Comparative Efficacy of Chondrosterum purpureum and Chemical Herbicides for Control of Resprouts in Tanoak and Bay Laurel
Abstract
:1. Introduction
2. Results
2.1. Laboratory
2.1.1. Bay Laurel Isolate Selection
2.1.2. Identification of Fungi Collected from Stumps
2.2. Field Trials
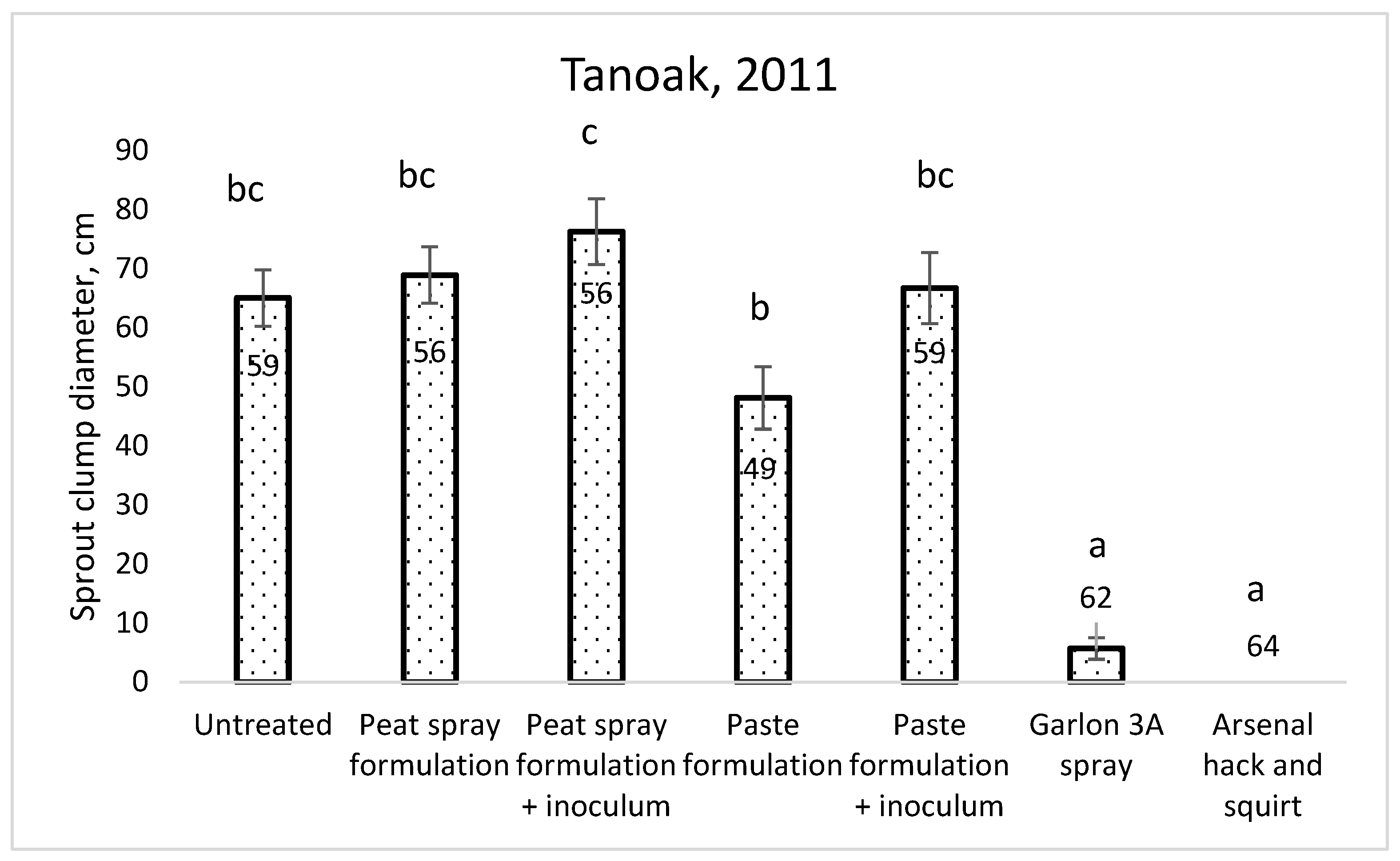
2.2.1. Tanoak
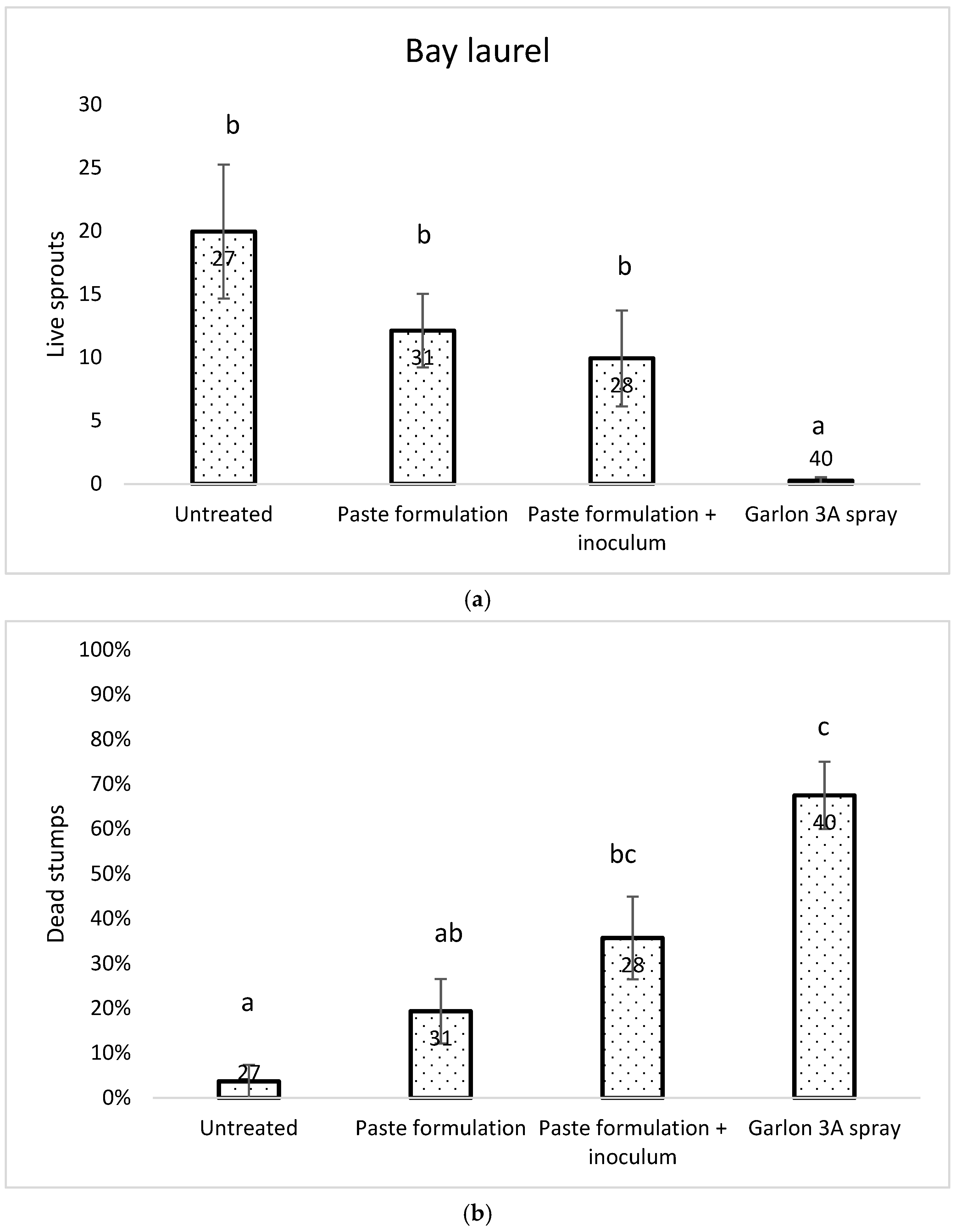
2.2.2. Bay Laurel
3. Discussion
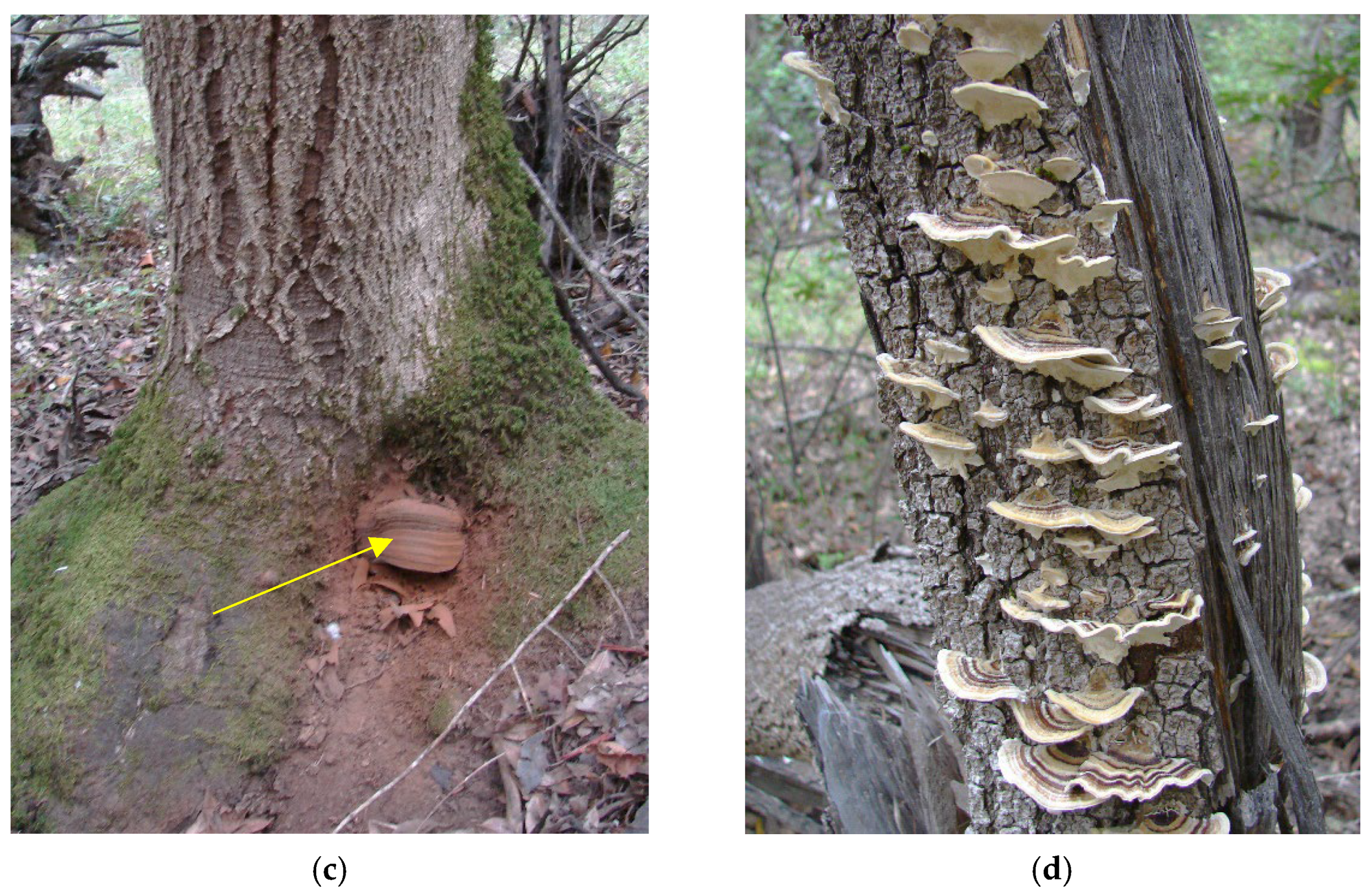
- Target tree species: the ability of C. purpureum to infect different tree species varies greatly because of the ability of a tree to resist the fungal infection and the physiological state of a tree species. There is evidence that the C. purpureum and other decay fungi can inflict the most severe damage to target tree species when the amount of soluble carbohydrates is the highest in wood (i.e., when the tree is growing intensively) [17,36]. Thus, it is important to time C. purpureum applications when the resistance of trees against the fungus is lowest for the best sprout control efficacy. Neither tanoak (Notholithocarpus densiflorus) nor bay laurel (Umbellularia californica) are listed as hosts for C. purpureum [19]. This may explain the delay of the response and possible resistance of tanoak and bay laurel to C. purpureum infection. Similar findings were observed on black locust (Robinia pseudoacacia) and sea buckthorn (Hippophae rhamnoides) because of either resistance of these trees to C. purpureum or a significantly delayed response to fungal infection [17];
