Identification of Inflammatory and Regulatory Cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis
Abstract
:1. Introduction
2. Results
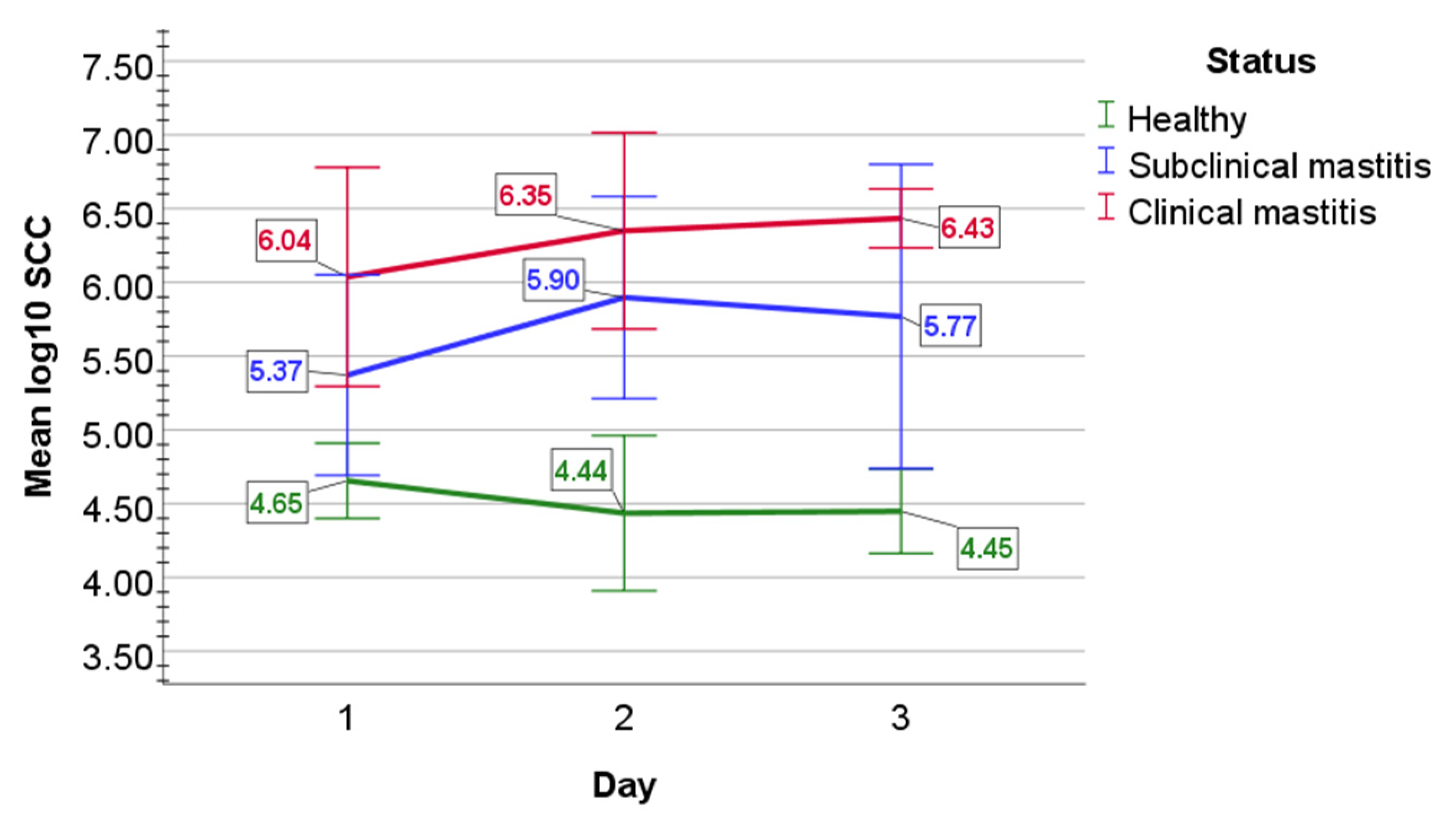
2.1. Evaluation of Milk Quality
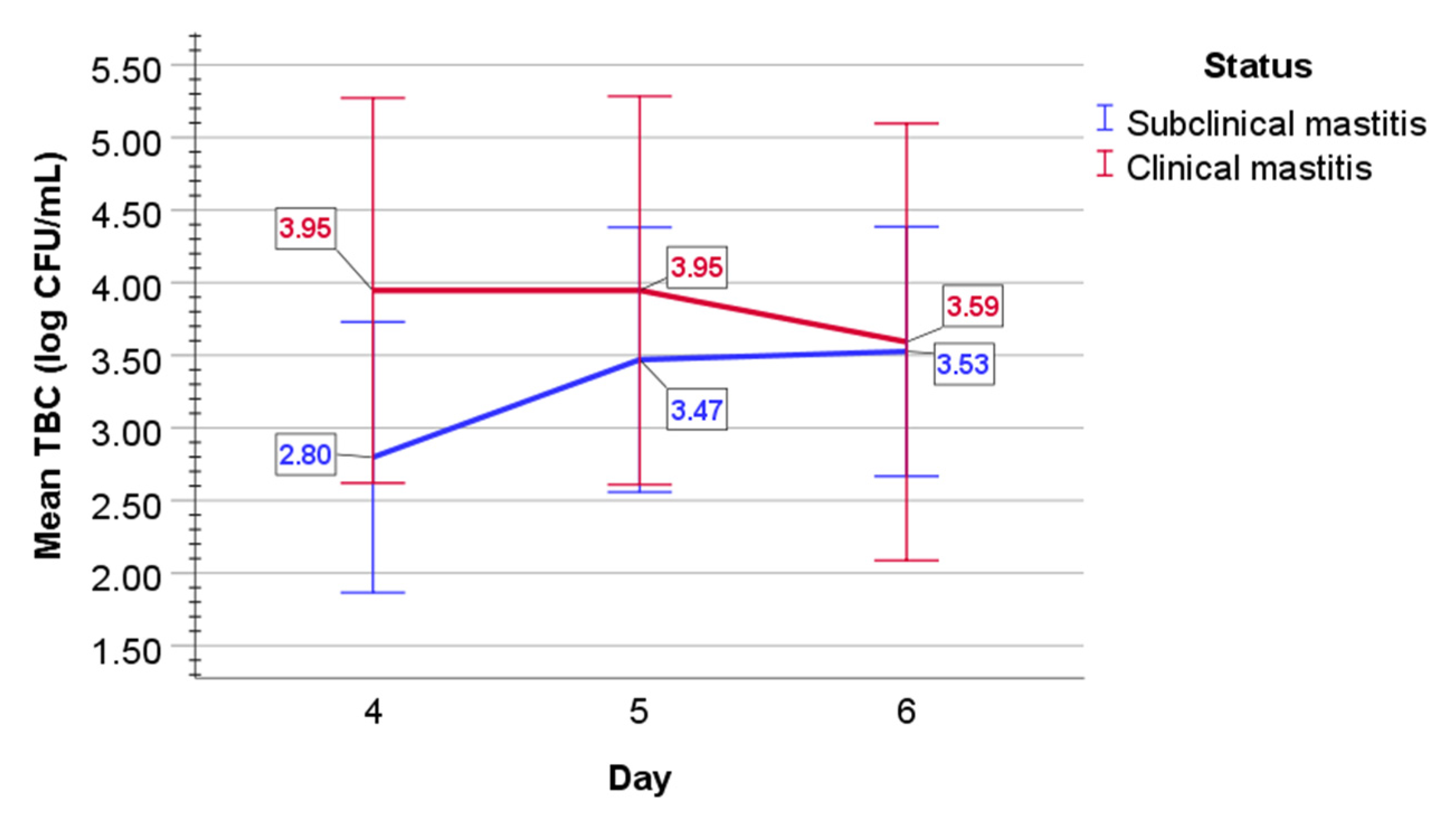
2.2. Bacteriological Examination
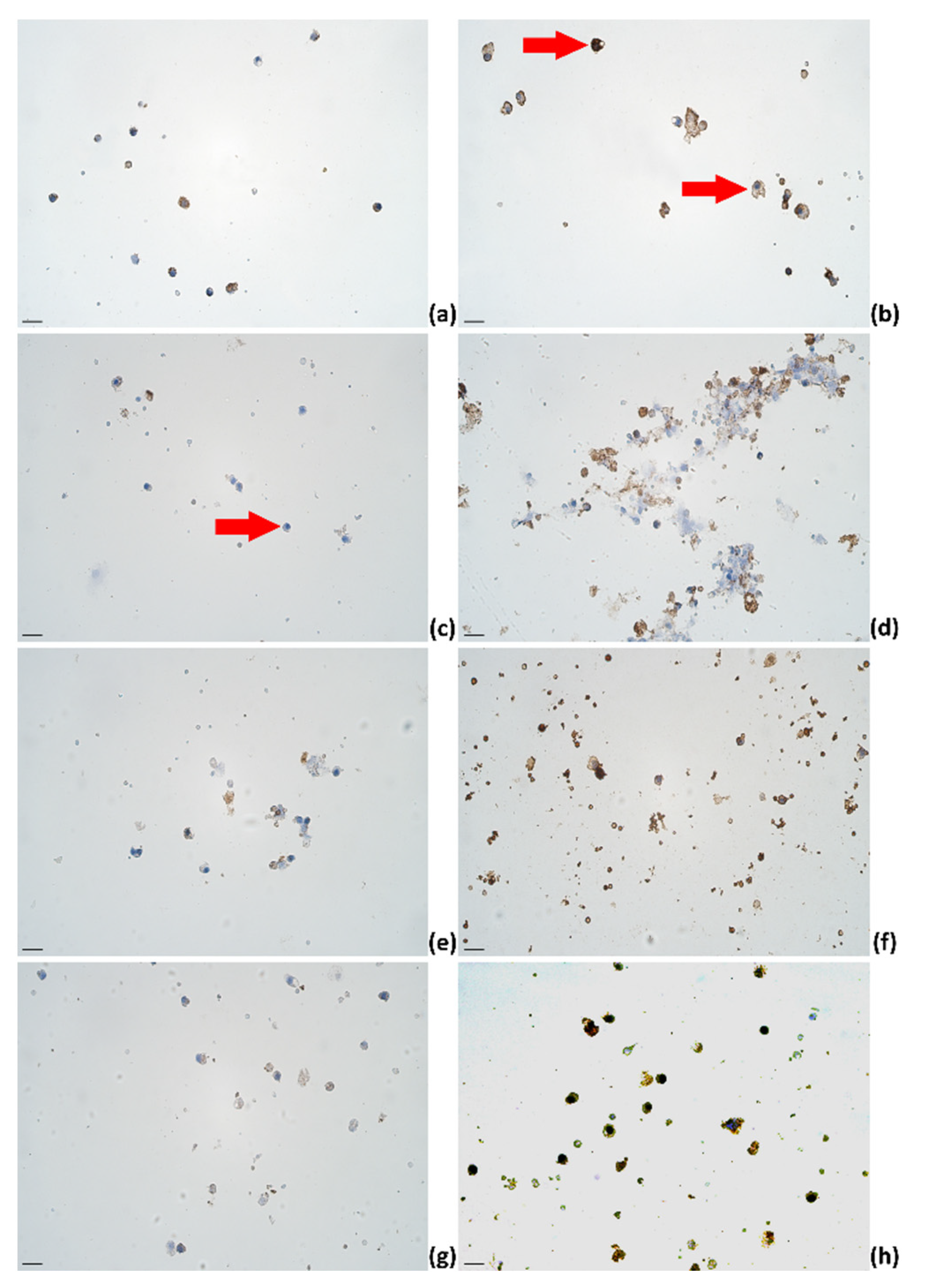
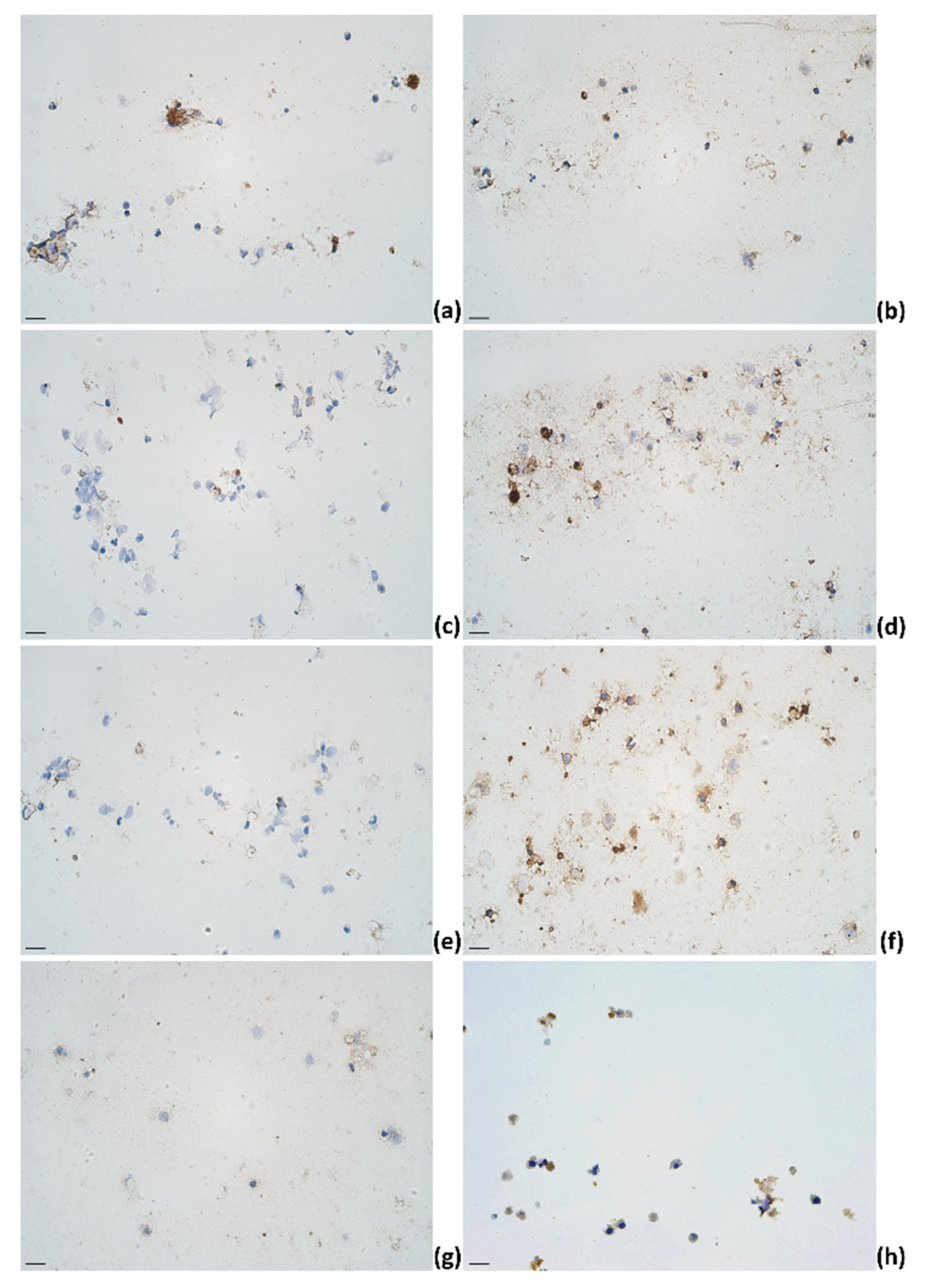
2.3. Identification of Cytokines Producing Cells in Milk of Dairy Cows
2.4. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Study Animals and Ethical Statements
4.2. Sample Collection
4.3. Differential Counting of Somatic Cells
4.4. Bacteriological Examination
4.5. Immunocytochemistry
4.6. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamoorthy, P.; Suresh, K.P.; Jayamma, K.S.; Shome, B.R.; Patil, S.S.; Amachawadi, R.G. An Understanding of the Global Status of Major Bacterial Pathogens of Milk Concerning Bovine Mastitis: A Systematic Review and Meta-Analysis (Scientometrics). Pathogens 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810. [Google Scholar] [CrossRef] [PubMed]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.-Y.; Leblond, A.; Haenni, M.; Gay, É. Antimicrobial Resistance in Bacteria Isolated from Mastitis in Dairy Cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis Treatment—Reduction in Antibiotic Usage in Dairy Cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A One Health Perspective on Dairy Production and Dairy Food Safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Imran, M. Diagnosis of Bovine Mastitis: From Laboratory to Farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, C.B.M.; Barreiro, J.R.; Moreno, J.F.G.; Porcionato, M.A.F.; Santos, M.V. Evaluation of Somatic Cell Count Thresholds to Detect Subclinical Mastitis in Gyr Cows. J. Dairy Sci. 2011, 94, 4406–4412. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, H.; Lassa, H.; Zastempowska, E.; Smulski, S.; Bis-Wencel, H. Etiological Agents of Bovine Mastitis in Poland. Med. Weter. 2020, 76, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, E.; Lassa, H.; Kłlossowska, A.; Smulski, S.; Markiewicz, H.; Kaczmarowski, M. Etiological Agents of Dairy Cows’ Mastitis in Western Part of Poland. Pol. J. Vet. Sci. 2006, 9, 191–194. [Google Scholar] [PubMed]
- Sztachańska, M.; Barański, W.; Janowski, T.; Pogorzelska, J.; Zduńczyk, S. Prevalence and Etiological Agents of Subclinical Mastitis at the End of Lactation in Nine Dairy Herds in North-East Poland. Pol. J. Vet. Sci. 2016, 19, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczorek-Łukowska, E.; Małaczewska, J.; Wójcik, R.; Naumowicz, K.; Blank, A.; Siwicki, A.K. Streptococci as the New Dominant Aetiological Factors of Mastitis in Dairy Cows in North-Eastern Poland: Analysis of the Results Obtained in 2013–2019. Ir. Vet. J. 2021, 74, 2. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Roeske, K.; Bakuła, Z.; Piech, T.; Wlazło, Ł.; Bochniarz, M.; Woch, P.; Krukowski, H. A Survey on the Incidence of Prototheca Mastitis in Dairy Herds in Lublin Province, Poland. J. Dairy Sci. 2019, 102, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, J.L. Etiological Agents of Bovine Mastitis. Vet. Microbiol. 1988, 16, 41–66. [Google Scholar] [CrossRef]
