Abstract
Bacterial infections are a significant cause of illness and death in different animals. However, these bacterial infections could be a source of human disease or illness if these pathogenic bacteria are present in companion animals. This study aimed to investigate the prevalence of pathogenic bacteria associated with different site infections in cats in the Republic of Korea. For this purpose, samples were collected from the skin/ear, urine, respiratory, and diarrheal stool origins of cats obtained between 2018 and 2019 from seven different laboratories and centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System. These samples were subjected to analysis for the identification and isolation of associated bacterial species using a bacterial culture approach. A total of 609 isolates were identified in four different cat samples. Among them, 267, 184, 57, and 101 were extracted from diarrheal stool, skin, urine, and respiratory samples, respectively. The findings of this study showed that Escherichia coli was the most prevalent species among isolated bacterial species of diarrheal stool and urine origin. Staphylococcus felis and Pasteurella multocida were most prevalent in the skin and respiratory tract, respectively. However, there was no significant difference in bacterial distribution among the different age groups in all samples. This is the first nationwide surveillance report that associates bacterial prevalence with their site of origin and helps in the prevention of bacterial infections in cats. Moreover, the pattern of bacterial prevalence could provide sufficient guidance for the selection of empirical antimicrobial therapy against infections in cats.
1. Introduction
Currently, pet consumerism and pet-owning households are increasing due to structural changes in the population, mainly due to increased single-person households, changes in lifestyle, and aging [1]. In 2019, the proportion of South Korean pet-owning households was 5.91 households (26.4%), and an estimation showed that the pet industry will grow from 1544 to 3498 billion during 2018–2027 [2]. These growing factors have led to an increased interest in the health and wellbeing of companion animals to ensure a healthy and sound symbiotic companionship between animals and owners.
Furthermore, the emergence of bacterial infections in companion animals is another growing concern, as it affects global morbidity and mortality [3]. The sharing of a common environment between companion animals, especially dogs and cats, and their owners can be a possible factor for bacterial transmission. Friedmann et al. and Kim et al. reported that companion animals might play an integral role in transferring infections due to their direct contact with humans [1,4].
Bacterial prevalence studies are also increasing with the growing pet industry and bacterial infections. There have been limited studies on bacterial prevalence in companion animals within the Korean veterinary market. Thus, it is difficult to treat site-specific bacterial infections, and there is a possible increase in the extent of antimicrobial resistance among companion animal pathogenic bacteria in veterinary practice. In the present study, we aimed to report the nationwide prevalence of pathogenic bacteria in diarrheal stool, skin, urine, and respiratory tract infection specimens collected from cats at seven different laboratories/centers of metropolitan cities of Korea and participating in the Korean Veterinary Antimicrobial Resistance Monitoring System.
2. Materials and Methods
2.1. Research Design and Sample Analysis
We conducted a prevalence study for bacterial isolates collected and identified by laboratories and centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System from 2018 to 2019. In general, the isolates were collected in proportion to the number of veterinary hospitals in each city (Table 1). The clinical samples were obtained from the fecal, skin/ear, urine, and respiratory origins of cats, placed on ice, and transported to laboratories/centers participating in the monitoring system within 6 h of their collection.

Table 1.
Total number of animal hospitals participated in this study from each city.
2.2. Isolation and Identification of Bacteria
The swabs were directly plated on 5% defibrinated sheep blood agar (Hangang, Gunpo, South Korea) and MacConkey agar plates (MAC, BD, Spark, Baltimore, MD, USA) and incubated overnight at 37°C for 24 h under aerobic and anaerobic conditions. Only 1–2 major colonies showing morphological differences in one culture plate were selected for further analysis [5].
All the colonies were analyzed with matrix-associated laser desorption ionization time-of-flight mass spectrometry (microflex LT/SH spectrometer, bioMérieux, Germany) for identification. However, Staphylococcal species were identified by polymerase chain reaction using a previously reported method of identification [6].