- Virulence of selected C. purpureum isolates: The efficacy of C. purpureum isolates varies, with some isolates more virulent in preventing sprouting than others [22,37,38]. In laboratory conditions, the laccase manganese peroxidase enzyme production of C. purpureum isolates has been shown to correlate with birch sprout control efficacy in field conditions [39]. In addition, crossbreeding of C. purpureum has been performed to increase biocontrol efficiency [40]. In our field trial at the tanoak site, we used isolate C. purpureum PFC2139, which was selected on the basis of its virulence, expressed as canker size on red alder (Alnus rubra) seedlings in greenhouse conditions [37]. The selection of C. purpureum isolate PFC2139 in our tanoak field trials is not necessarily “ecologically fit” to control tanoak resprouts; hence, there is a potential to explore the use of native decay fungi occurring in southwest Oregon forests. This includes using Stereum hirsutum, closely related to C. purpureum. This fungus has already been shown to be an aggressive and common colonizer of wounds in hardwood tree species. In addition, S. hirsutum was also found in association with an unusual decline of tanoak sprouts [41], and therefore, it may possess the potential to control stump sprouting in tanoak. In the case of bay laurel field trials, the C. purpureum isolate was selected on the basis of the bay laurel stem inoculations under greenhouse conditions at the Canadian Forest Service, Pacific Forestry Centre. On the basis