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakuła, Z.; Wlazło, Ł.; Woch, P. Prevalence of Prototheca Spp. on Dairy Farms in Poland—A Cross-Country Study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alluwaimi, A.M. The Cytokines of Bovine Mammary Gland: Prospects for Diagnosis and Therapy. Res. Vet. Sci. 2004, 77, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N.; Valand, P.; Nauriyal, D.S.; Joshi, C.G. Immunomodulation of IL-1, IL-6 and IL-8 Cytokines by Prosopis Juliflora Alkaloids during Bovine Sub-Clinical Mastitis. 3 Biotech 2018, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Niedziela, D.A.; Leonard, F.C.; Keane, O.M. The in Vitro Host Cell Immune Response to Bovine-Adapted Staphylococcus Aureus Varies According to Bacterial Lineage. Sci. Rep. 2019, 9, 6134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannerman, D.D. Pathogen-Dependent Induction of Cytokines and Other Soluble Inflammatory Mediators during Intramammary Infection of Dairy Cows. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochniarz, M.; Piech, T.; Kocki, T.; Iskra, M.; Krukowski, H.; Jagielski, T. Tryptophan, Kynurenine and Kynurenic Acid Concentrations in Milk and Serum of Dairy Cows with Prototheca Mastitis. Animals 2021, 11, 3608. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Mantovani, A. Ligands and Receptors of the Interleukin-1 Family in Immunity and Disease. Front. Immunol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska-Tomala, K.K.; Siemieniuch, M.J.; Szóstek, A.Z.; Korzekwa, A.J.; Woclawek-Potocka, I.; Galváo, A.M.; Okuda, K.; Skarzynski, D.J. Lipopolysaccharides, Cytokines, and Nitric Oxide Affect Secretion of Prostaglandins and Leukotrienes by Bovine Mammary Gland Epithelial Cells. Domest. Anim. Endocrinol. 2012, 43, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Karo-Atar, D.; Bitton, A.; Benhar, I.; Munitz, A. Therapeutic Targeting of the Interleukin-4/Interleukin-13 Signaling Pathway: In Allergy and Beyond. BioDrugs 2018, 32, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Ezzat Alnakip, M.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Caamaño-Antelo, S.; Calo-Mata, P.; Barros-Velázquez, J. The Immunology of Mammary Gland of Dairy Ruminants between Healthy and Inflammatory Conditions. J. Vet. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeb, L.E.M.; Egholm, C.; Impellizzieri, D.; Ridder, F.; Boyman, O. Regulation of Neutrophils in Type 2 Immune Responses. Curr. Opin. Immunol. 2018, 54, 115–122. [Google Scholar] [CrossRef]
- Fonseca, I.; Silva, P.V.; Lange, C.C.; Guimarães, M.F.M.; Weller, M.M.D.C.A.; Sousa, K.R.S.; Lopes, P.S.; Guimarães, J.D.; Guimarães, S.E.F. Expression Profile of Genes Associated with Mastitis in Dairy Cattle. Genet. Mol. Biol. 2009, 32, 776–781. [Google Scholar] [CrossRef]
- Bochniarz, M.; Zdzisińska, B.; Wawron, W.; Szczubiał, M.; Dąbrowski, R. Milk and Serum IL-4, IL-6, IL-10, and Amyloid A Concentrations in Cows with Subclinical Mastitis Caused by Coagulase-Negative Staphylococci. J. Dairy Sci. 2017, 100, 9674–9680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellati, T.J.; Sahay, B. Cells of Innate Immunity: Mechanisms of Activation. