhyay, C. Antimicrobial susceptibility pattern in diabetic foot ulcer: A pilot study. Ann. Med. Health Sci. Res. 2014, 4, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakant, P.; Verma, A.K.; Misra, R.; Prasad, K.N.; Chand, G.; Mishra, A.; Agarwal, G.; Agarwal, A.; Mishra, S.K. Changing microbiological profile of pathogenic bacteria in diabetic foot infections: Time for a rethink on which empirical therapy to choose? Diabetologia 2011, 54, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Nithyalakshmi, J. A comparative study of Diabetic and Non-diabetic wound infections with special reference to MRSA and ESBL. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 546–554. [Google Scholar]
- Kupfer, M.; Jatzwauk, L.; Monecke, S.; Möbius, J.; Weusten, A. MRSA in a large German University Hospital: Male gender is a significant risk factor for MRSA acquisition. GMS Krankenhhyg. Interdiszip. 2010, 5, Doc11. [Google Scholar]
- Busaidi, I.; Abdulhadi, N.; Coppell, K. Care of patients with diabetic foot disease in Oman? Sultan Qaboos Univ. Med. J. 2016, 16, e270–e276. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Sharma, A.K.; Mann, J. Diabetic Foot Disease—Incidence and Risk Factors: A Clinical Study. J. Foot Ankle Surg. 2016, 3, 41–46. [Google Scholar]
- Diller, R.; Sonntag, A.K.; Mellmann, A.; Grevener, K.; Senninger, N.; Kipp, F.; Friedrich, A.W. Evidence for cost reduction based on pre-admission MRSA screening in general surgery. Int. J. Hyg. Environ. Health 2008, 211, 205–212. [Google Scholar] [CrossRef]
- Hoefnagels-Schuermans, A.; Borremans, A.; Peetermans, W.; Van Lierde, S.; Reybrouck, G.; Van Eldere, J. Origin and transmission of methicillin-resistant Staphylococcus aureus in an endemic situation: Differences between geriatric and intensive-care patients. J. Hosp. Inf. 1997, 36, 209–222. [Google Scholar] [CrossRef]
- Shukla, S.; Nixon, M.; Acharya, M.; Korim, M.T.; Pandey, R. Incidence of MRSA surgical-site infection in MRSA carriers in an orthopaedic trauma unit. J. Bone Jt. Surg. Br. 2009, 91, 225–228. [Google Scholar] [CrossRef]
- Mairghani, M.; Elmusharaf, K.; Patton, D.; Burns, J.; Eltahir, O.; Jassim, G.; Moore, Z. The prevalence and incidence of diabetic foot ulcers among five countries in the Arab world: A systematic review. J. Wound Care 2017, 26, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Ko, S.H.; Lee, S.H.; Cho, J.H.; Moon, S.D.; Jang, S.A.; Son, H.S.; Song, K.H.; Cha, B.Y.; Son, H.Y.; et al. Incidence of diabetic foot and associated risk factors in type 2 diabetic patients: A five year observational study. Korean Diabetes J. 2009, 33, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Olsen, K.; Sangvik, M.; Simonsen, G.S.; Sollid, J.U.E.; Sundsfjord, A.; Thune, I.; Furberg, A. Prevalence and population structure of Staphylococcus aureus nasal carriage in healthcare workers in a general population. The Tromsø Staph and Skin Study. Epidemiol. Infect. 2013, 141, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawcharoenporn, T.; Tice, A.D.; Grandinetti, A.; Chow, D. Risk factors for community-associated methicillin-resistant Staphylococcus aureus cellulitis--and the value of recognition. Hawaii Med. J. 2010, 69, 232–236. [Google Scholar]
- Chan, G.; Iliopoulos, E.; Jain, A.; Turki, M.; Trompeter, A. Infection after operative fixation of tibia plateau fractures. A risk factor analysis. Injury 2019, 50, 2089–2092. [Google Scholar] [CrossRef]
- Meier, K.; Nordestgaard, A.T.; Eid, A.I.; Kongkaewpaisan, N.; Lee, J.M.; Kongwibulwut, M.; Han, K.R.; Kokoroskos, N.; Mendoza, A.E.; Saillant, N.; et al. Obesity as protective against, rather than a risk factor for, postoperative Clostridium difficile infection: A nationwide retrospective analysis of 1,426,807 surgical patients. J. Trauma Acute Care Surg. 2019, 86, 1001–1009. [Google Scholar] [CrossRef]