2.3. Data and Statistical Analysis
Data were analyzed using Excel (Microsoft Office, USA) and GraphPad Prism 7 software (San Diego, CA, USA). Categorical variables were expressed as numbers and percentages. The threshold for statistical significance was set to a p-value of less than 0.05.
3. Results
3.1. Prevalence Pattern among Different Age Groups
A total of 609 bacteria were isolated from cats’ skin, diarrheal stool, urine, and respiratory samples during 2018–2019, nationwide from veterinary hospital-visited cats. The overall prevalence of bacteria was higher (45.2%) among cats aged 1–5 years, followed by 27.9% in cats from the 1-year age group. Bacterial isolates from diarrheal stool and skin samples were more frequently isolated than the other cat samples (Table 2). However, the prevalence pattern was different with variation in age groups, and most of the strains were isolated from cats aged less than 5 years. However, 23% of the bacteria were isolated from urine samples of 6–10-year age group cats.

Table 2.
Number of isolates from different samples and age groups of cats.
3.2. Prevalence of Bacteria in Diarrheal Stool Samples
Overall, 267 isolates were collected and identified from diarrheal cat samples. Among the identified isolates, approximately 92.9% belonged to 13 different bacterial species. Among all identified bacteria from diarrheal stool samples, Escherichia coli was the most prevalent, with a prevalence of 65.5%. The prevalence of the identified bacteria was similar among the different regions. However, in cities where many bacteria are isolated, various strains were identified (Table 3).

Table 3.
Most prevalent bacterial species in diarrheal stool samples of cats during 2018–2019.
Comparison of prevalence among different age groups of cats showed that the overall bacterial distribution pattern was similar in all age groups of cats, except for the older age group of cats. However, some bacterial species may differ in their prevalence among different age groups, such as Klebsiella pneumoniae and Clostridium difficile, which were most prevalent in cats aged 11–15 years, followed by the 1–5-year and <1-year age groups. Enterococcus faecium was most prevalent in cats aged 11–15 years, followed by the 6–10-year, 1–5-year, and <1-year age groups, as shown in Figure 1.
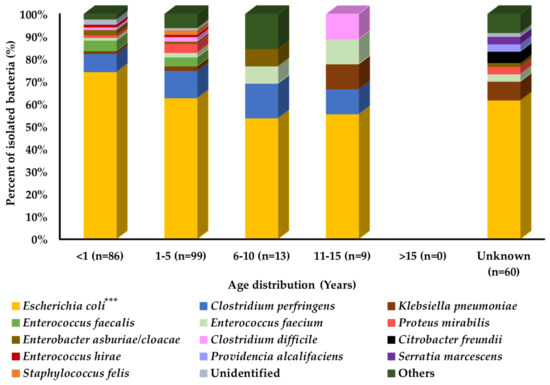
Figure 1.
Bacterial prevalence (%) of isolates from cats’ diarrheal stool samples by age group (n = 267). In graph, “n” presents number of recovered isolates; * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.3. Prevalence of Bacteria in Skin Samples
Overall, 184 isolates from a variety of bacterial species (46 species) were collected and identified in cat skin samples. In this case, Staphylococcus felis (26.1%) was the most prevalent, followed by Staphylococcus pseudintermedius (12.5%) and Staphylococcus schleiferi (8.2%). However, seven bacterial species had a relatively low prevalence range of 2–5.5%. The identified bacterial species followed a similar pattern of prevalence in different cities (Table 4).

Table 4.
Most prevalent bacterial species in skin samples of cats during 2018–2019.
Prevalence analysis with age variation in cats showed that each bacterial species had a different distribution pattern among different age groups, as shown in Figure 2. E. coli, Staphylococcus hominis, and Streptococcus canis were the most prevalent and isolated bacteria from the old age group of cats. Staphylococcus aureus, S. schleiferi, and Staphylococcus simulans were the most prevalent in the younger age group of cats. However, S. pseudintermedius and S. felis were equally distributed among all age groups.