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 258–274. ISBN 978-0-12-386457-4. [Google Scholar]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A Panoramic Review of IL-6: Structure, Pathophysiological Roles and Inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Shaheen, T.; Bilal Ahmad, S.; Rehman, M.U.; Muzamil, S.; Razak Bhat, R.; Hussain, I.; Bashir, N.; Mir, M.U.R.; Paray, B.A.; Dawood, M.A.O. Investigations on Cytokines and Proteins in Lactating Cows with and without Naturally Occurring Mastitis. J. King Saud Univ. Sci. 2020, 32, 2863–2867. [Google Scholar] [CrossRef]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic Messenger Molecules: Cytokines, Interferons, and Chemokines. J. Allergy Clin. Immunol. 2010, 125, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.R.; Fox, L.K.; Muller, F.J.; Rinaldi, M.; Zeng, R.; Bannerman, D.D. Factors Associated with Concentrations of Select Cytokine and Acute Phase Proteins in Dairy Cows with Naturally Occurring Clinical Mastitis. J. Dairy Sci. 2010, 93, 2458–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junttila, I.S.; Watson, C.; Kummola, L.; Chen, X.; Hu-Li, J.; Guo, L.; Yagi, R.; Paul, W.E. Efficient Cytokine-Induced IL-13 Production by Mast Cells Requires Both IL-33 and IL-3. J. Allergy Clin. Immunol. 2013, 132, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cua, D.J.; Tato, C.M. Innate IL-17-Producing Cells: The Sentinels of the Immune System. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Dungan, L.S.; Mills, K.H.G. Caspase-1-Processed IL-1 Family Cytokines Play a Vital Role in Driving Innate IL-17. Cytokine 2011, 56, 126–132. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, Y.-J.; Chung, Y. Targeting IL-17 in Autoimmunity and Inflammation. Arch. Pharm. Res. 2016, 39, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kolls, J.K. Interluekin-17A (IL17A). Gene 2017, 614, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roussel, P.; Cunha, P.; Porcherie, A.; Petzl, W.; Gilbert, F.B.; Riollet, C.; Zerbe, H.; Rainard, P.; Germon, P. Investigating the Contribution of IL-17A and IL-17F to the Host Response during Escherichia Coli Mastitis. Vet. Res. 2015, 46, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcherie, A.; Gilbert, F.B.; Germon, P.; Cunha, P.; Trotereau, A.; Rossignol, C.; Winter, N.; Berthon, P.; Rainard, P. IL-17A Is an Important Effector of the Immune Response of the Mammary Gland to Escherichia Coli Infection. J. Immunol. 2016, 196, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-M. Tumor Necrosis Factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 2572. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour Necrosis Factor Signalling in Health and Disease. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-Gamma: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-Gene Expression of pro-Inflammatory Cytokines (TNF-α, IL-1β and IL-6) via TLRs Following NF-ΚB and MAPKs in Bovine Mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Diesterbeck, U.S.; König, S.; Brügemann, K.; Schlez, K.; Zschöck, M.; Wolter, W.; Czerny, C.-P. Flow Cytometric Differential Cell Counts in Milk for the Evaluation of Inflammatory Reactions in Clinically Healthy and Subclinically Infected Bovine Mammary Glands. J. Dairy Sci. 2011, 94, 5033–5044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellnitz, O.; Baumert, A.; Saudenowa, M.; Bruckmaier, R.M. Immune Response of Bovine Milk Somatic Cells to Endotoxin in Healthy Quarters with Normal and Very Low Cell Counts. J. Dairy Res. 2010, 77, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Sumon, S.M.M.R.; Parvin, M.S.; Ehsan, M.A.; Islam, M.T. Relationship between Somatic Cell Counts and Subclinical Mastitis in Lactating Dairy Cows. Vet. World 2020, 13, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Berardo, N.; Giraudo, J.; Nader-Macías, M.E.F.; Bogni, C. Bovine Mastitis Prevention: Humoral and Cellular Response of Dairy Cows Inoculated with Lactic Acid Bacteria at the Dry-off Period. Benef. Microbes 2017, 8, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Lyman, R.L.; Hockett, M.; Rodriguez, R.; Dos Santos, M.V.; Anderson, K.L. Using Milk Leukocyte Differentials for Diagnosis of Subclinical Bovine Mastitis. J. Dairy Res. 2017, 84, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, J.R.; Hardin, D.; Steevens, B.; Randle, R.; Tyler, J.W. Use of Somatic Cell Counts and California Mastitis Test Results from Individual Quarter Milk Samples to Detect Subclinical Intramammary Infection in Dairy Cattle from a Herd with a High Bulk Tank Somatic Cell Count. J. Am. Vet. Med. Assoc. 2004, 224, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Hayashi, T.; Mizuta, M.; Ebara, S.; Kiku, Y.; Ozawa, T.; Matsubara, T.; Ito, I.; Kitamura, D.; Mizuta, R. Increased Concentration of High-Mobility Group Box 1 Protein in Milk Is Related to the Severity of Bovine Mastitis. Vet. Res. Commun. 2011, 35, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Rice-Ficht, A.C.; Estes, D.M. Bovine Type 1 and Type 2 Responses. Vet. Immunol. Immunopathol. 1998, 63, 45–55. [Google Scholar] [CrossRef]
- Pellegrino, M.; Rodriguez, N.; Vivas, A.; Giraudo, J.; Bogni, C. Staphylococcus Aureus Avirulent Mutant Vaccine Induces Humoral and Cellular Immune Responses on Pregnant Heifers. Vaccine 2016, 34, 3356–3362. [Google Scholar] [CrossRef]
- Commins, S.; Steinke, J.W.; Borish, L. The Extended IL-10 Superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef]
- Taylor, A.; Akdis, M.; Joss, A.; Akkoç, T.; Wenig, R.; Colonna, M.; Daigle, I.; Flory, E.; Blaser, K.; Akdis, C.A. IL-10 Inhibits CD28 and ICOS Costimulations of T Cells via Src Homology 2 Domain-Containing Protein Tyrosine Phosphatase 1. J. Allergy Clin. Immunol. 2007, 120, 76–83. [Google Scholar] [CrossRef]
- Atkins, E. Fever: The Old and the New. J. Infect. Dis. 1984, 149, 339–348. [Google Scholar] [CrossRef]
- Beeson, P.B. Temperature-Elevating Effect of a Substance Obtained from Polymorphonuclear Leucocytes. J. Clin. Investig. 1948, 27, 524. [Google Scholar] [PubMed]
- Fukuyama, K.; Islam, M.A.; Takagi, M.; Ikeda-Ohtsubo, W.; Kurata, S.; Aso, H.; Vignolo, G.; Villena, J.; Kitazawa, H. Evaluation of the Immunomodulatory Ability of Lactic Acid Bacteria Isolated from Feedlot Cattle Against Mastitis Using a Bovine Mammary Epithelial Cells In Vitro Assay. Pathogens 2020, 9, 410. [Google Scholar] [CrossRef]
- Ru, K.; Su, F.; Zheng, Y.; Zhang, Y.; Luo, Y.; Guo, Z.; He, X.; Liu, X.; Zhang, J.; Liu, J.; et al. Inducible Expression of Enhanced Green Fluorescent Protein by Interleukin-1α, Interleukin-1β and Toll-like Receptor 2 Promoters in Goat Mammary Epithelial Cells in Response to Bacterial Challenges. Vet. J. 2015, 203, 85–91. [Google Scholar] [CrossRef]