- Neidhart, S.; Zaatreh, S.; Klinder, A.; Redanz, S.; Spitzmuller, R.; Holtfreter, S.; Warnke, P.; Alozie, A.; Henck, V.; Gohler, A.; et al. Predictors of colonization with Staphylococcus species among patients scheduled for cardiac and orthopedic interventions at tertiary care hospitals in north-eastern Germany—A prevalence screening study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 633–641. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Veves, A.; Giurini, J.M.; Logerfo, F.W. The Diabetic Foot, 2nd ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 978–1007. [Google Scholar]
- Lin, S.D.; Lin, C.J.; Wang, A.H.; Zhao, S.; Yan, L.; Wang, P.H.; Du, Y.M.; Wang, Z.J.; Xiao, Z.H.; Ma, X.Y. A multicentre survey on the diabetic foot and its neuropathy in China. Zhonghua Yi Xue Za Zhi 2007, 87, 1241–1244. [Google Scholar]
- Giurato, L.; Meloni, M.; Izzo, V.; Uccioli, L. Osteomyelitis in diabetic foot: A comprehensive overview. World J. Diabetes 2017, 8, 135–142. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, N.; Ma, Y.F.; Jiang, Y.; Zhao, X.Q.; Xie, G.P.; Hu, Y.J.; Qin, C.H.; Yu, B. Clinical characteristics and treatment of extremity chronic osteomyelitis in Southern China: A retrospective analysis of 394 consecutive patients. Medicine 2015, 94, e1874. [Google Scholar] [CrossRef] [PubMed]
- Sarkissian, E.J.; Gans, I.; Gunderson, M.A.; Myers, S.H.; Spiegel, D.A.; Flynn, J.M. Community-acquired methicillin-resistant Staphylococcus aureus musculoskeletal infections: Emerging trends over the past decade. J. Pediatric Orthop. 2016, 36, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.R.; Shetty, V.; Kumar, A.M.; Shetty, S.M.; Shetty, A. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: Experience from India. J. Pediatric Orthop. Part B 2013, 22, 158–166. [Google Scholar] [CrossRef]
- Kok, E.Y.; Vallejo, J.G.; Sommer, L.M.; Rosas, L.; Kaplan, S.L.; Hulten, K.G.; McNeil, J.C. Association of vancomycin MIC and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob. Agents Chemother. 2018, 62, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stańkowska, M.; Garbacz, K.; Piechowicz, L.; Bronk, M. Dissemination of t437-sccmeciv and coagulase-negative t037-SCCmecIII types among borderline oxacillin-resistant Staphylococcus aureus isolated from skin infections and diabetic foot ulcers. Infect. Drug Resist. 2019, 10, 3197–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katkowska, M.; Garbacz, K.; Kopala, W.; Schubert, J.; Bania, J. Genetic diversity and antimicrobial resistance of Staphylococcus aureus from recurrent tonsillitis in children. APMIS 2020, 128, 211–219. [Google Scholar] [CrossRef]
- Lodise, T.P.; McKinnon, P.S. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 2005, 52, 113–122. [Google Scholar] [CrossRef]
- Rozgonyi, F.; Kocsis, E.; Kristóf, K.; Nagy, K. Is MRSA more virulent than MSSA? Clin. Microbiol. Infect. 2007, 13, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Gannepalli, A.; Ayinampudi, B.K.; Baghirath, P.V.; Reddy, G.V. Actinomycotic Osteomyelitis of Maxilla Presenting as Oroantral Fistula: A Rare Case Report. Case Rep. Dent. 2015, 2015, 689240. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Haddad, A.J. Cervicofacial actinomycosis—Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Sezer, B.; Akdeniz, B.G.; Günbay, S.; Hilmioğlu-Polat, S.; Başdemir, G. Actinomycosis osteomyelitis of the jaws: Report of four cases and a review of the literature. J. Dent. Sci. 2017, 12, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaper, N.C.; Apelqvist, J.; Bakker, K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr. Diab. Rep. 2003, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Tille, P. Bailey & Scott’s Diagnostic Microbiology, 13th ed.; Mosby, Inc.: St. Louis, MO, USA, 2014. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2020. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 1 January 2020).
