Pseudomonas aeruginosa Infections in Cancer Patients
Abstract
1. Introduction
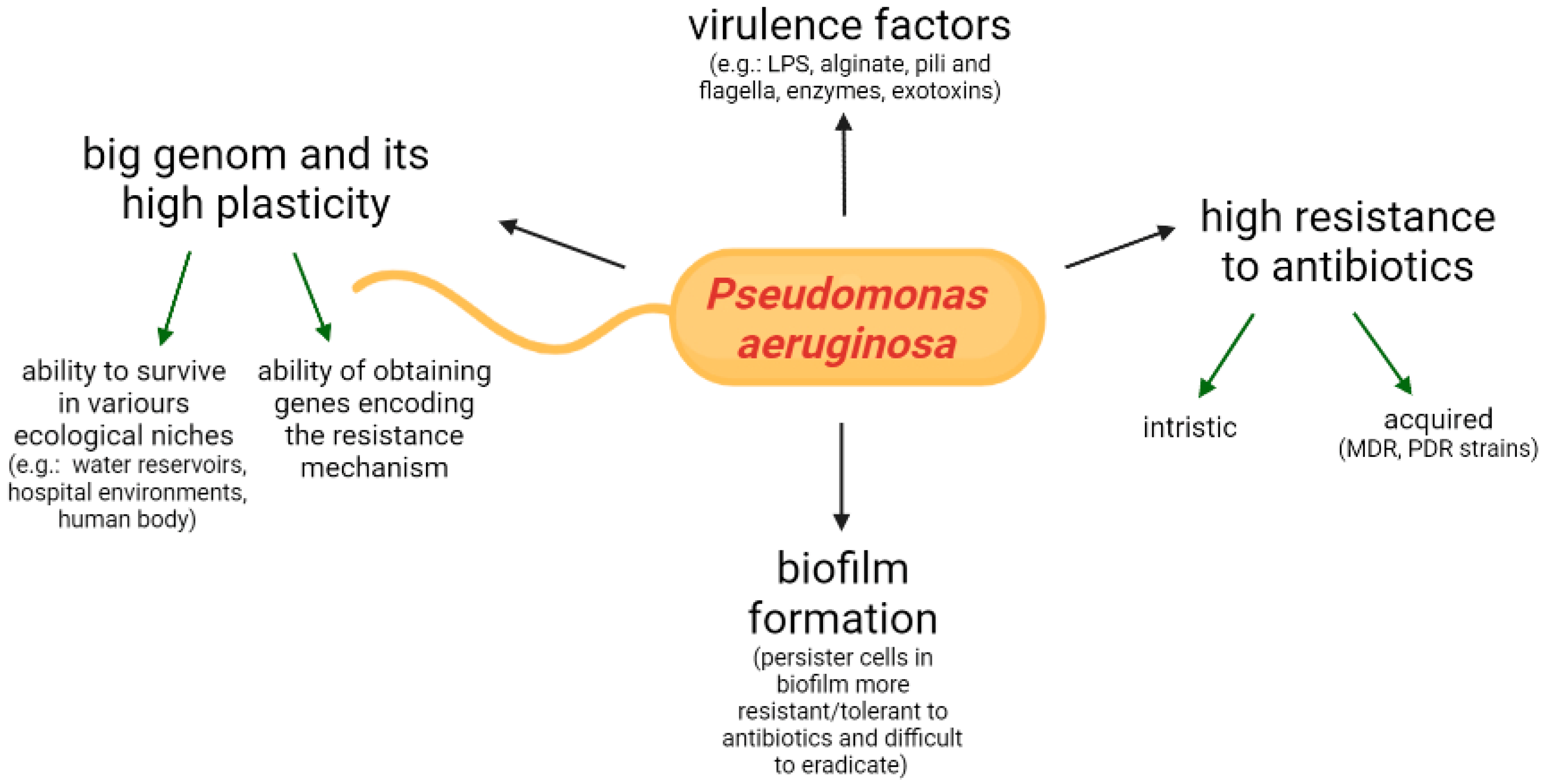
2. Features of Microorganism, Virulence Determinants
3. P. aeruginosa Mechanisms of Resistance
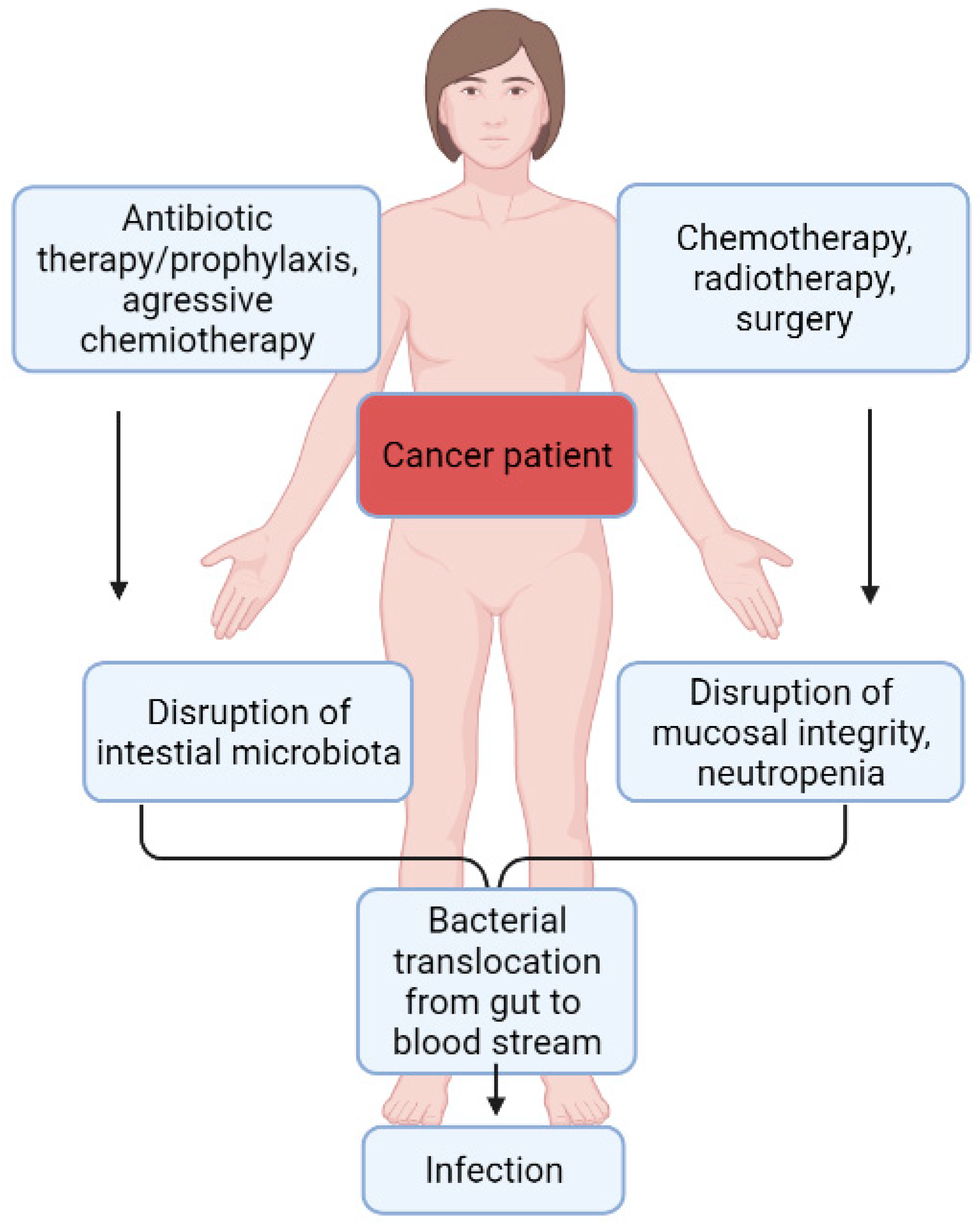
4. P. aeruginosa Colonization
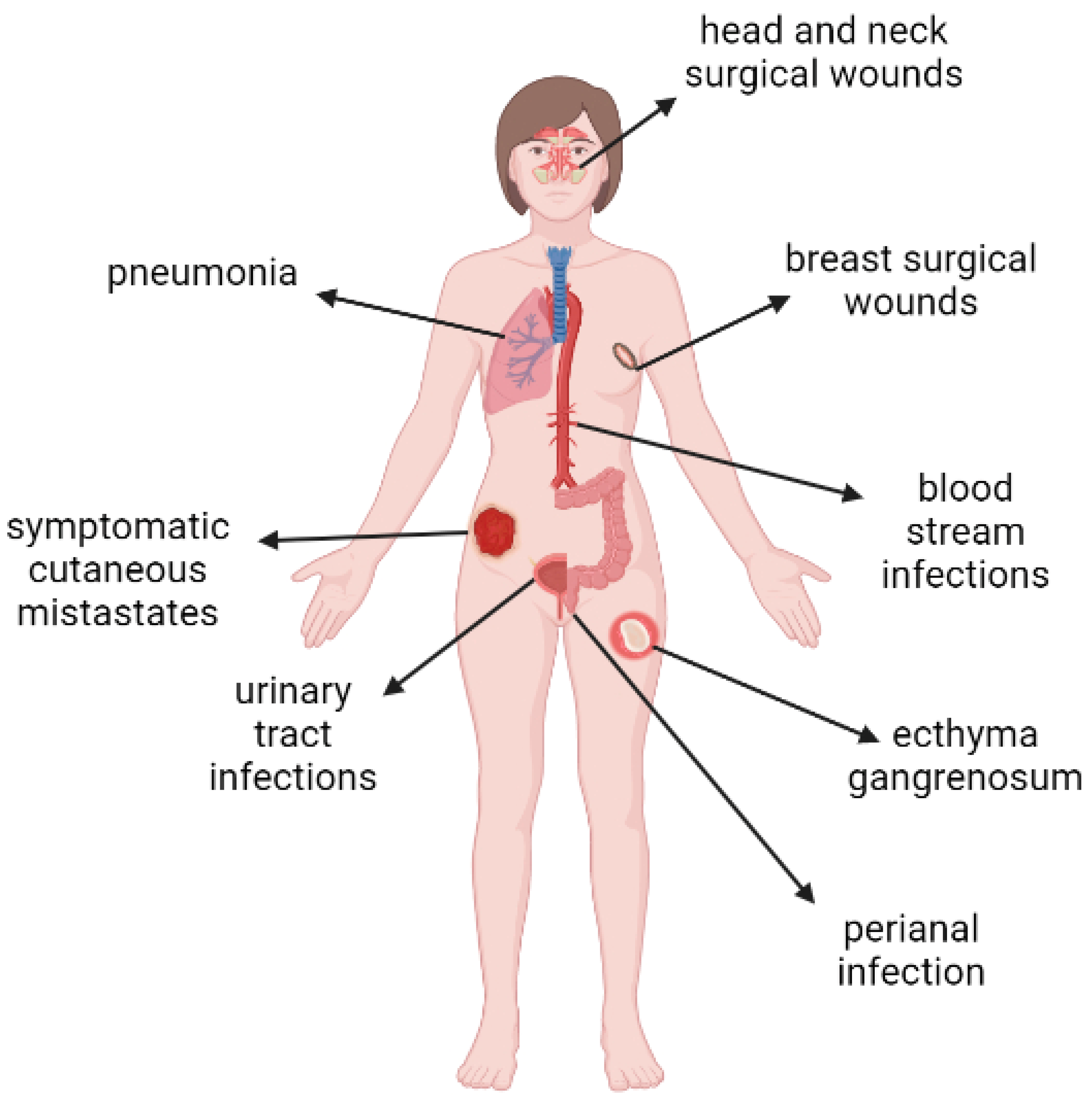
5. P. aeruginosa as an Etiological Factor of Infections in Cancer Patients
6. The Old and the New Antibiotics against P. aeruginosa
7. Treatment of P. aeruginosa Infections in Cancer Patients
8. Prevention of P. aeruginosa Infection in Cancer Patients
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G., Jr.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 5 (Suppl. 5), S335–S339. [Google Scholar] [CrossRef]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Catalina Gomez-Puerto, M.; Calum, H.; et al. The environmental occurrence of Pseudomonas aeruginosa. Apmis 2020, 128, 220–231. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.; Hébraud, M.; Dapkevicius, M.; Maltez, L.; Pereira, J.E.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Genomic and Metabolic Characteristics of the Pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 12892. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Shimizu, M.; Moriyama, K.; Wiener-Kronish, J.P. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: A review. Crit. Care 2014, 18, 668. [Google Scholar] [CrossRef]
- Faure, E.; Kwong, K.; Nguyen, D. Pseudomonas aeruginosa in Chronic Lung Infections: How to Adapt Within the Host? Front. Immunol. 2018, 9, 2416. [Google Scholar] [CrossRef]
- Wolf, P.; Elsässer-Beile, U. Pseudomonas exotoxin A: From virulence factor to anti-cancer agent. Int. J. Med. Microbiol. 2009, 299, 161–176. [Google Scholar] [CrossRef]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Dalmolin, T.; Lima-Morales, D.; Barth, A. Plasmid-mediated Colistin Resistance: What Do We Know? J. Infect. 2018, 1, 16–22. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Masoud, S.M.; Mohamed, D.S.; Waly, N.G.; Shafik, E.A.; Mohareb, D.A.; Elkady, A.; Elbadr, M.M.; Hetta, H.F. Prevalence and Some Possible Mechanisms of Colistin Resistance Among Multidrug-Resistant and Extensively Drug-Resistant. Infect. Drug Resist. 2020, 13, 323–332. [Google Scholar] [CrossRef]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Ohmagari, N.; Hanna, H.; Graviss, L.; Hackett, B.; Perego, C.; Gonzalez, V.; Dvorak, T.; Hogan, H.; Hachem, R.; Rolston, K.; et al. Risk factors for infections with multidrug-resistant Pseudomonas aeruginosa in patients with cancer. Cancer 2005, 104, 205–212. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef]
- Okuda, J.; Hayashi, N.; Okamoto, M.; Sawada, S.; Minagawa, S.; Yano, Y.; Gotoh, N. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na,K-ATPase regulator, FXYD3. Infect. Immun. 2010, 78, 4511–4522. [Google Scholar] [CrossRef]