of the inoculation results, we selected isolate PFC2249 for further formulation using the same technology used for C. purpureum isolate PFC2139 in tanoak trials. As with tanoak trials, the C. purpureum isolate 2249 that was isolated from a canker on Prunus spp. for use in our bay laurel field trials is not necessarily “ecologically fit” to control bay laurel. This may explain the delay to the response or possible resistance of bay laurel to C. purpureum isolate PFC2249 infection and control of bay laurel resprouts; hence, there is a potential to explore the use of native decay fungi occurring in northern California forests. This includes using the most common decay fungi Ganoderma applanatum, Schizophyllum commune, and Trametes versicolor naturally occurring in bay laurel trials.
- Environmental conditions: In laboratory conditions, the optimum growth temperature for C. purpureum is 24–25 °C, but in extreme temperatures (e.g., 0 °C and over 35 °C), growth is severely inhibited [42]. However, in the field conditions, C. purpureum is able to withstand high temperatures (30–40 °C) [43]. Because of logistical reasons, we have treated tanoak and bay laurel stumps with C. purpureum in our field trials during the summer (temperatures were as high as 35 °C); hence, there was a delay in sprout control in the first 1–2 years post-treatment. Our results corroborate those of Hamberg and Hantula [44], who found a similar delay in the sprout efficacy control of C. purpureum (i.e., the growth of mycelia within the stump).