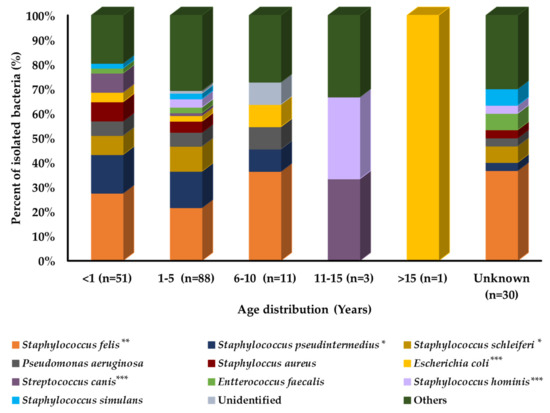
Figure 2.
Bacterial prevalence (%) of isolates from cats’ skin samples by age group (n = 184). In graph, “n” presents number of recovered isolates; * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.4. Prevalence of Bacteria in Urine Samples
Overall, 57 isolates were collected and identified in the urine samples of cats. Among the bacterial species isolated from urine samples, E. coli (17.5%) was the most prevalent, and E. faecium (8.8%) and S. felis (7%) were the second and third most prevalent species in urine samples from all cats, respectively. The overall prevalence of these three species was 33.3%, whereas the prevalence of other species ranged from 5.3% to 3.5%. However, it is difficult to compare the prevalence of individual species among different regions. The number of isolated strains was insufficient (Table 5).

Table 5.
Most prevalent bacterial species in urine samples of cats during 2018–2019.
Among all age groups, bacteria were isolated only from the middle-aged groups (1–5 and 6–10 years). Among these two age groups, E. coli was the most prevalent strain. However, some bacterial species were more prevalent in cats aged 6–10 years compared with cats aged 1–5 years, including S. felis, S. pseudintermedius, S. canis, and K. pneumoniae. However, a large number of bacterial species showed a higher prevalence in the 1–5-year age group of cats, as shown in Figure 3.
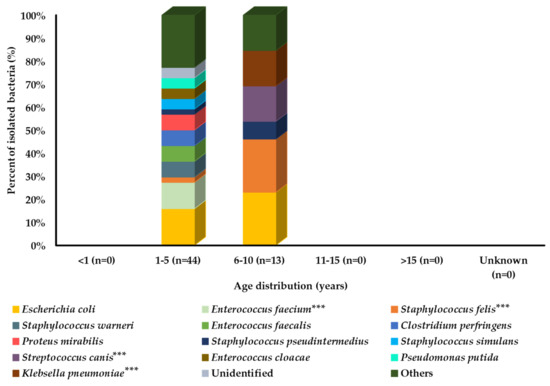
Figure 3.
Bacterial prevalence (%) of isolates from cats’ urine samples by age group (n = 57). In graph, “n” presents number of recovered isolates; * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.5. Prevalence of Bacteria from the Respiratory Tract
Overall, 101 isolates were collected and identified from the respiratory tract of cats. A wide variety of bacterial species were identified in the respiratory specimens of cats. Different from other samples, P. multocida was the most prevalent (13.9%) bacterial species isolated from respiratory samples of cats, followed by S. felis (11.9%), S. pseudintermedius (9.9%), and E. coli (7.9%). However, the prevalence of other bacterial species was relatively lower in this case than in the other samples collected from the cats. Moreover, Pasteurella dagmatis (4%) and Bordetella bronchiseptica (3%) were two important respiratory tract pathogenic bacteria isolated from the respiratory specimens of cats (Table 6).

Table 6.
Most prevalent bacterial species in respiratory samples of cats during 2018–2019.
A prevalence study with age group variation showed that the prevalence of isolated bacteria was different among the groups (Figure 4). Among all age groups of cats, a large number of bacterial species were identified in the younger age group (<1 year). P. dagmatics, B. bronchiseptica, S. canis, and E. faecium were more prevalent in younger cats than in the other age groups. In contrast, P. multocida and Proteus mirabilis showed relatively higher prevalence in the middle and S. felis in the older age groups of cats. Pseudomonas aeruginosa and E. coli were more prevalent in the 6–10-year age group of cats. However, S. pseudintermedius was equally prevalent in the younger and middle-aged groups of cats compared with the older age group.