- Gómez-Zorrilla, S.; Camoez, M.; Tubau, F.; Cañizares, R.; Periche, E.; Dominguez, M.A.; Ariza, J.; Peña, C. Prospective observational study of prior rectal colonization status as a predictor for subsequent development of Pseudomonas aeruginosa clinical infections. Antimicrob. Agents Chemother. 2015, 59, 5213–5219. [Google Scholar] [CrossRef]
- Mendes, E.T.; Salomão, M.C.; Tomichi, L.M.; Oliveira, M.S.; Graça, M.; Rossi, F.; Sapadao, F.; Guimarães, T.; Rocha, V.; Costa, S.F. Effectiveness of surveillance cultures for high priority multidrug-resistant bacteria in hematopoietic stem cell transplant units. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e77. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Klasa, A.; Piekarska, A.; Prejzner, W.; Bieniaszewska, M.; Hellmann, A. Colonization with multidrug-resistant bacteria increases the risk of complications and a fatal outcome after allogeneic hematopoietic cell transplantation. Ann. Hematol. 2018, 97, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Tuschak, C.; Nickel, S.; Krupa, E.; Lehner-Reindl, V.; Höller, C. Prevalence, antimicrobial susceptibility, and genetic diversity of Pseudomonas aeruginosa as intestinal colonizer in the community. Infect. Dis. 2015, 47, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roldán, L.; Bellés, A.; Bueno, J.; Azcona-Gutiérrez, J.M.; Rojo-Bezares, B.; Torres, C.; Castillo, F.J.; Sáenz, Y.; Seral, C. Pseudomonas aeruginosa Isolates from Spanish Children: Occurrence in Faecal Samples, Antimicrobial Resistance, Virulence, and Molecular Typing. Biomed Res. Int. 2018, 2018, 8060178. [Google Scholar] [CrossRef]
- Gómez-Zorrilla, S.; Camoez, M.; Tubau, F.; Periche, E.; Cañizares, R.; Dominguez, M.A.; Ariza, J.; Peña, C. Antibiotic pressure is a major risk factor for rectal colonization by multidrug-resistant Pseudomonas aeruginosa in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 5863–5870. [Google Scholar] [CrossRef]
- Andremont, A.; Marang, B.; Tancrède, C.; Baume, D.; Hill, C. Antibiotic treatment and intestinal colonization by Pseudomonas aeruginosa in cancer patients. Antimicrob. Agents Chemother. 1989, 33, 1400–1402. [Google Scholar] [CrossRef]
- Willmann, M.; Klimek, A.M.; Vogel, W.; Liese, J.; Marschal, M.; Autenrieth, I.B.; Peter, S.; Buhl, M. Clinical and treatment-related risk factors for nosocomial colonisation with extensively drug-resistant Pseudomonas aeruginosa in a haematological patient population: A matched case control study. BMC Infect. Dis. 2014, 14, 650. [Google Scholar] [CrossRef][Green Version]
- Yang, J.J.; Wang, J.T.; Cheng, A.; Chuang, Y.C.; Sheng, W.H. Impact of broad-spectrum antimicrobial treatment on the ecology of intestinal flora. J. Microbiol. Immunol. Infect. 2018, 51, 681–687. [Google Scholar] [CrossRef]
- Alagna, L.; Palomba, E.; Mangioni, D.; Bozzi, G.; Lombardi, A.; Ungaro, R.; Castelli, V.; Prati, D.; Vecchi, M.; Muscatello, A.; et al. Multidrug-Resistant Gram-Negative Bacteria Decolonization in Immunocompromised Patients: A Focus on Fecal Microbiota Transplantation. Int. J. Mol. Sci. 2020, 21, 5619. [Google Scholar] [CrossRef]