4. Materials and Methods
4.1. Laboratory
4.1.1. Bay Laurel Isolate Selection
4.1.2. Identification of Fungi Collected from Stumps
4.2. Field Trials
4.2.1. Tanoak
4.2.2. Bay Laurel
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, T.; Horta Jung, M.; Webber, J.F.; Kageyama, K.; Hieno, A.; Masuya, H.; Uematsu, S.; Pérez Sierra, A.; Harris, A.R.; Forster, J.; et al. The destructive tree pathogen Phytophthora ramorum originates from the Laurosilva forests of East Asia. J. Fungi 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, N.J.; LeBoldus, J.M.; Hamelin, R.C. Ecology and evolution of the Sudden Oak Death pathogen Phytophthora ramorum. Annu. Rev. 2019, 57, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.C.; Haas, S.E.; Kruskamp, N.; Dillon, W.W.; Swiecki, T.J.; Rizzo, D.M.; Frankel, S.J.; Meentemeyer, R.K. The Magnitude of Regional-Scale Tree Mortality Caused by the Invasive Pathogen Phytophthora ramorum. Earths Future 2020, 8, e2020EF001500. [Google Scholar] [CrossRef]
- Kanaskie, A.; Goheen, E.; Osterbauer, N.; McWilliams, M.; Hansen, E.; Sutton, W. Eradication of Phytophthora ramorum in Oregon-status after 6 years. In Proceedings of the Sudden Oak Death Third Science Symposium, Santa Rosa, CA, USA, 5–9 March 2007; Frankel, S.J., Kliejunas, J.T., Palmieri, K.M., Eds.; USDA, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2008; pp. 15–17. [Google Scholar]
- Hansen, E.M.; Sutton, W. Persistence of Phytophthora ramorum after eradication efforts in Oregon tanoak forests. In Proceedings of the Sudden Oak Death Second Science Symposium: The State of Our Knowledge, Monterey, CA, USA, 18–21 January 2005; Frankel, S.J., Shea, P.J., Haverty, M.I., Eds.; USDA, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2006; p. 516. [Google Scholar]
- Elliott, M.; Chastagner, G.; Shamoun, S.F.; Sumampong, G.; Goheen, E.; Kanaskie, A. Biological control of tanoak and bay laurel resprouts using the fungus Chondrostereum purpureum. In Proceedings of the Sudden Oak Death Fifth Science Symposium, Petaluma, CA, USA, 19–22 June 2012; Frankel, S.J., Palmieri, K.M., Alexander, J.M., Eds.; USDA, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2013; pp. 134–136. [Google Scholar]
- Davidson, J.M.; Shaw, C.G. Pathways of movement for Phytophthora ramorum, the causal agent of Sudden Oak Death. In Sudden Oak Death Online Symposium; USDA, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2003. Available online: www.apsnet.org/online/SOD (accessed on 2 December 2021). [CrossRef]
- Stein, W.I. Umbellularia californica. In Silvics of North America: 1. Conifers; 2: Hardwoods. Agriculture Handbook 654; Burns, R.M., Honkala, B.H., Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2l, p. 778. [Google Scholar]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood: Its Biology and Ecology; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Spiers, A.G.; Hopcroft, D.H. Factors affecting Chondrostereum purpureum infection in Salix. Eur. J. For. Pathol. 1988, 18, 257–2278. [Google Scholar] [CrossRef]
- Rayner, A.D.M. Fungal colonization of hardwood stumps from natural sources. II. Basidiomycetes. Trans. Br. Mycol. Soc. 1977, 69, 303–312. [Google Scholar] [CrossRef]
- Wall, R.E. Pathological effects of Chondrostereum purpureum in inoculated yellow birch and beech. Can. J. Plant Pathol. 1991, 13, 81–87. [Google Scholar] [CrossRef]
- Bishop, G.C. Infection of cherry trees and production of a toxin that causes foliar silvering by different isolates of Chondrostereum purpureum. Aust. J. Agric. Res. 1979, 30, 659–665. [Google Scholar] [CrossRef]
- Becker, E.; Shamoun, S.F.; Hintz, W.E. Efficacy and environmental fate of Chondrostereum purpureum used as a biological control for red alder (Alnus rubra). Biol. Control 2005, 33, 269–277. [Google Scholar] [CrossRef]
- Shamoun, S.F.; Hintz, W.E. Development and registration of Chondrostereum purpureum as a mycoherbicide for hardwood weeds in conifer reforestation sites and utility rights-of-way. In Proceedings of the IVth International Bioherbicides Workshop Programme and Abstracts, Glasgow, Scotland, 6–7 August 1998; University of Strathclyde: Glasgow, UK, 1998; p. 14. [Google Scholar]
- Hamberg, L.; Saksa, T.; Hantula, J. Role and function of Chondrostereum purpureum in biocontrol of trees. Appl. Microbiol. Biotechnol. 2021, 105, 431–440. [Google Scholar] [CrossRef]
- Lygis, V.; Bakys, R.; Burokiene, D.; Vasiliauskaite, I. Chondrostereum purpureum–based control of stump sprouting of seven hardwood species in Lithuania. Balt. For. 2012, 18, 41–55. [Google Scholar]
- Bellgard, S.E.; Johnson, V.W.; Than, D.J.; Anand, N.; Winks, C.J.; Ezeta, G.; Dodd, S.L. Use of the silver leaf fungus Chondrostereum purpureum for biological control of stump-sprouting, riparian weedy tree species in New Zealand. Aust. Plant Pathol. 2014, 43, 321–326. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 23 February 2022).