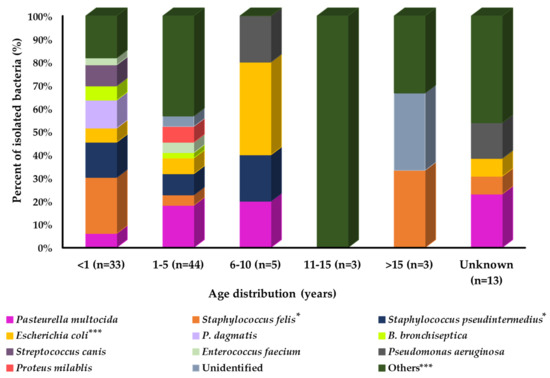
Figure 4.
Bacterial prevalence (%) of isolates from cat’s respiratory samples by age group (n = 101). In graph, “n” presents number of recovered isolates; * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
Companion animals are important for their owners’ mental and physical health. However, the close contact between owners and companion animals may be a source of zoonotic infection transmission if these animals are carriers of virulent and resistant bacteria. Hence, prevalence monitoring studies are important to guide therapeutic decisions and the development of updated strategies to control infections.
In this report, we aimed to study the first nationwide prevalence of different bacteria isolated from different samples of cats in Korea. The distribution of bacterial species was dependent on the sampling site. However, among our isolated species, the most prevalent bacterial species were E. coli in diarrheal stool and urine samples, S. felis in skin samples, and P. multocida in respiratory tract samples of cats.
Moreover, the pattern of bacterial prevalence among cats with age variation was evaluated to elucidate the prevalence transition with age. The age-dependent prevalence transition study could assist veterinarians in identifying infectious and beneficial bacteria associated with cats from different age groups. According to the findings of our study, cats aged less than 5 years were more vulnerable to infections, with an increased prevalence of a variety of bacterial species compared with the other age groups.
As only a limited number of studies have reported previously on the bacterial prevalence in cats and age association, it is difficult to compare the present findings with other reports. However, our correlation of bacterial prevalence with age was in contrast to a prevalence study in house cats in Western Turkey. In this study, Muz et al. reported that there was no statistical difference observed between the prevalence of the pathogen in different age groups [7]. Furthermore, in our study, the most commonly isolated species of bacteria were E. coli, S. felis, Enterococcus spp., and P. mirabilis in cats, which agreed with the findings of Jung et al. in stray and hospital admitted cats of South Korea [8].
In the present study, our findings were consistent with those of a previous study by Polish researchers. Bierowiec et al. reported that S. pseudintermedius is the least prevalent bacteria in cats among the Staphylococcus class of bacteria [9]. In previous reports, the prevalence of S. pseudintermedius in dogs was 90%, which is higher than that in cats with a prevalence range of 2.49–8.8% [10,11,12,13,14,15]. Our results, together with those of previous reports, showed that S. pseudintermedius might not be a natural microbiota of cats.
Among diarrheal stool samples, E. coli was the most prevalent. Among the bacteria isolated from diarrheal stool samples, Clostridium perfringens was less prevalent than E. coli. The results of the present study are consistent with prior presumptions from North California that C. perfringens is of less clinical importance [16]. However, opposing results have also been reported in diarrheic cats by Oh et al. 2021. According to this study, C. perfringens was the predominant bacteria being isolated from the diarrheic cats [17]. These discrepancies could be related to varying factors, including regions, environmental conditions, methodologies, and concurrent diseases, thereby affecting the prevalence of bacterial species.
A comparison of the results obtained in this study showed a similar trend to that of international studies. Similar to the ComPath project of Europe [18], S. felis and S. pseudintermedius were the major isolates from skin samples, followed by S. schleiferi and P. aeruginosa.