- Aragón-Sánchez, J.; Lipksy, B.; Lázaro-Martínez, J. Diagnosing diabetic foot osteomyelitis: Is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet. Med. 2011, 28, 191–194. [Google Scholar] [CrossRef]
- Lipsky, B. Osteomyelitis of the foot in diabetic patients. Clin. Infect. Dis. 1997, 25, 1318–1326. [Google Scholar] [CrossRef]
- Kothari, N.A.; Pelchovitz, D.P.; Meyer, P.J. Imaging of musculoskeletal infections. Radiol. Clin. N. Am. 2001, 39, 653–671. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y.J. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 1 July 2021).
| Variable | Number of Patients with DFU Infected S. aureus (n = 201) | Percentage (%) | |
|---|---|---|---|
| Sex | Female | 51 | 25.4 |
| Male | 150 | 74.6 | |
| Age (years) | <40 years | 3 | 1.5 |
| 40–60 years | 66 | 32.8 | |
| >60 years | 132 | 65.7 | |
| Diabetes type | 1 | 41 | 20.4 |
| 2 | 158 | 78.6 | |
| 3 | 2 | 1.0 | |
| Diabetes duration | <5 years | 12 | 5.9 |
| 5–9 years | 29 | 14.4 | |
| >9 years | 160 | 79.6 | |
| BMI (kg/m2) | Normal weight | 42 | 20.9 |
| Overweight | 54 | 26.9 | |
| Obesity class 1 | 56 | 27.9 | |
| Obesity class 2 | 33 | 16.4 | |
| Obesity class 3 | 16 | 7.9 | |
| HbA1c level (mmol/mol) | <7 | 56 | 27.9 |
| 7.1–7.9 | 35 | 17.4 | |
| ≥8 | 110 | 54.7 | |
| CRP (mg/L) | <5 | 82 | 40.8 |
| 5–99 | 94 | 46.8 | |
| ≥99 | 25 | 12.4 | |
| Bacteria Co-Isolated with S. aureus | Number | Percentage (%) |
|---|---|---|
| Only Staphylococcus aureus | 66 | 32.8 |
| Gram-positive cocci | ||
| Coagulase negative staphylococci | ||
| Staphylococcus epidermidis | 6 | 3 |
| Staphylococcus haemolyticus | 5 | 2.5 |
| Staphylococcus capitis | 3 | 1.5 |
| Staphylococcus hominis | 1 | 0.5 |
| Staphylococcus simulans | 1 | 0.5 |
| Beta-hemolytic streptococci | ||
| group A | 2 | 1 |
| group B | 16 | 8 |
| group C | 6 | 3 |
| group G | 13 | 6.5 |
| group E | 1 | 0.5 |
| Alpha-hemolytic streptococci | 4 | 2 |
| Enterococcus faecalis | 12 | 6 |
| Enterococcus faecium | 6 | 3 |
| Enterococcus durans | 1 | 0.5 |
| Gram-negative bacilli | ||
| Enterobacteriaceae | ||
| Escherichia coli | 22 | 11 |
| Proteus mirabilis | 16 | 8 |
| Proteus vulgaris | 2 | 1 |
| Enterobacter cloacae | 12 | 6 |
| Enterobacter hermannii | 1 | 0.5 |
| Morganella morganii | 5 | 2.5 |
| Klebsiella pneumoniae | 5 | 2.5 |
| Klebsiella oxytoca | 4 | 2 |
| Citrobacter koseri | 3 | 1.5 |
| Citrobacter freundii | 3 | 1.5 |
| Serratia marcescens | 5 | 2.5 |
| Raoutella planticola | 1 | 0.5 |
| Non-fermenting bacilli | ||
| Pseudomonas aeruginosa | 17 | 8.5 |
| Pseudomonas putida | 1 | 0.5 |
| Acinetobacter baumannii | 5 | 2.5 |