- Faedda, R.; Agosteo, G.E.; Schena, L.; Mosca, S.; Frisullo, S.; Magnano di San Leo, G.; Cacciola, S.O. Colletotrichum clavatum sp. nov. identified as the causal agent of olive anthracnose in Italy. Phytopathol. Mediterr. 2011, 50, 283–302. [Google Scholar]
- Shamoun, S.F. From laboratory bench to marketplace- The “Chontrol®” story: A legacy to government-academia-industry partnership. In Strategic Technology Management-Building Bridges between Science, Engineering and Business Management, 2nd ed.; Tesar, G., Anderson, S.W., Ghosh, S., Bramorski, T., Eds.; Imperial College Press: London, UK, 2008; pp. 429–437. [Google Scholar]
- Jobidon, R. Comparative efficacy of biological and chemical control of the vegetative reproduction in Betula papyrifera and Prunus pensylvanica. Biol. Control 1998, 11, 22–28. [Google Scholar] [CrossRef]
- de Jong, M.D. The BioChon story: Development of Chondrostereum purpureum to suppress stump sprouting in hardwoods. Mycologist 2000, 14, 58–62. [Google Scholar] [CrossRef]
- van der Meersschaut, D.; Lust, N. Comparison of mechanical, biological and chemical methods for controlling black cherry (Prunus serotina) in Flanders (Belgium). Silva Gandav. 1997, 62, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Hamberg, L.; Malmivaara-Lamsa, M.; Lofstrom, I.; Hantula, J. Effects of of a biocontrol agent Chondrostereum purpureum on sprouting of Sorbus aucuparia sprouting and Populus tremula after four growing seasons. BioControl 2014, 59, 125–137. [Google Scholar] [CrossRef]
- Bourdot, G.W.; Hurrell, G.A.; Gianotti, A.F.; Saville, D.J. Chondrostereum purpureum and Fusarium tumidum independently reduce regrowth in gorse (Ulex europaeus). Biocontrol Sci. Tech. 2006, 16, 307–327. [Google Scholar] [CrossRef]
- Strunz, G.M.; Bethell, R.; Dumas, M.T.; Boyonoski, N. On a new synthesis of sterpurene and the bioactivity of some related Chondrostereum purpureum sesquiterpene metabolites. Can. J. Chem. 1997, 75, 742–753. [Google Scholar] [CrossRef]
- Shamoun, S.F.; Wall, R.E. Characterization of Canadian isolates of Chondrostereum purpureum by protein contents, API ZYM and isozyme analyses. Europ. J. Forest Pathol. 1996, 26, 333–342. [Google Scholar] [CrossRef]
- Myari, K.; Okuno, T.; Sawai, K. Purification and properties of endopolygalactruronase I from Stereum purpureum, a factor inducing sliver-leaf symptoms on apple trees. Agric. Biol. Chem. 1985, 49, 111–118. [Google Scholar]
- Harper, G.J.; Comeau, P.G.; Hintz, W.E.; Wall, R.E.; Prasad, R.; Becker, E.B. Chondrostereum purpureum as a biological control agent in forest vegetation management. II. Efficacy on Sitka alder and aspen in western Canada. Can. J. For. Res. 1999, 29, 852–858. [Google Scholar] [CrossRef]
- Korzeniewicz, R.; Baranowska, M.; Kwaśna, H.; Niedbała, G.; Behnke-Borowczyk, J. Communities of Fungi in Black Cherry Stumps and Effects of Herbicide. Plants 2020, 9, 1126. [Google Scholar] [CrossRef]
- Wall, R.E. Fructification of Chondrostereum purpureum on hardwoods inoculated for biological control. Can. J. Plant Pathol. 1997, 19, 181–184. [Google Scholar] [CrossRef]
- Roy, V.; Dubeau, D.; Auger, I. Biological control of intolerant hardwood competition: Silvicultural efficacy of Chondrostereum purpureum and worker productivity in conifer plantations. For. Ecol. Manag. 2010, 259, 1571–1579. [Google Scholar] [CrossRef]
- Becker, E.B.; Ball, A.L.; Dumas, M.T.; Pitt, D.G.; Wall, R.E.; Hintz, W.E. Chondrostereum purpureum as a biological control agent in forest vegetation management. III. Infection survey of a national field trial. Can. J. For. Res. 1999, 29, 859–865. [Google Scholar] [CrossRef]
- Menkis, A.; Vasaitis, R.; Ostbrant, I.; Pliura, A.; Stenlid, J. Tests with wood decay fungi to control sprouting from cut stumps infected by Dutch elm disease. Balt. For. 2017, 23, 270–273. [Google Scholar]
- Stanislawek, S.D.; Long, P.G.; Davis, L.K. Sugar content of xylem sap and susceptibilityof willow to Chondrostereum purpureum. N. Z. J. Bot. 1987, 25, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.E.; Macey, D.; Sela, E. Virulence and interfertility of Chondrostereum purpureum isolates. Biol. Control 1996, 7, 205–211. [Google Scholar] [CrossRef]
- Hamberg, L.; Hantula, J. The efficacy ofsix elite isolates of the fungus Chondrostereum purpureum against the sprouting of European aspen. J. Environ. Manag. 2016, 171, 217–224. [Google Scholar] [CrossRef]
- Vartiamaki, H.; Maijala, P.; Uotila, A.; Hantula, J. Characterization of growth and enzyme production of Chondrostereum purpureum isolates and correlation of these characteristics with their capability to prevent sprouting of birch in field. Biol. Control 2008, 47, 46–54. [Google Scholar] [CrossRef]
- Hamberg, L.; Vartiamaki, H.; Hantula, J. Breeding increases the efficacy of Chondrostereum purpureum in the sprout control of birch. PLoS ONE 2015, 10, e0117381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.M.; Volger, D.R.; Mayhew, D. Unusual Decline of Tanoak Sprouts; USDA, Forest Service, Pacific Southwest Forest and Range Experiment Station: Albany, CA, USA, 1988.