- Piotrowska-Tomala, K.K.; Bah, M.M.; Jankowska, K.; Lukasik, K.; Warmowski, P.; Galvao, A.M.; Skarzynski, D.J. Lipopolysaccharides, Cytokines, and Nitric Oxide Affect Secretion of Prostaglandins and Leukotrienes by Bovine Mammary Gland during Experimentally Induced Mastitis in Vivo and in Vitro. Domest. Anim. Endocrinol. 2015, 52, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Peli, A.; Scagliarini, A.; Britti, D.; Boari, A. Detection of Proinflammatory and Regulatory Cytokines in Bovine Milk Using RT-PCR. Vet. Res. Commun. 2003, 27 (Suppl. 1), 779–781. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Dellinger, J.D.; Cullor, J.S.; Stott, J.L. Bovine Milk Lymphocytes Display the Phenotype of Memory T Cells and Are Predominantly CD8+. Cell. Immunol. 1994, 156, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Düvel, A.; Frank, C.; Schnapper, A.; Schuberth, H.-J.; Sipka, A. Classically or Alternatively Activated Bovine Monocyte-Derived Macrophages in Vitro Do Not Resemble CD163/Calprotectin Biased Macrophage Populations in the Teat. Innate Immun. 2012, 18, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Herbert, J.M.; Savi, P.; Laplace, M.C.; Lalé, A.; Dol, F.; Dumas, A.; Labit, C.; Minty, A. IL-4 and IL-13 Exhibit Comparable Abilities to Reduce Pyrogen-Induced Expression of Procoagulant Activity in Endothelial Cells and Monocytes. FEBS Lett. 1993, 328, 268–270. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, K.; Kataoka, S.; Yamanaka, H.; Kirisawa, R.; Iwai, H. Detection of Cytokines in Bovine Colostrum. Vet. Immunol. Immunopathol. 2000, 76, 183–190. [Google Scholar] [CrossRef]
- Sakemi, Y.; Tamura, Y.; Hagiwara, K. Interleukin-6 in Quarter Milk as a Further Prediction Marker for Bovine Subclinical Mastitis. J. Dairy Res. 2011, 78, 118–121. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, J.; Han, B.; Barkema, H.W.; Cobo, E.R.; Kastelic, J.P.; Zhou, M.; Shi, Y.; Wang, J.; Yang, R.; et al. Klebsiella Pneumoniae Isolated from Bovine Mastitis Is Cytopathogenic for Bovine Mammary Epithelial Cells. J. Dairy Sci. 2020, 103, 3493–3504. [Google Scholar] [CrossRef] [PubMed]
- Riollet, C.; Rainard, P.; Poutrel, B. Cells and Cytokines in Inflammatory Secretions of Bovine Mammary Gland. In Biology of the Mammary Gland; Mol, J.A., Clegg, R.A., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2002; pp. 247–258. ISBN 978-0-306-46832-2. [Google Scholar]
- Boudjellab, N.; Chan-Tang, H.S.; Zhao, X. Bovine Interleukin-1 Expression by Cultured Mammary Epithelial Cells (MAC-T) and Its Involvement in the Release of MAC-T Derived Interleukin-8. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 127, 191–199. [Google Scholar] [CrossRef]
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The Role of Innate Immune Response and Microbiome in Resilience of Dairy Cattle to Disease: The Mastitis Model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef]
- Alluwaimi, A.M.; Cullor, J.S. Cytokines Gene Expression Patterns of Bovine Milk During Middle and Late Stages of Lactation. J. Vet. Med. Ser. B 2002, 49, 105–110. [Google Scholar] [CrossRef]