- Napeñas, J.J.; Brennan, M.T.; Bahrani-Mougeot, F.K.; Fox, P.C.; Lockhart, P.B. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 48–59. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Itoh, K. Significance of Pseudomonas aeruginosa colonization of the gastrointestinal tract. Intern. Med. 2003, 42, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Markou, P.; Apidianakis, Y. Pathogenesis of intestinal Pseudomonas aeruginosa infection in patients with cancer. Front. Cell. Infect. Microbiol. 2014, 3, 115. [Google Scholar] [CrossRef]
- Nesher, L.; Rolston, K.V.; Shah, D.P.; Tarrand, J.T.; Mulanovich, V.; Ariza-Heredia, E.J.; Chemaly, R.F. Fecal colonization and infection with Pseudomonas aeruginosa in recipients of allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2015, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, S.L.; Hancock, R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef]
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Archana Bhat, K.; Kotian, K.H. Bacterial Infection among Cancer Patients: Analysis of Isolates and Antibiotic Sensitivity Pattern. Int. J. Microbiol. 2021, 2021, 8883700. [Google Scholar] [CrossRef] [PubMed]
- Tofas, P.; Samarkos, M.; Piperaki, E.T.; Kosmidis, C.; Triantafyllopoulou, I.D.; Kotsopoulou, M.; Pantazatou, A.; Perlorentzou, S.; Poulli, A.; Vagia, M.; et al. Pseudomonas aeruginosa bacteraemia in patients with hematologic malignancies: Risk factors, treatment and outcome. Diagn. Microbiol. Infect. Dis. 2017, 88, 335–341. [Google Scholar] [CrossRef]
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Marchesi, F.; Cavallo, I.; Toma, L.; Sivori, F.; Papa, E.; Spadea, A.; Cafarella, G.; Terrenato, I.; Prignano, G.; et al. The Impact of Bacterial Biofilms on End-Organ Disease and Mortality in Patients with Hematologic Malignancies Developing a Bloodstream Infection. Microbiol. Spectr. 2021, 9, e0055021. [Google Scholar] [CrossRef]
- Chen, S.; Lin, K.; Li, Q.; Luo, X.; Xiao, M.; Chen, M.; Zhu, H.; Chen, Y.; Wu, X.; Zeng, Y.; et al. A practical update on the epidemiology and risk factors for the emergence and mortality of bloodstream infections from real-world data of 3014 hematological malignancy patients receiving chemotherapy. J. Cancer 2021, 12, 5494–5505. [Google Scholar] [CrossRef]
- Yao, J.-F.; Li, N.; Jiang, J. Clinical Characteristics of Bloodstream Infections in Pediatric Acute Leukemia: A Single-center Experience with 231 Patients. Chin. Med. J. 2017, 130, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Gudiol, C.; Garcia-Vidal, C.; Ardanuy, C.; Carratalà, J. Bloodstream infections in patients with solid tumors: Epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine 2014, 93, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, I.; Abi-Said, D.; Bodey, G.P.; Rolston, K.V.; Tarrand, J.J.; Samonis, G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: Retrospective analysis of 245 episodes. Arch. Intern. Med. 2000, 160, 501–509. [Google Scholar] [CrossRef]