Blanco et al. and Bartges et al. reported that bacterial urinary tract infections (UTIs) in cats are relatively rare events due to a variety of host defense mechanisms and that the reported prevalence varies, depending on the exclusion and inclusion criteria of the investigators [19,20]. Studies on cats with UTIs have reported that the overall bacterial prevalence of positive bacterial urine cultures is less than 3% [21,22,23].
In our study, we analyzed the relatively low bacterial prevalence in urine samples due to the small sample size compared with the other samples of cats. In this study, bacteria identified from UTIs can be expected to cause bacterial UTI in cats. E. coli is the most prevalent bacteria among the isolated bacteria, followed by E. faecium and S. felis in the urine samples of cats, echoing the results of other published reports in cats [20,24].
Moreover, S. felis was first recognized in feline clinical specimens and is considered a normal commensal organism present on the skin, conjunctival sac and eyelid margins, and saliva of normal healthy cats [25,26,27,28]. Litster et al. reported the strains of S. felis isolated from clinical specimens of cats with cystitis [24]. Consistent with these reports, we isolated S. felis as the most prevalent bacteria in skin samples of cats. In urine samples, it was the third most prevalent bacteria. The Staphylococcal species isolated from urine samples were S. simulans, S. canis, and S. pseudintermedius. Takahashi et al. in Japan reported their little pathogenic relativity with UTIs [29].
Furthermore, among the isolates collected from the respiratory tract samples of cats, P. multocida was the most prevalent, followed by S. felis, S. pseudintermedius, E. coli, P. dagmatis, B. bronchiseptica, S. canis, and E. faecium. In previous reports from the United Kingdom and the United States, Dolieslager et al. and Freshwater reported that Pasteurella species are not only part of the normal flora in cats but also an important zoonotic agent. According to them, subtypes of Pasteurella spp. have also been associated with human infections, including P. multocida, P. canis, Pseudomonas septica, Pasteurella stomatis, and P. dagmatis [30,31]. According to previous reports, P. multocida can be isolated from the pyothorax or subcutaneous abscesses and causative agents of secondary lower respiratory tract (LRT) infections in cats [32,33,34]. Other studies have reported that cat ownership by immunocompetent or immunocompromised persons carries the risk of P. multocida infections due to its transmission through cat bite, scratch, or respiratory secretions [35,36,37]. Based on our findings, some of the identified bacteria in respiratory samples were previously reported as common pathogenic bacteria in cats associated with LRT infections [38,39,40]. Different groups of researchers have reported that B. bronchiseptica is the primary pathogen in cats and in upper respiratory tract infections and is a co-pathogen with respiratory viruses/mycoplasma [41,42,43].
In our study, we isolated bacteria not only pathogenic to cats but also to humans. Overgaauw et al. reported that cat–owner companionship has significantly fewer positive health effects [44]. This study showed that the presence of zoonotic parasites and pathogens, including bacteria, viruses, and fungi, in healthy cats and dogs transmitted to humans through scratching, biting, licking, sneezing, and handling of pets consequently induced mild or severe bacterial infections. Another previous study reported that P. multocida, Salmonella, S. canis, H. pylori, P. aeruginosa, K. pneumonia, Bordetella, and Corynebacterium bovis are pathogenic bacteria associated with bacterial infections in humans and are transmitted through companion animals [44].
The companion animal population, especially cats, is likely to be more vulnerable to infectious diseases, leading to increased opportunities for antimicrobial administration. The emergence of antimicrobial resistance is another growing concern in veterinary practice. Thus, prevalence studies can guide the selection of appropriate treatments for each case and reduce bacterial resistance. Moreover, the findings of our study can create a paradigm for future studies on bacterial prevalence and antimicrobial susceptibility not only in cats but also in other companion animals, such as dogs.