| Stenotrophomonas maltophilia | 1 | 0.5 |
| Alcaligenes faecalis | 1 | 0.5 |
| Anaerobes non spore-forming | ||
| Peptostreptococcus anaerobius | 7 | 3.5 |
| Bacteroides fragilis | 4 | 2 |
| Prevotella bivia | 2 | 1 |
| Prevotella melaninogenica | 1 | 0.5 |
| Veillonella parvula | 1 | 0.5 |
| Propionibacterium propionicum | 1 | 0.5 |
| Anaerobes spore-forming | ||
| Clostridium sp. | 32 | 15.9 |
| Actinomyces sp. | 5 | 2.5 |
| One Bacterium Co-Isolated with S. aureus | Two Bacteria Co-Isolated with S. aureus | Three Bacteria Co-Isolated with S. aureus | n (%) (n = 135) |
|---|---|---|---|
| Enterobacteriaceae | 37 (27.4) | ||
| Beta-hemolytic streptococci | 13 (9.6) | ||
| Enterococci | 5 (3.7) | ||
| Spore-forming anaerobes | 3 (2.2) | ||
| Coagulase negative staphylococci | 2 (1.5) | ||
| Non-spore-forming anaerobes | 2 (1.5) | ||
| Alpha-hemolytic streptococci | 2 (1.5) | ||
| Non-fermenting bacilli | 0 (0) | ||
| Actinomyces sp. | 0 (0) | ||
| Enterobacteriaceae | Anaerobes spore-forming | 11 (8.2) | |
| Non-fermenting bacilli | Coagulase negative staphylococci | 7 (5.2) | |
| Enterobacteriaceae | Non-fermenting bacilli | 7 (5.2) | |
| Enterobacteriaceae | Coagulase negative staphylococci | 6 (4.4) | |
| Enterobacteriaceae | Beta-hemolytic streptococci | 4 (3) | |
| Beta-hemolytic streptococci | Non-fermenting bacilli | 4 (3) | |
| Enterobacteriaceae | Alpha- hemolytic streptococci | 2 (1.5) | |
| Beta-hemolytic streptococci | Actinomyces sp. | 1 (0.7) | |
| Enterobacteriaceae | Actinomyces sp. | 1 (0.7) | |
| Beta-hemolytic streptococci | Enterococci | Anaerobes non-spore-forming | 8 (5.9) |
| Non-fermenting bacilli | Beta-hemolytic streptococci | Anaerobes spore-forming | 7 (5.2) |
| Enterobacteriaceae | Anaerobes spore-forming | Anaerobes non-spore-forming | 6 (4.4) |
| Enterobacteriaceae | Enterococci | Anaerobes spore-forming | 4 (3) |
| Enterobacteriaceae | Enterococci | Actinomyces sp. | 1 (0.7) |
| Beta-hemolytic streptococci | Enterococci | Actinomyces sp. | 1 (0.7) |
| Spore-forming anaerobes | Coagulase negative staphylococci | Actinomyces sp. | 1 (0.7) |
| Bacteria Co-Isolated with S. aureus | MSSA | MRSA | Total | p-Value | OR [95% CI] |
|---|---|---|---|---|---|
| (n = 170) | (n = 31) | (n = 201) | |||
| Gram-positive cocci | |||||
| Coagulase negative staphylococci | 13 | 3 | 16 | 0.7181 | 1.29 [0.35–4.84] |
| Streptococci | 38 | 4 | 42 | 0.3367 | 0.51 [0.17–1.56] |
| Enterococci | 13 | 6 | 19 | 0.0510 | 2.90 [1.01–8.33] |
| Gram-negative bacilli | |||||
| Enterobacteriaceae | 65 | 15 | 80 | 0.3217 | 1.51 [0.70–3.27] |