- Ekramoddullah, A.; Shamoun, S.F.; Wall, R.E. Comparison of Canadian isolates of Chondrostereum purpureum with respect to temperature response, virulence and protein profiles. Can. J. Plant Pathol. 1996, 15, 7–13. [Google Scholar] [CrossRef]
- Dumas, M.T.; Wood, J.E.; Mitchell, E.G.; Boyonski, N.W. Control of stump sprouting of Populus tremuloides and P. gradidentata by inoculation with Chondrostereum purpureum. Biol. Control 1997, 10, 37–41. [Google Scholar] [CrossRef]
- Hamberg, L.; Hantula, J. The biocontrol efficacy of Chondrostereum purpureum is not sensitive to prevailing environmental conditions in boreal forests. For. Ecol. Manag. 2020, 456, 117646. [Google Scholar] [CrossRef]
- Adaskaveg, J.; University of California, Riverside, CA, USA. Personal communication, 2012.
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- White, H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; European Environment Agency: Copenhagen, Denmark, 2021; Available online: https://www.R-project.org/ (accessed on 6 October 2021).


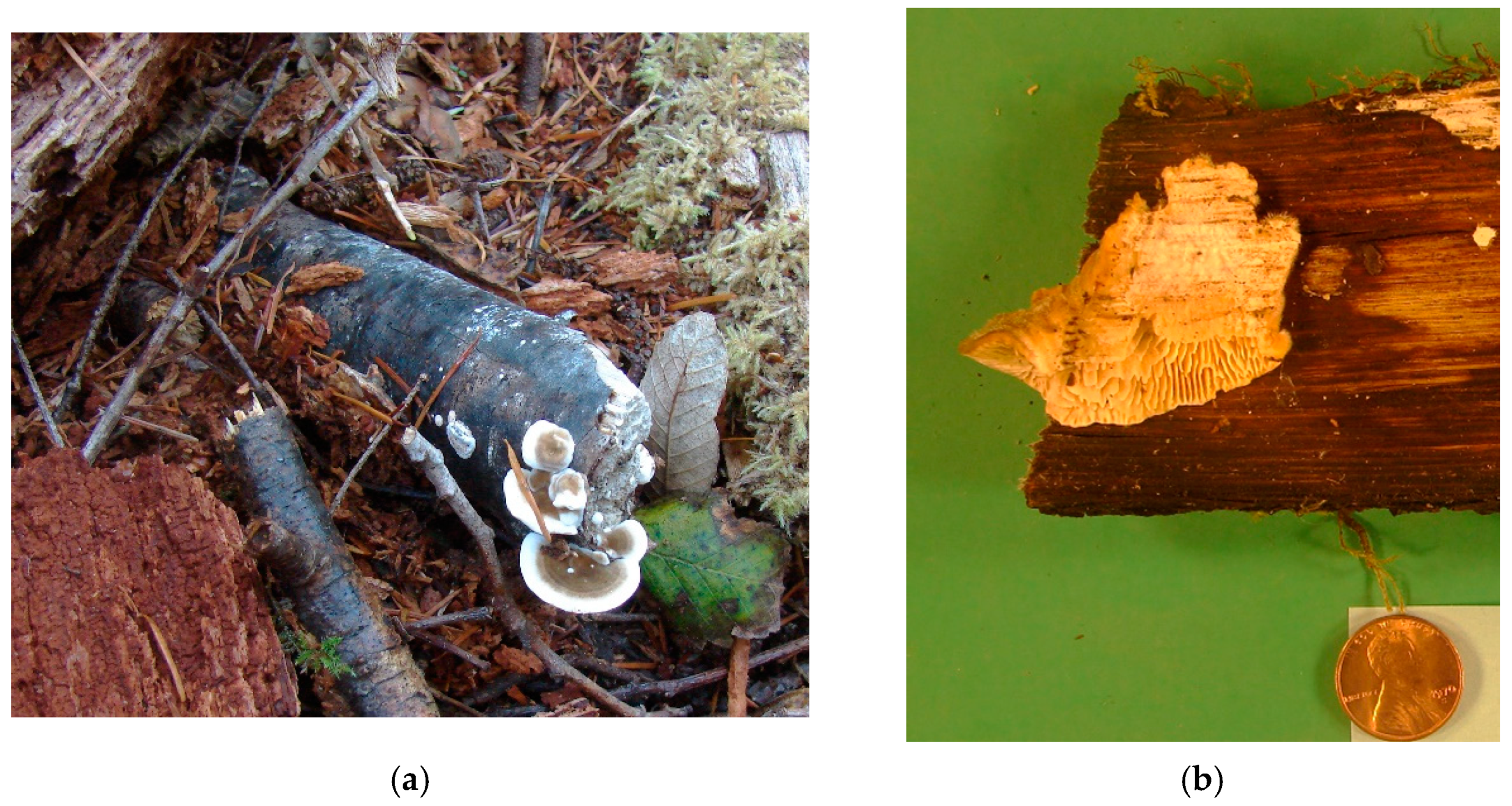
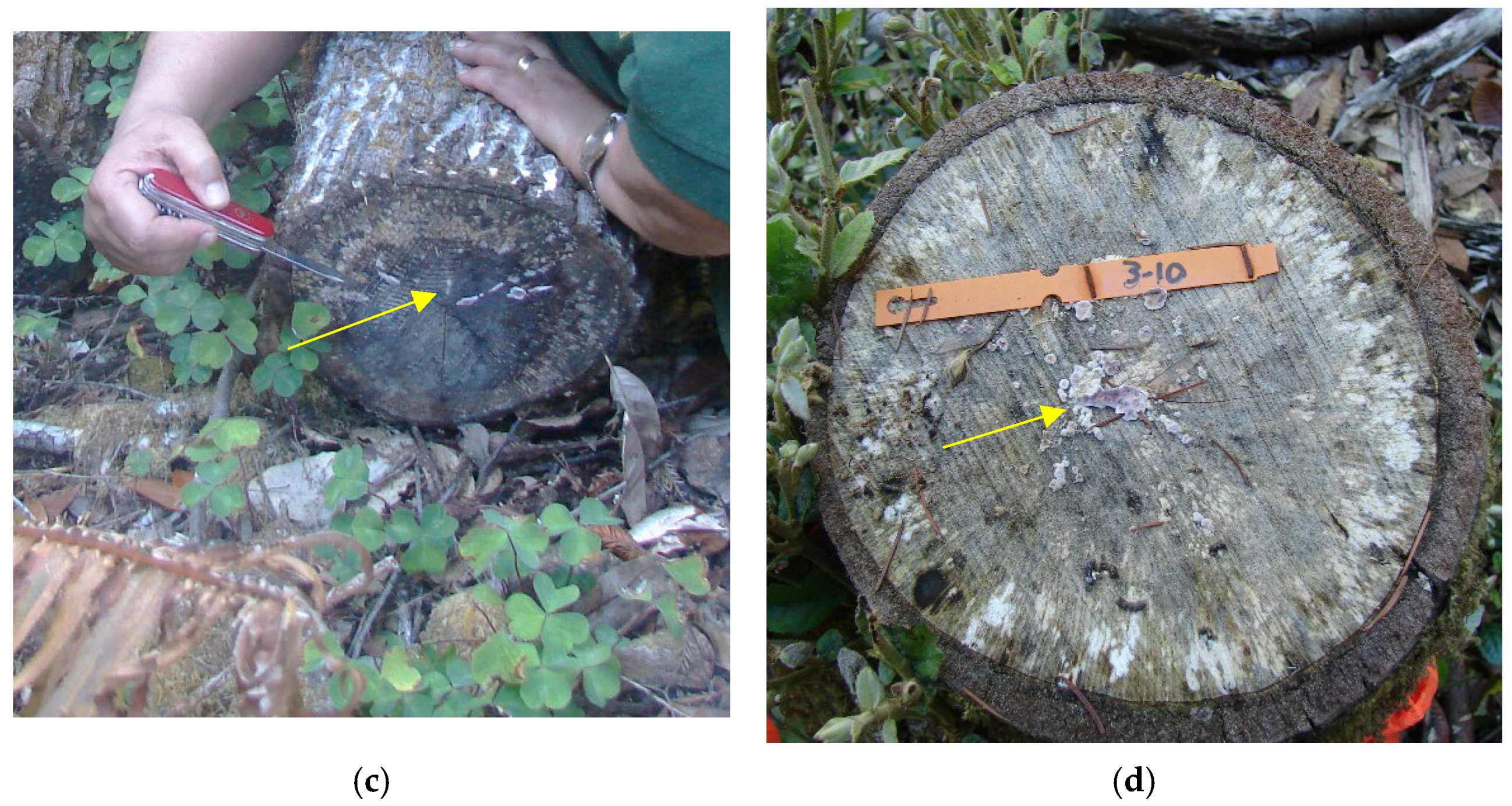


| 2010 | Sprouting Treatment |
| Live sprouts | 388.95, 0.000 *** |
| Height of tallest sprout | 366.41, 0.000 *** |
| Number of dead sprouts | 65.129, 0.000 *** |
| Deer browse | 117, 0.000 *** |
| Stump diameter | 4.3668, 0.6272 |
| 2011 | Sprouting Treatment |
| Live sprouts | 286.97, 0.000 *** |
| Sprout clump diam | 516.51, 0.000 *** |
| Height of tallest sprout | 485.06, 0.000 *** |
| Number of dead sprouts | 114.58, 0.000 *** |
| 2014 | Sprouting Treatment |
| Live sprouts | 23.101, 0.001 ** |
| Height of tallest sprout | 24.711, 0.004 ** |
| Number of dead sprouts | 28.818, 0.000 *** |
| Fungi | 20.952, 0.002 ** |
| 2014 | Sprouting Treatment | Cutting Treatment | Interaction |