- Alluwaimi, A.M.; Leutenegger, C.M.; Farver, T.B.; Rossitto, P.V.; Smith, W.L.; Cullor, J.S. The Cytokine Markers in Staphylococcus Aureus Mastitis of Bovine Mammary Gland. J. Vet. Med. Ser. B 2003, 50, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bannerman, D.D.; Paape, M.J.; Huang, M.-K.; Zhao, X. Characterization of Cytokine Expression in Milk Somatic Cells during Intramammary Infections with Escherichia Coli or Staphylococcus Aureus by Real-Time PCR. Vet. Res. 2006, 37, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannerman, D.D.; Chockalingam, A.; Paape, M.J.; Hope, J.C. The Bovine Innate Immune Response during Experimentally-Induced Pseudomonas Aeruginosa Mastitis. Vet. Immunol. Immunopathol. 2005, 107, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Kauf, A.C.W.; Rosenbusch, R.F.; Paape, M.J.; Bannerman, D.D. Innate Immune Response to Intramammary Mycoplasma Bovis Infection. J. Dairy Sci. 2007, 90, 3336–3348. [Google Scholar] [CrossRef] [Green Version]
- Bannerman, D.D.; Paape, M.J.; Hare, W.R.; Hope, J.C. Characterization of the Bovine Innate Immune Response to Intramammary Infection with Klebsiella Pneumoniae. J. Dairy Sci. 2004, 87, 2420–2432. [Google Scholar] [CrossRef] [Green Version]
- Rainard, P.; Cunha, P.; Martins, R.P.; Gilbert, F.B.; Germon, P.; Foucras, G. Type 3 Immunity: A Perspective for the Defense of the Mammary Gland against Infections. Vet. Res. 2020, 51, 129. [Google Scholar] [CrossRef]
- Rainard, P.; Cunha, P.; Ledresseur, M.; Staub, C.; Touzé, J.-L.; Kempf, F.; Gilbert, F.B.; Foucras, G. Antigen-Specific Mammary Inflammation Depends on the Production of IL-17A and IFN-γ by Bovine CD4+ T Lymphocytes. PLoS ONE 2015, 10, e0137755. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Cunha, P.; Gilbert, F.B. Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland. PLoS ONE 2016, 11, e0154172. [Google Scholar] [CrossRef] [PubMed]
- Herry, V.; Gitton, C.; Tabouret, G.; Répérant, M.; Forge, L.; Tasca, C.; Gilbert, F.B.; Guitton, E.; Barc, C.; Staub, C.; et al. Local Immunization Impacts the Response of Dairy Cows to Escherichia Coli Mastitis. Sci. Rep. 2017, 7, 3441. [Google Scholar] [CrossRef] [PubMed]
- Tassi, R.; McNeilly, T.N.; Fitzpatrick, J.L.; Fontaine, M.C.; Reddick, D.; Ramage, C.; Lutton, M.; Schukken, Y.H.; Zadoks, R.N. Strain-Specific Pathogenicity of Putative Host-Adapted and Nonadapted Strains of Streptococcus Uberis in Dairy Cattle. J. Dairy Sci. 2013, 96, 5129–5145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, L.M.; Shafer-Weaver, K.; DeRosa, D. Immunobiology of the Mammary Gland. J. Dairy Sci. 1997, 80, 1851–1865. [Google Scholar] [CrossRef]
- Rainard, P.; Paape, M. Sensitization of the Bovine Mammary Gland to Escherichia Coli Endotoxin. Vet. Res. 1997, 28, 231–238. [Google Scholar] [PubMed]
- Hisaeda, K.; Hagiwara, K.; Eguchi, J.; Yamanaka, H.; Kirisawa, R.; Iwai, H. Interferon-Gamma and Tumor Necrosis Factor-Alpha Levels in Sera and Whey of Cattle with Naturally Occurring Coliform Mastitis. J. Vet. Med. Sci. 2001, 63, 1009–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, K.H.; VandeHaar, M.J.; Burton, J.L.; Liesman, J.S.; Erskine, R.J.; Elsasser, T.H. Clinical Responses to Intramammary Endotoxin Infusion in Dairy Cows Subjected to Feed Restriction. J. Dairy Sci. 2002, 85, 1724–1731. [Google Scholar] [CrossRef]
- Lehtolainen, T.; Røntved, C.; Pyörälä, S. Serum Amyloid A and TNF Alpha in Serum and Milk during Experimental Endotoxin Mastitis. Vet. Res. 2004, 35, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Persson Waller, K.; Colditz, I.G.; Lun, S.; Ostensson, K. Cytokines in Mammary Lymph and Milk during Endotoxin-Induced Bovine Mastitis. Res. Vet. Sci. 2003, 74, 31–36. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Innate Immune Response of Bovine Mammary Gland to Pathogenic Bacteria Responsible for Mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef] [PubMed]