| Non-fermenting bacilli | 16 | 8 | 24 | 0.0162 | 3.35 [1.29–8.70] |
| Anaerobes | |||||
| Non-spore-forming anaerobes | 13 | 6 | 19 | 0.0510 | 2.90 [1.01–8.33] |
| Spore-forming anaerobes | 30 | 2 | 32 | 0.1796 | 0.32 [0.07–1.42] |
| Actinomyces sp. | 5 | 0 | 5 | 0.5996 | 0.00 [0.00–NaN] |
| Variable | Number of Patients with DFU Infected S. aureus (n = 201) | MSSA Infection (n = 170) | MRSA Infection (n = 31) | p-Value | |
|---|---|---|---|---|---|
| Sex | Female | 51 | 49 | 2 | 0.0067 |
| Male | 150 | 121 | 29 | ||
| Age (years) | <40 years | 3 | 2 | 1 | 0.4705 |
| 40–60 years | 66 | 57 | 9 | ||
| >60 years | 132 | 111 | 21 | ||
| Diabetes type | 1 | 41 | 40 | 1 | 0.0157 |
| 2 | 158 | 128 | 30 | ||
| 3 | 2 | 2 | 0 | ||
| Diabetes duration | <5 years | 12 | 10 | 2 | 0.1196 |
| 5–9 years | 29 | 28 | 1 | ||
| >9 years | 160 | 132 | 28 | ||
| BMI (kg/m2) | Normal weight | 42 | 24 | 18 | <<0.0001 |
| Overweight | 54 | 46 | 8 | ||
| Obesity class 1 | 56 | 54 | 2 | ||
| Obesity class 2 | 33 | 33 | 0 | ||
| Obesity class 3 | 16 | 13 | 3 | ||
| HbA1c level (mmol/mol) | <7 | 56 | 42 | 14 | 0.0835 |
| 7.1–7.9 | 35 | 31 | 4 | ||
| ≥8 | 110 | 97 | 13 | ||
| CRP (mg/L) | <5 | 82 | 66 | 16 | 0.1647 |
| 5–99 | 94 | 80 | 14 | ||
| ≥99 | 25 | 24 | 1 | ||
| MSSA (n = 170) | MRSA (n = 31) | p-Value | OR [95% CI] | |
|---|---|---|---|---|
| Osteitis (−) (n = 94) | 91 | 3 | <<0.0001 | 10.5 [3.075–35.858] |
| Osteitis (+) (n = 107) | 79 | 28 | ||
| osteitis <50% (n = 50) | 41 | 9 | 0.012 | 6.585 [1.694–25.603] |
| osteitis >50% (n = 57) | 38 | 19 | <<0.0001 | 14.615 [4.087–52.269] |
| Bacteria Co-Isolated with S. aureus | Osteitis (+) | Osteitis (−) | Total | p-Value | OR [95% CI] |
|---|---|---|---|---|---|
| (n = 107) | (n = 94) | (n = 201) | |||
| Gram-positive cocci | |||||
| Coagulase negative staphylococci | 7 | 9 | 16 | 0.4472 | 0.66 [0.24–1.85] |
| streptococci | 23 | 19 | 42 | 0.8633 | 1.08 [0.55–2.14] |
| Enterococci | 14 | 5 | 19 | 0.0891 | 2.68 [0.93–7.75] |
| Gram-negative bacilli | |||||
| Enterobacteriaceae | 45 | 35 | 80 | 0.5638 | 1.22 [0.69–2.16] |
| Non-fermenting bacilli | 13 | 11 | 24 | 1.0000 | 1.04 [0.44–2.45] |
| Anaerobes | |||||
| Non spore-forming anaerobes | 13 | 6 | 19 | 0.0510 | 2.9 [1.01–8.33] |
| Spore-forming anaerobes | 16 | 16 | 32 | 0.7038 | 0.86 [0.40–1.83] |
| Actinomyces sp. | 5 | 0 | 5 | 0.0620 | Inf [NaN–Inf] |
| Antibiotics Group Resistance | Osteitis (+) | Osteitis (−) | p-Value | OR [95% CI] |
|---|---|---|---|---|
| (n = 107) | (n = 94) | |||
| Beta-lactams | 74 | 69 | 0.0001 | 0.32 [0.18–0.57] |
| Macrolides | 32 | 40 | 0.0769 | 0.58 [0.32–1.03] |