| Live sprouts | 30.7640, 0.000 *** | 4.3083, 0.03793 * | 0.0102, 0.99973 |
| Height of tallest sprout | 14.1139, 0.002754 ** | 0.4721, 0.492005 | 0.5370, 0.910691 |
| Number of dead sprouts | 50.294, 0.000 *** | 13.185, 0.000 *** | 1.219, 0.7484413 |
| Deer browse | 77.839, 0.000 *** | 4.841, 0.027789 * | 11.921, 0.007659 ** |
| Stump diameter | 1.14941, 0.7652 | 0.26954, 0.6036 | 1.45238, 0.6933 |
| 2016 | Sprouting Treatment | Cutting Treatment | Interaction |
| Live sprouts | 45.267, 0.000 *** | 1.959, 0.1617 | 5.538, 0.1364 |
| Loose bark | 9.4759, 0.02359 * | 2.0471, 0.15250 | 5.8841, 0.11739 |
| Decay fungi present | 14.2855, 0.002541 ** | 0.0150, 0.902537 | 3.3625, 0.339032 |
| Dead (no sprouting) | 36.978, 0.000 *** | 0.225, 0.6355 | 3.725, 0.2927 |
| Isolate ID | Year Isolated | Host | Location | Collector | Identifier |
|---|---|---|---|---|---|
| PFC2249 | 2001 | Prunus dulcis | Modesto CA | J. E. Adaskaveg | J. E. Adaskaveg |
| PFC2367 | 2001 | Prunus persica | Parlier CA | T. Michailides | J. E. Adaskaveg |
| PFC2434 | 2002 | Prunus dulcis | Modesto CA | J. E. Adaskaveg | J. E. Adaskaveg |
| Treatment | Description |
|---|---|
| Control | No treatment. |
| ChontrolTM liquid w/ inoculum a | Peat spray formulation containing Chondrostereum purpureum isolate PFC2139 105 to 107 Colony Forming Units (CFU) per L. |
| ChontrolTM liquid w/o inoculum | Peat spray formulation only. |
| ChontrolTM paste w/ inoculum | Paste formulation containing Chondrostereum purpureum isolate PFC2139 1 × 102 CFU per gram. |
| ChontrolTM paste w/o inoculum | Paste formulation only. |
| Garlon 3A b | Apply triclopyr (Garlon 3A (Amine)), cut 50–50 with water, plus dye to all exposed cambium immediately after cutting (within 30 min). Exposed cambium includes the stump surface and bark tears that occurred during falling. |