- De, U.K.; Mukherjee, R. Expression of Cytokines and Respiratory Burst Activity of Milk Cells in Response to Azadirachta Indica during Bovine Mastitis. Trop. Anim. Health Prod. 2009, 41, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, B. Act on the Protection of Animals Used for Scientific or Educational Purposes—Legal Regulation Review. 2016. Available online: https://doi.org/10.12775/PYEL.2015.004 (accessed on 29 November 2021).
- Rainard, P.; Foucras, G.; Boichard, D.; Rupp, R. Invited Review: Low Milk Somatic Cell Count and Susceptibility to Mastitis. J. Dairy Sci. 2018, 101, 6703–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.; Neglia, G.; Campanile, G.; Marchi, M.D. Milk Somatic Cell Count and Its Relationship with Milk Yield and Quality Traits in Italian Water Buffaloes. J. Dairy Sci. 2020, 103, 5485–5494. [Google Scholar] [CrossRef] [PubMed]
- Jakiel, M.; Jesiołkiewicz, E.; Ptak, E. Zależność między zawartością komórek somatycznych a cechami wydajności mlecznej w mleku krów rasy PHF odmiany czarno-białej. Rocz. Nauk. Pol. Tow. Zootech. 2011, 7, 9. [Google Scholar]
- Hsu, S.M.; Raine, L.; Fanger, H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Gulbe, G.; Pilmane, M.; Saulīte, V.; Doniņa, S.; Jermolajevs, J.; Peškova, L.; Valdovska, A. Cells and Cytokines in Milk of Subclinically Infected Bovine Mammary Glands after the Use of Immunomodulatory Composition GLP 810. Mediat. Inflamm. 2020, 2020, 8238029. [Google Scholar] [CrossRef]
- Stefanini, M.; De Martino, C.; Zamboni, L. Fixation of Ejaculated Spermatozoa for Electron Microscopy. Nature 1967, 216, 173–174. [Google Scholar] [CrossRef]
- Boutinaud, M.; Rulquin, H.; Keisler, D.H.; Djiane, J.; Jammes, H. Use of Somatic Cells from Goat Milk for Dynamic Studies of Gene Expression in the Mammary Gland. J. Anim. Sci. 2002, 80, 1258–1269. [Google Scholar] [CrossRef] [Green Version]
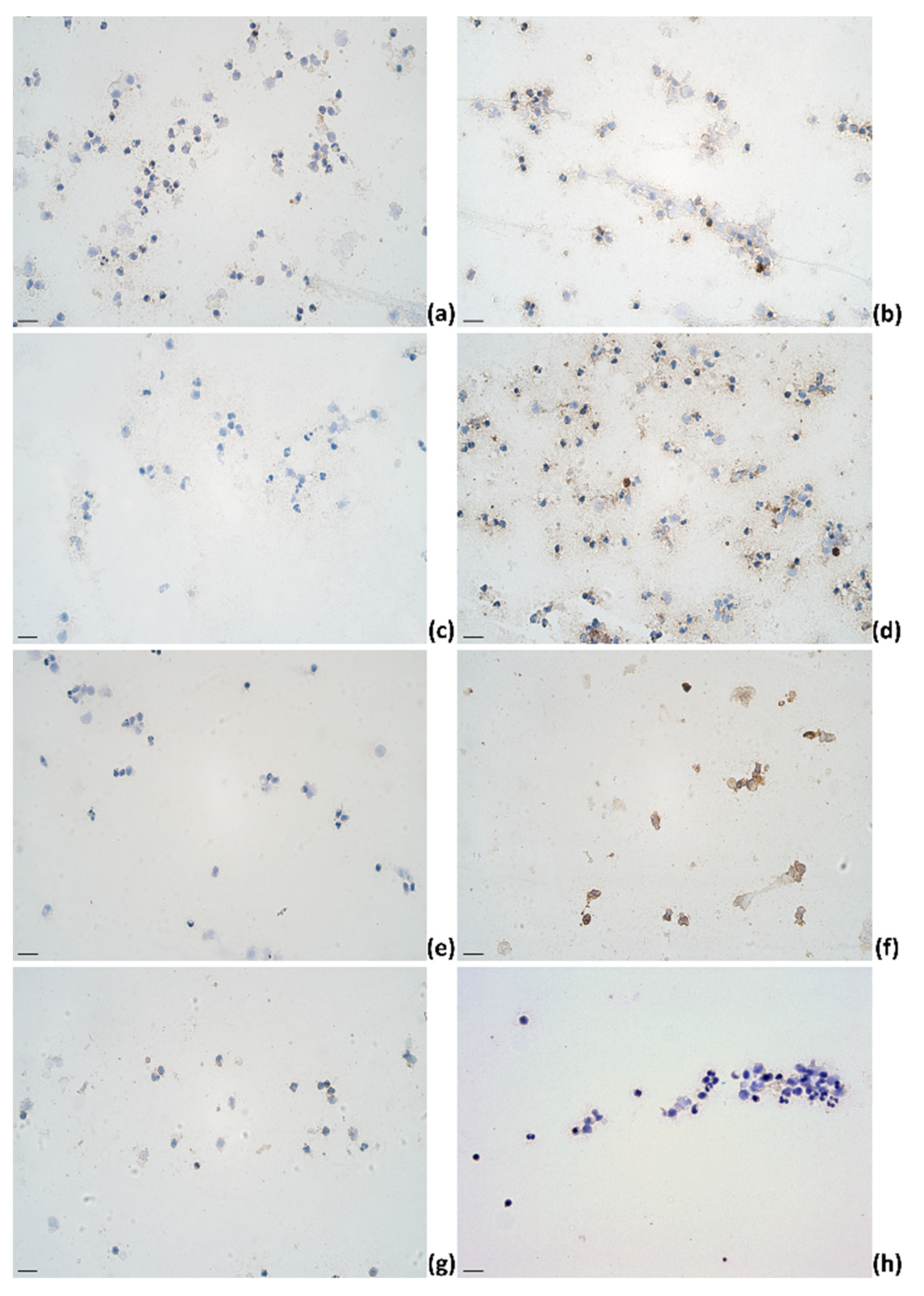
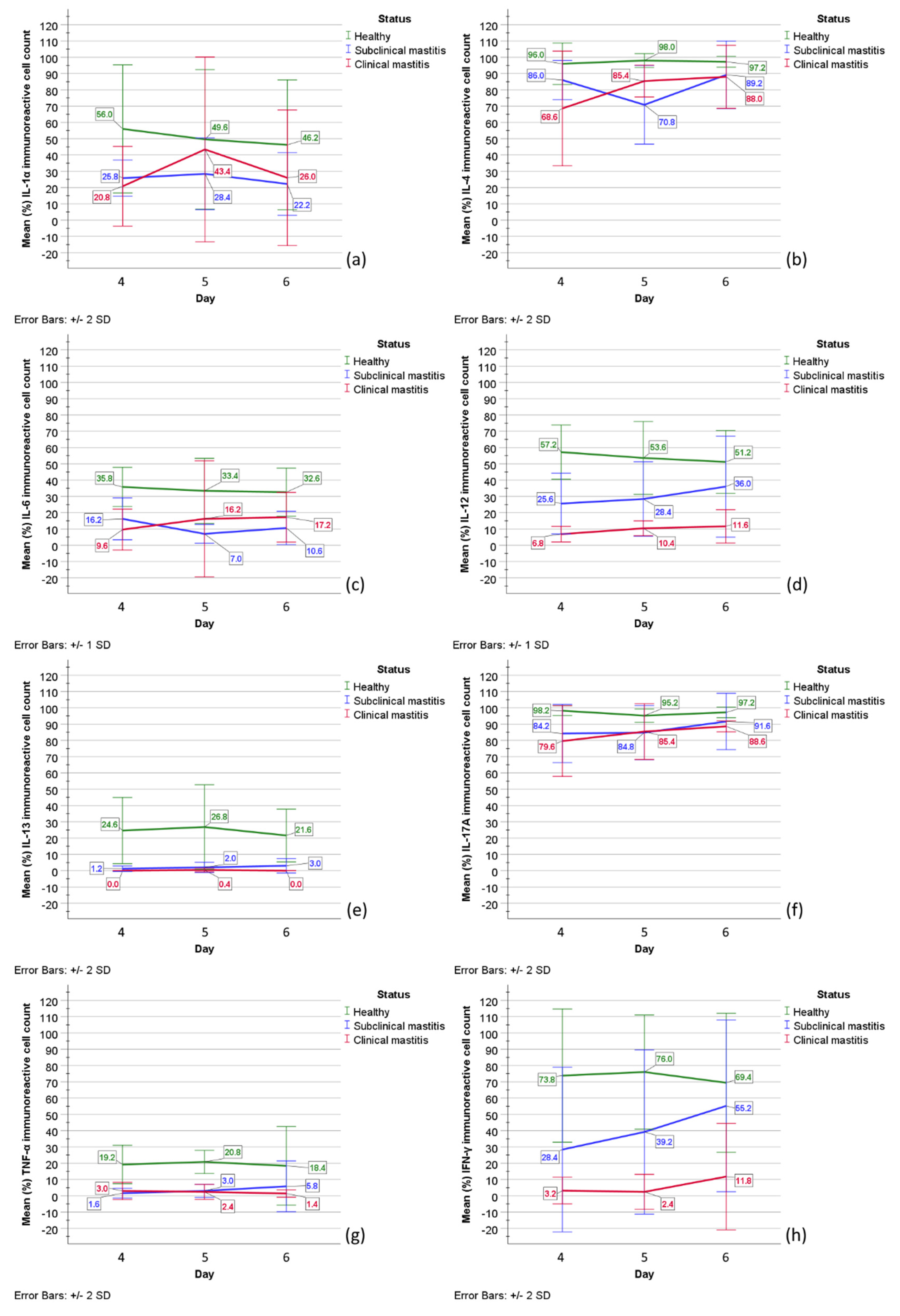
| IL-1α | IL-4 | IL-6 | IL-12 | |||||||||||||||||||||||||||||||||
| Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | |||||||||||||||||||||||||
| ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | |
| Healthy | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 15 | 41 | 44 | 15 | 34 | 51 | 14 | 33 | 54 | 51 | 45 | 4 | 64 | 34 | 2 | 54 | 43 | 3 | 18 | 18 | 64 | 18 | 16 | 67 | 16 | 17 | 67 | 29 | 28 | 43 | 28 | 26 | 46 | 21 | 30 | 49 |
| +/− | 56 */44 | 49/51 | 47/54 | 96/4 | 98/2 | 97/3 | 36/64 | 34/67 | 33/67 | 57/43 | 54/46 | 51/49 | ||||||||||||||||||||||||
| Subclinical mastitis | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 6 | 20 | 74 | 8 | 21 | 71 | 5 | 17 | 78 | 24 | 64 | 12 | 15 | 56 | 29 | 31 | 55 | 14 | 5 | 11 | 84 | 3 | 4 | 93 | 1 | 9 | 89 | 10 | 16 | 74 | 12 | 16 | 72 | 14 | 22 | 64 |
| +/− | 26/74 | 29/71 | 22/78 | 88/12 | 71 */29 | 86/14 | 16/84 | 7/93 | 10/89 | 26/74 | 28/72 | 36/64 | ||||||||||||||||||||||||
| Clinical mastitis | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 1 | 20 | 79 | 6 | 37 | 57 | 3 | 23 | 74 | 19 | 56 | 31 | 17 | 69 | 15 | 23 | 65 | 12 | 1 | 9 | 90 | 6 | 10 | 84 | 2 | 15 | 83 | 2 | 5 | 93 | 3 | 7 | 90 | 2 | 10 | 88 |