5. Limitation and Future Perspectives
This study has some limitations such as the lack of information about the number of samples by region and season. Moreover, information about the sex, condition, and health status of cats was not provided, and several human-relevant bacteria have not been recovered in the study, such as EHEC (Enterohemorrhagic E. coli) and Salmonella, which might be due to a lack of specific culture media and growth conditions tested. However, prevalence data obtained from the present study could serve as a reference to start bacterial susceptibility and antimicrobial resistance monitoring in cats and be used to guide veterinarians’ decisions in clinical practices for the selection of antimicrobial agents to treat bacterial infections in cats throughout Korea. The findings of this study provide an improved understanding of bacterial prevalence for site-specific disorders among cats and can support veterinarians when listing differential diagnoses. Moreover, these findings can assist in the prioritization of therapeutics and health control strategies. Bacterial prevalence studies are not only important to attenuate the overuse of antimicrobials in veterinary practices but also help to reduce the spread of bacterial infections among companion animals and humans. There is a need to study the associated infections and susceptibility profiles of these bacteria in the future.
Author Contributions
Conceptualization, S.-K.L. and D.C.M.; methodology, N.B., J.-H.C., and S.-J.K.; software, D.C.M., N.B., J.-H.C., and S.-J.K.; validation, N.B. and J.-H.C.; formal analysis, H.-J.S., S.-S.Y., and M.-C.G.; investigation, D.C.M., N.B., H.-J.S., H.-S.P., M.-C.G., and S.-J.K.; data curation, D.C.M. and S.-S.Y.; writing—original draft preparation, D.C.M. and N.B.; writing—review and editing, S.-S.Y. and S.-K.L.; supervision, S.-S.Y. and S.-K.L.; project administration, S.-S.Y. and S.-K.L.; funding acquisition; S.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food, and Rural Affairs, Korea (grant number: N-1543081-2017-24-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank all the laboratories and centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Friedmann, E.; Gee, N.R.; Simonsick, E.M.; Studenski, S.; Resnick, B.; Barr, E.; Kitner-Triolo, M.; Hackney, A. Pet ownership patterns and successful aging outcomes in community dwelling older adults. Front. Vet. Sci. 2020, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Seo, G.; Kim, H.; Kim, W.; Ji, I. The Estimation of Current and Future Market Size of Pet Related Industries. Korean J. Agric. Manag. Policy 2018, 45, 611–629. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Kim, J.; Chun, B.C. Association between companion animal ownership and overall life satisfaction in Seoul, Korea. PLoS ONE 2021, 16, e0258034. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. Laboratory Methods for Use in Mastitis Work; International Dairy Federation Bulletin; International Dairy Federation: Schaerbeek, Belgium, 1981; Volume 132. [Google Scholar]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clinic. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Muz, M.N.; Erat, S.; Mumcuoglu, K.Y. Protozoan and Microbial Pathogens of House Cats in the Province of Tekirdag in Western Turkey. Pathogens 2021, 10, 1114. [Google Scholar] [CrossRef]
- Jung, W.K.; Shin, S.; Park, Y.K.; Lim, S.-K.; Moon, D.-C.; Park, K.T.; Park, Y.H. Distribution and antimicrobial resistance profiles of bacterial species in stray cats, hospital-admitted cats, and veterinary staff in South Korea. BMC Vet. Res. 2020, 16, 109. [Google Scholar] [CrossRef]
- Bierowiec, K.; Miszczak, M.; Korzeniowska-Kowal, A.; Wzorek, A.; Płókarz, D.; Gamian, A. Epidemiology of Staphylococcus pseudintermedius in cats in Poland. Sci. Rep. 2021, 11, 18898. [Google Scholar] [CrossRef]
- Rubin, J.E.; Chirino-Trejo, M. Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. J. Vet. Diagn. Invest. 2011, 23, 351–354. [Google Scholar] [CrossRef]
- Ma, G.C.; Worthing, K.A.; Ward, M.P.; Norris, J.M. Commensal staphylococci including methicillin-resistant Staphylococcus aureus from dogs and cats in remote New South Wales, Australia. Microb. Ecol. 2020, 79, 164–174. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, Y.K.; Shin, S.; Park, Y.H.; Park, K.T. Characterization of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Dogs in Veterinary Hospitals in Korea. Int. J. Appl. Res. Vet. Med. 2018, 16. Available online: https://www.researchgate.net/publication/330654805_Characterization_of_Methicillin-Resistant_Staphylococcus_pseudintermedius_Isolated_from_Dogs_in_Veterinary_Hospitals_in_Korea (accessed on 28 January 2022).