- Islas-Muñoz, B.; Volkow-Fernández, P.; Ibanes-Gutiérrez, C.; Villamar-Ramírez, A.; Vilar-Compte, D.; Cornejo-Juárez, P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int. J. Infect. Dis. 2018, 71, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, B.K.; Kim, S.k.; Han, S.B.; Lee, J.W.; Lee, D.-G.; Chung, N.-G.; Cho, B.; Jeong, D.C.; Kang, J.H. Clinical characteristics and outcomes of Pseudomonas aeruginosa bacteremia in febrile neutropenic children and adolescents with the impact of antibiotic resistance: A retrospective study. BMC Infect. Dis. 2017, 17, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, Q.; Liu, L.; Ma, R.; Chen, J.; Shen, Y.; Zhu, G.; Jiang, E.; Mi, Y.; Han, M.; et al. Risk Factors and Outcomes of Antibiotic-resistant Pseudomonas aeruginosa Bloodstream Infection in Adult Patients With Acute Leukemia. Clin. Infect. Dis. 2020, 71, S386–S393. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nadal, G.; Puerta-Alcalde, P.; Gudiol, C.; Cardozo, C.; Albasanz-Puig, A.; Marco, F.; Laporte-Amargós, J.; Moreno-García, E.; Domingo-Doménech, E.; Chumbita, M.; et al. Inappropriate Empirical Antibiotic Treatment in High-risk Neutropenic Patients With Bacteremia in the Era of Multidrug Resistance. Clin. Infect. Dis. 2020, 70, 1068–1074. [Google Scholar] [CrossRef]
- Gudiol, C.; Albasanz-Puig, A.; Laporte-Amargós, J.; Pallarès, N.; Mussetti, A.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; et al. Clinical Predictive Model of Multidrug Resistance in Neutropenic Cancer Patients with Bloodstream Infection Due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e02494-19. [Google Scholar] [CrossRef]
- Sampson, M.M.; Nanjappa, S.; Greene, J.N. Mucositis and oral infections secondary to gram negative rods in patients with prolonged neutropenia. IDCases 2017, 9, 101–103. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Eckardt, P.A.; Lemos-Ramirez, J.C.; Niu, J. Ecthyma Gangrenosum of Scrotum in a Patient with Neutropenic Fever: A Case Report. Am. J. Case Rep. 2019, 20, 1369–1372. [Google Scholar] [CrossRef]
- Martínez-Longoria, C.A.; Rosales-Solis, G.M.; Ocampo-Garza, J.; Guerrero-González, G.A.; Ocampo-Candiani, J. Ecthyma gangrenosum: A report of eight cases. An. Bras. Dermatol. 2017, 92, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Cheng, A.; Huang, S.Y.; Sheng, W.H.; Liu, J.H.; Ko, B.S.; Yao, M.; Chou, W.C.; Lin, H.C.; Chen, Y.C.; et al. Clinical and microbiological characteristics of perianal infections in adult patients with acute leukemia. PLoS ONE 2013, 8, e60624. [Google Scholar] [CrossRef] [PubMed]
- Morita, F.; Hirai, Y.; Suzuki, K.; Uehara, Y.; Mitsuhashi, K.; Takahashi, M.; Watanabe, S.; Naito, T. The First Case of Pseudomonas aeruginosa Bacteremic Pneumonia in a Cancer Patient Receiving Pegfilgrastim. Intern. Med. 2017, 56, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef]