| +/− | 21/79 | 43/57 | 26/74 | 75/31 | 86/15 | 88/12 | 10/90 | 16/84 | 17/83 | 7/93 | 10/90 | 12/88 | ||||||||||||||||||||||||
| IL-13 | IL-17A | TNF-α | IFN-γ | |||||||||||||||||||||||||||||||||
| Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | Day 4 | Day 5 | Day 6 | |||||||||||||||||||||||||
| ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | ++ | + | 0 | |
| Healthy | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 4 | 20 | 76 | 4 | 23 | 73 | 3 | 19 | 79 | 17 | 81 | 2 | 21 | 74 | 5 | 23 | 74 | 3 | 1 | 18 | 81 | 2 | 19 | 79 | 2 | 16 | 82 | 42 | 31 | 26 | 42 | 43 | 24 | 35 | 35 | 30 |
| +/− | 24/76 | 27/73 | 22/79 | 98 * /2 | 95/5 | 97/3 | 19/81 | 21/79 | 18/82 | 73/26 | 76/24 | 70/30 | ||||||||||||||||||||||||
| Subclinical mastitis | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 0 | 1 | 99 | 0 | 2 | 98 | 0 | 3 | 97 | 10 | 74 | 16 | 11 | 74 | 15 | 17 | 75 | 8 | 0 | 2 | 98 | 0 | 3 | 97 | 0 | 6 | 94 | 12 | 16 | 72 | 16 | 23 | 60 | 14 | 42 | 49 |
| +/− | 1/99 | 2/98 | 3/97 | 84/16 | 85/15 | 92/8 | 2/98 | 3/97 | 6/94 | 28/72 | 40/60 | 26/49 | ||||||||||||||||||||||||
| Clinical mastitis | ||||||||||||||||||||||||||||||||||||
| Mean (%) | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 3 | 77 | 19 | 5 | 81 | 14 | 7 | 82 | 11 | 0 | 3 | 97 | 0 | 2 | 98 | 0 | 1 | 99 | 1 | 4 | 95 | 1 | 2 | 98 | 4 | 8 | 88 |
| +/− | 0/100 | 0/100 | 0/100 | 80/19 | 86/14 | 89/11 | 3/97 | 2/98 | 1/99 | 5/95 | 3/98 | 12/88 | ||||||||||||||||||||||||
| Cytokine | p-Value | ||||
|---|---|---|---|---|---|
| D | S | S × D | |||
| Healthy | Subclinical Mastitis | Clinical Mastitis | |||
| IL-1α | 0.253 | 0.294 | 0.068 | 0.071 | 0.019 * |
| IL-4 | 0.728 | 0.085 | 0.125 | <0.001 * | 0.031 * |
| IL-6 | 0.892 | 0.081 | 0.65 | 0.073 | 0.896 |
| IL-12 | 0.614 | 0.494 | 0.557 | 0.004 * | 0.852 |
| IL-13 | 0.442 | 0.147 | 0.111 | <0.001 * | 0.909 |
| IL-17A | 0.007 * | 0.084 | 0.374 | 0.015 * | 0.063 |
| TNF-α | 0.842 | 0.294 | 0.255 | <0.001 * | 0.759 |
| IFN-γ | 0.586 | 0.156 | 0.178 | <0.001 * | 0.184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitenberga-Verza, Z.; Pilmane, M.; Šerstņova, K.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Identification of Inflammatory and Regulatory Cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis. Pathogens 2022, 11, 372. https://doi.org/10.3390/pathogens11030372
Vitenberga-Verza Z, Pilmane M, Šerstņova K, Melderis I, Gontar Ł, Kochański M, Drutowska A, Maróti G, Prieto-Simón B. Identification of Inflammatory and Regulatory Cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis. Pathogens. 2022; 11(3):372. https://doi.org/10.3390/pathogens11030372
Chicago/Turabian StyleVitenberga-Verza, Zane, Māra Pilmane, Ksenija Šerstņova, Ivars Melderis, Łukasz Gontar, Maksymilian Kochański, Andżelika Drutowska, Gergely Maróti, and Beatriz Prieto-Simón. 2022. "Identification of Inflammatory and Regulatory Cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis" Pathogens 11, no. 3: 372. https://doi.org/10.3390/pathogens11030372
APA StyleVitenberga-Verza, Z., Pilmane, M., Šerstņova, K., Melderis, I., Gontar, Ł., Kochański, M., Drutowska, A., Maróti, G., & Prieto-Simón, B. (2022). Identification of Inflammatory and Regulatory Cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis. Pathogens, 11(3), 372. https://doi.org/10.3390/pathogens11030372