| Lincosamides | 30 | 33 | 0.2907 | 0.72 [0.40–1.31] |
| Fluoroquinolones | 33 | 10 | 0.0005 | 3.75 [1.73–8.12] |
| Aminoglycosides | 2 | 0 | 0.4996 | Inf [NaN–Inf] |
| Tetracyclines | 2 | 0 | 0.4996 | Inf [NaN–Inf] |
| Glycopeptides | 0 | 0 | 1.0000 | Inf [NaN–Inf] |
| Variable | Number of Patients with DFU Infected S. aureus (n = 201) | Osteitis (+) (n = 107) | Osteitis (−) (n = 94) | p-Value | |
|---|---|---|---|---|---|
| Sex | Female | 51 | 23 | 28 | 0.1963 |
| Male | 150 | 84 | 66 | ||
| Age (years) | <40 years | 3 | 1 | 2 | 0.8603 |
| 40–60 years | 66 | 35 | 31 | ||
| >60 years | 132 | 71 | 61 | ||
| Diabetes type | 1 | 41 | 20 | 21 | 0.4969 |
| 2 | 158 | 85 | 73 | ||
| 3 | 2 | 2 | 0 | ||
| Diabetes duration | <5 years | 12 | 6 | 6 | 0.9644 |
| 5–9 years | 29 | 15 | 14 | ||
| >9 years | 160 | 86 | 74 | ||
| BMI (kg/m2) | Normal weight | 42 | 35 | 7 | 0.0002 |
| Overweight | 54 | 28 | 26 | ||
| Obesity class 1 | 56 | 23 | 33 | ||
| Obesity class 2 | 33 | 13 | 20 | ||
| Obesity class 3 | 16 | 8 | 8 | ||
| HbA1c level (mmol/mol) | <7 | 56 | 33 | 23 | 0.4886 |
| 7.1–7.9 | 35 | 16 | 19 | ||
| ≥8 | 110 | 58 | 52 | ||
| CRP (mg/L) | <5 | 82 | 39 | 43 | 0.1038 |
| 5–99 | 94 | 50 | 44 | ||
| ≥99 | 25 | 18 | 7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańkowska, M.; Garbacz, K.; Korzon-Burakowska, A.; Bronk, M.; Skotarczak, M.; Szymańska-Dubowik, A. Microbiological, Clinical and Radiological Aspects of Diabetic Foot Ulcers Infected with Methicillin-Resistant and -Sensitive Staphylococcus aureus. Pathogens 2022, 11, 701. https://doi.org/10.3390/pathogens11060701
Stańkowska M, Garbacz K, Korzon-Burakowska A, Bronk M, Skotarczak M, Szymańska-Dubowik A. Microbiological, Clinical and Radiological Aspects of Diabetic Foot Ulcers Infected with Methicillin-Resistant and -Sensitive Staphylococcus aureus. Pathogens. 2022; 11(6):701. https://doi.org/10.3390/pathogens11060701
Chicago/Turabian StyleStańkowska, Maria, Katarzyna Garbacz, Anna Korzon-Burakowska, Marek Bronk, Monika Skotarczak, and Anna Szymańska-Dubowik. 2022. "Microbiological, Clinical and Radiological Aspects of Diabetic Foot Ulcers Infected with Methicillin-Resistant and -Sensitive Staphylococcus aureus" Pathogens 11, no. 6: 701. https://doi.org/10.3390/pathogens11060701
APA StyleStańkowska, M., Garbacz, K., Korzon-Burakowska, A., Bronk, M., Skotarczak, M., & Szymańska-Dubowik, A. (2022). Microbiological, Clinical and Radiological Aspects of Diabetic Foot Ulcers Infected with Methicillin-Resistant and -Sensitive Staphylococcus aureus. Pathogens, 11(6), 701. https://doi.org/10.3390/pathogens11060701