- Szymankiewicz, M.; Nowikiewicz, T.; Biedka, M. Significance of Infections in Implant Loss After Breast Reconstruction in the Course of Breast Cancer Treatment. Pol. J. Microbiol. 2019, 68, 343–351. [Google Scholar] [CrossRef]
- Ramos-Zayas, A.; López-Medrano, F.; Urquiza-Fornovi, I.; Zubillaga, I.; Gutiérrez, R.; Sánchez-Aniceto, G.; Acero, J.; Almeida, F.; Galdona, A.; Morán, M.J.; et al. The Impact of Healthcare-Associated Infections in Patients Undergoing Oncological Microvascular Head and Neck Reconstruction: A Prospective Multicentre Study. Cancers 2021, 13, 2109. [Google Scholar] [CrossRef]
- Virgen, C.A.; Barker, C.A.; Lacouture, M.E. The microbial flora of clinically infected cutaneous metastases: A retrospective study. Clin. Exp. Dermatol. 2020, 45, 722–726. [Google Scholar] [CrossRef]
- Cornejo-Juárez, P.; González-Oros, I.; Mota-Castañeda, P.; Vilar-Compte, D.; Volkow-Fernández, P. Ventilator-associated pneumonia in patients with cancer: Impact of multidrug resistant bacteria. World J. Crit. Care Med. 2020, 9, 43–53. [Google Scholar] [CrossRef]
- Gudiol, C.; Durà-Miralles, X.; Aguilar-Company, J.; Hernández-Jiménez, P.; Martínez-Cutillas, M.; Fernandez-Avilés, F.; Machado, M.; Vázquez, L.; Martín-Dávila, P.; de Castro, N.; et al. Co-infections and superinfections complicating COVID-19 in cancer patients: A multicentre, international study. J. Infect. 2021, 83, 306–313. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Sepulcri, C.; Giacobbe, D.R.; Peghin, M. Treatment of Bloodstream Infections Due to Gram-Negative Bacteria with Difficult-to-Treat Resistance. Antibiotics 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Skalweit, M.J. Profile of ceftolozane/tazobactam and its potential in the treatment of complicated intra-abdominal infections. Drug Des Devel Ther 2015, 9, 2919–2925. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamma, P.D.; Hsu, A.J. Defining the Role of Novel β-Lactam Agents That Target Carbapenem-Resistant Gram-Negative Organisms. J. Pediatric. Infect. Dis. Soc. 2019, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.G.; Shortridge, D.; Sader, H.S.; Castanheira, M. Activity of Meropenem-Vaborbactam against Bacterial Isolates Causing Pneumonia in Patients in U.S. Hospitals during 2014 to 2018. Antimicrob. Agents Chemother. 2020, 64, e02177-e19. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Chen, L.; Hao, B.; Press, E.G.; Alkrouk, A.; Potoski, B.A.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Evaluation of the In Vitro Activity of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Meropenem-Resistant Pseudomonas aeruginosa Isolates. Antimicrob. Agents Chemother. 2016, 60, 3227–3231. [Google Scholar] [CrossRef]
- Grupper, M.; Sutherland, C.; Nicolau, D.P. Multicenter Evaluation of Ceftazidime-Avibactam and Ceftolozane-Tazobactam Inhibitory Activity against Meropenem-Nonsusceptible Pseudomonas aeruginosa from Blood, Respiratory Tract, and Wounds. Antimicrob. Agents Chemother. 2017, 61, e00875-e17. [Google Scholar] [CrossRef]