| Hack and squirt c | Inject imazapyr (Arsenal®) cut 50–50 with water, 1 hack (1 mL solution/hack) per 3 inches diameter) plus dye using the hack-and-squirt method. Hacks were made at or below stump height (40 cm). |
| Treatment | Description |
|---|---|
| Control | No treatment. |
| Chontrol™ paste w/inoculum a | Paste formulation containing Chondrostereum purpureum isolate PFC2249 1 × 102 CFU per gram. |
| ChontrolTM paste w/o inoculum | Paste formulation only. |
| Garlon 4 Ultra b | Apply triclopyr (Garlon 4 Ultra (Amine)), 30% in oil, diesel fuel, or kerosene, plus dye to all exposed cambium immediately after cutting (within 30 min). Exposed cambium includes the stump surface and bark tears that occurred during falling and the exposed sapwood area on trees with the girdling treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© Her Majesty the Queen in Right of Canada, as represented by the Minister of Natural Resources, 2022.
Share and Cite
Shamoun, S.F.; Elliott, M. Comparative Efficacy of Chondrosterum purpureum and Chemical Herbicides for Control of Resprouts in Tanoak and Bay Laurel. Pathogens 2022, 11, 485. https://doi.org/10.3390/pathogens11050485
Shamoun SF, Elliott M. Comparative Efficacy of Chondrosterum purpureum and Chemical Herbicides for Control of Resprouts in Tanoak and Bay Laurel. Pathogens. 2022; 11(5):485. https://doi.org/10.3390/pathogens11050485
Chicago/Turabian StyleShamoun, Simon Francis, and Marianne Elliott. 2022. "Comparative Efficacy of Chondrosterum purpureum and Chemical Herbicides for Control of Resprouts in Tanoak and Bay Laurel" Pathogens 11, no. 5: 485. https://doi.org/10.3390/pathogens11050485
APA StyleShamoun, S. F., & Elliott, M. (2022). Comparative Efficacy of Chondrosterum purpureum and Chemical Herbicides for Control of Resprouts in Tanoak and Bay Laurel. Pathogens, 11(5), 485. https://doi.org/10.3390/pathogens11050485