- Mirza, H.C.; Hortaç, E.; Koçak, A.A.; Demirkaya, M.H.; Yayla, B.; Güçlü, A.; Başustaoğlu, A. In vitro activity of ceftolozane-tazobactam and ceftazidime-avibactam against clinical isolates of meropenem-non-susceptible Pseudomonas aeruginosa: A two-centre study. J. Glob. Antimicrob. Resist. 2020, 20, 334–338. [Google Scholar] [CrossRef]
- Shortridge, D.; Pfaller, M.A.; Streit, J.M.; Flamm, R.K. Antimicrobial activity of ceftolozane/tazobactam tested against contemporary (2015-2017) Pseudomonas aeruginosa isolates from a global surveillance programme. J. Glob. Antimicrob. Resist. 2020, 21, 60–64. [Google Scholar] [CrossRef]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Skłodowski, K.; Król, G.; Zakrzewska, M.; Czarnowski, M.; Kot, P.; Fortunka, K.; Góźdź, S.; et al. New β-Lactam Antibiotics and Ceragenins - A Study to Assess Their Potential in Treatment of Infections Caused by Multidrug-Resistant Strains of Pseudomonas aeruginosa. Infect. Drug Resist. 2021, 14, 5681–5698. [Google Scholar] [CrossRef]
- Saran, O.; Sulik-Tyszka, B.; Basak, G.W.; Wróblewska, M.M. Activity of Ceftolozane/Tazobactam Against Gram-Negative Rods of the Family Enterobacteriaceae and Pseudomonas Spp. Isolated from Onco-Hematological Patients Hospitalized in a Clinical Hospital in Poland. Med. Sci. Monit. 2019, 25, 305–311. [Google Scholar] [CrossRef]
- Streling, A.P.; Al Obaidi, M.M.; Lainhart, W.D.; Zangeneh, T.; Khan, A.; Dinh, A.Q.; Hanson, B.; Arias, C.A.; Miller, W.R. Evolution of Cefiderocol Non-Susceptibility in Pseudomonas aeruginosa in a Patient Without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 2021, 73, e4472–e4474. [Google Scholar] [CrossRef] [PubMed]
- Emeraud, C.; Escaut, L.; Boucly, A.; Fortineau, N.; Bonnin, R.A.; Naas, T.; Dortet, L. Aztreonam plus Clavulanate, Tazobactam, or Avibactam for Treatment of Infections Caused by Metallo-β-Lactamase-Producing Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2019, 63, e00010-19. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Reygaert, W.C. Gram Negative Bacteria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Almutairy, R.; Aljrarri, W.; Noor, A.; Elsamadisi, P.; Shamas, N.; Qureshi, M.; Ismail, S. Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics 2020, 9, 485. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: For Whom, When, and How. Front. Public Health 2019, 7, 151. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Cantón, R. New microbiological aspects of fosfomycin. Rev. Esp. Quimioter 2019, 32 (Suppl. 1), 8–18. [Google Scholar]
- Trecarichi, E.M.; Tumbarello, M. Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: Current epidemiology and clinical impact. Curr. Opin. Infect. Dis. 2014, 27, 200–210. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; de Castro, E.M.; Mata, E.; Biosca, M.; Custodio, A.; Espinosa, J.; Vázquez, E.G.; Henao, F.; Ayala de la Peña, F. SEOM clinical practice guideline: Management and prevention of febrile neutropenia in adults with solid tumors (2018). Clin. Transl. Oncol. 2019, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Walsh, T.J. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: Three major threats to hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12762. [Google Scholar] [CrossRef] [PubMed]
- Albasanz-Puig, A.; Gudiol, C.; Puerta-Alcalde, P.; Ayaz, C.M.; Machado, M.; Herrera, F.; Martín-Dávila, P.; Laporte-Amargós, J.; Cardozo, C.; Akova, M.; et al. Impact of the Inclusion of an Aminoglycoside to the Initial Empirical Antibiotic Therapy for Gram-Negative Bloodstream Infections in Hematological Neutropenic Patients: A Propensity-Matched Cohort Study (AMINOLACTAM Study). Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Buurma, V.; Shah, M.; Fahim, G. Evaluation of studies on extended versus standard infusion of beta-lactam antibiotics. Am J Health Syst Pharm 2019, 76, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef]
- Barreto, J.N.; Aitken, S.L.; Krantz, E.M.; Nagel, J.L.; Dadwal, S.S.; Seo, S.K.; Liu, C. Variation in Clinical Practice and Attitudes on Antibacterial Management of Fever and Neutropenia in Patients With Hematologic Malignancy: A Survey of Cancer Centers Across the United States. Open Forum Infect. Dis. 2022, 9, ofac005. [Google Scholar] [CrossRef]
- Averbuch, D.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Orasch, C.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: Guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica 2013, 98, 1836–1847. [Google Scholar] [CrossRef]
- Tan, B.H.; Guzman, M.R.T.; Donato, L.K.S.; Kalimuddin, S.; Lee, W.H.L.; Tan, A.L.; Wong, G.C. Impact of an alternating first-line antibiotics strategy in febrile neutropenia. PLoS ONE 2018, 13, e0208039. [Google Scholar] [CrossRef]
- Sidler, J.A.; Frei, R.; Tschudin-Sutter, S.; Dangel, M.; Battegay, M.; Weisser, M.; Passweg, J.; Widmer, A.F. Is admission screening for Pseudomonas aeruginosa useful in haematologic patients? A prospective study with 1310 patients. Clin. Microbiol. Infect. 2015, 21, 572-e1. [Google Scholar] [CrossRef][Green Version]
- Araoka, H.; Kimura, M.; Abe, M.; Takahashi, N.; Yoneyama, A. Appropriate sampling sites for the surveillance of multidrug-resistant Pseudomonas aeruginosa colonization. Jpn J. Infect. Dis. 2014, 67, 118–119. [Google Scholar] [CrossRef][Green Version]
- van Belkum, A.; Burnham, C.-A.D.; Rossen, J.W.A.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020, 18, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bucaneve, G.; Micozzi, A.; Menichetti, F.; Martino, P.; Dionisi, M.S.; Martinelli, G.; Allione, B.; D’Antonio, D.; Buelli, M.; Nosari, A.M.; et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N. Engl. J. Med. 2005, 353, 977–987. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Prevention and treatment of cancer-related infections. Version 1. 2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1457 (accessed on 7 June 2022).
- Mikulska, M.; Averbuch, D.; Tissot, F.; Cordonnier, C.; Akova, M.; Calandra, T.; Ceppi, M.; Bruzzi, P.; Viscoli, C. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J. Infect. 2018, 76, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Egan, G.; Robinson, P.D.; Martinez, J.P.D.; Alexander, S.; Ammann, R.A.; Dupuis, L.L.; Fisher, B.T.; Lehrnbecher, T.; Phillips, B.; Cabral, S.; et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Med. 2019, 8, 4536–4546. [Google Scholar] [CrossRef]
- Hakki, M.; Humphries, R.M.; Hemarajata, P.; Tallman, G.B.; Shields, R.K.; Mettus, R.T.; Doi, Y.; Lewis, J.S. Fluoroquinolone Prophylaxis Selects for Meropenem-nonsusceptible Pseudomonas aeruginosa in Patients With Hematologic Malignancies and Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2019, 68, 2045–2052. [Google Scholar] [CrossRef]
- Satlin, M.J.; Chen, L.; Douglass, C.; Hovan, M.; Davidson, E.; Soave, R.; La Spina, M.; Gomez-Arteaga, A.; van Besien, K.; Mayer, S.; et al. Colonization With Fluoroquinolone-Resistant Enterobacterales Decreases the Effectiveness of Fluoroquinolone Prophylaxis in Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2021, 73, 1257–1265. [Google Scholar] [CrossRef]
- Bow, E.J. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr. Opin. Infect. Dis. 2011, 24, 545–553. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, Z.; Riaz, M.; Zeshan, B.; Wattoo, J.I.; Aslam, M.N. Evaluation of Antibiotic Resistance and Virulence Genes among Clinical Isolates of Pseudomonas aeruginosa from Cancer Patients. Asian Pac. J. Cancer Prev. 2020, 21, 1333–1338. [Google Scholar] [CrossRef]

| Group of Antibiotics | Old | New |
|---|---|---|
| β-lactams | Ceftazidime | Ceftolozane/tazobactam |
| Cefepime | Ceftazidime/avibactam | |
| Piperacilin/tazobactam | Meropenem/vaborbactam | |
| Imipenem | Imipenem/relebactam | |
| Meropenem | Cefiderocol | |
| Aztreonam | ||
| Fluoroquinolones | Ciprofloxacin | - |
| Levofloxacin | ||
| Aminoglycosides | Amikacin | Plazomycin |
| Tobramycin | ||
| Polomyxins | Colistin | - |
| Phosphonates | Fosfomycin | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, P.; Durnaś, B.; Mańkowska, A.; Król, G.; Wollny, T.; Bucki, R. Pseudomonas aeruginosa Infections in Cancer Patients. Pathogens 2022, 11, 679. https://doi.org/10.3390/pathogens11060679
Paprocka P, Durnaś B, Mańkowska A, Król G, Wollny T, Bucki R. Pseudomonas aeruginosa Infections in Cancer Patients. Pathogens. 2022; 11(6):679. https://doi.org/10.3390/pathogens11060679
Chicago/Turabian StylePaprocka, Paulina, Bonita Durnaś, Angelika Mańkowska, Grzegorz Król, Tomasz Wollny, and Robert Bucki. 2022. "Pseudomonas aeruginosa Infections in Cancer Patients" Pathogens 11, no. 6: 679. https://doi.org/10.3390/pathogens11060679
APA StylePaprocka, P., Durnaś, B., Mańkowska, A., Król, G., Wollny, T., & Bucki, R. (2022). Pseudomonas aeruginosa Infections in Cancer Patients. Pathogens, 11(6), 679. https://doi.org/10.3390/pathogens11060679