- Hanselman, B.A.; Kruth, S.A.; Rousseau, J.; Weese, J.S. Coagulase positive staphylococcal colonization of humans and their household pets. Can. Vet. J. 2009, 50, 954. [Google Scholar] [PubMed]
- Han, J.-I.; Rhim, H.; Yang, C.-H.; Park, H.-M. Molecular characteristics of new clonal complexes of Staphylococcus pseudintermedius from clinically normal dogs. Vet. Q. 2018, 38, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, G.C.; Morris, D.O.; Abraham, J.L.; Shofer, F.S.; Rankin, S.C. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 2008, 19, 142–149. [Google Scholar] [CrossRef]
- Queen, E.V.; Marks, S.L.; Farver, T.B. Prevalence of Selected Bacterial and Parasitic Agents in Feces from Diarrheic and Healthy Control Cats from Northern California. J. Vet. Intern. Med. 2012, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-I.; Seo, K.-W.; Kim, D.-H.; Cheon, D.-S. Prevalence, co-infection and seasonality of fecal enteropathogens from diarrheic cats in the Republic of Korea (2016–2019): A retrospective study. BMC Vet. Res. 2021, 17, 367. [Google Scholar] [CrossRef]
- Morrissey, I.; Moyaert, H.; de Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of bacterial pathogens isolated from respiratory tract infections in dogs and cats across Europe: ComPath results. Vet. Microbiol. 2016, 191, 44–51. [Google Scholar] [CrossRef]
- Blanco, L.; Bartges, J. Understanding and eradicating bacterial urinary tract infections. Vet. Med. 2001, 96, 776. [Google Scholar]
- Bartges, J.; Barsanti, J. Bacterial Urinary Tract Infection in Cats. Kirks Curr. Vet. Ther. 2000, 13, 880–882. [Google Scholar]
- Lekcharoensuk, C.; Osborne, C.A.; Lulich, J.P. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J. Am. Vet. Med. Assoc. 2001, 218, 1429–1435. [Google Scholar] [CrossRef]
- Kruger, J.; Osborne, C.A.; Goyal, S.M.; Wickstrom, S.; Johnston, G.; Fletcher, T.F.; Brown, P. Clinical evaluation of cats with lower urinary tract disease. J. Am. Vet. Med. Assoc. 1991, 199, 211–216. [Google Scholar] [PubMed]
- Buffington, C.; Chew, D.J.; Kendall, M.S.; Scrivani, P.V.; Thompson, S.B.; Blaisdell, J.; Woodworth, B. Clinical evaluation of cats with nonobstructive urinary tract diseases. J. Am. Vet. Med. Assoc. 1997, 210, 46–50. [Google Scholar] [PubMed]
- Litster, A.; Moss, S.M.; Honnery, M.; Rees, B.; Trott, D.J. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: Recognition of Staphylococcus felis as a possible feline urinary tract pathogen. Vet. Microbiol. 2007, 121, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lilenbaum, W.; Nunes, E.; Azeredo, M. Prevalence and antimicrobial susceptibility of staphylococci isolated from the skin surface of clinically normal cats. Lett. Appl. Microbiol. 1998, 27, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Lilenbaum, W.; Esteves, A.; Souza, G. Prevalence and antimicrobial susceptibility of staphylococci isolated from saliva of clinically normal cats. Lett. Appl. Microbiol. 1999, 28, 448–452. [Google Scholar] [CrossRef]
- Igimi, S.; Kawamura, S.; Takahashi, E.; Mitsuoka, T. Staphylococcus felis, a new species from clinical specimens from cats. Int. J. Syst. Evol. Microbiol. 1989, 39, 373–377. [Google Scholar] [CrossRef]
- Espínolaz, M.; Lilenbaum, W. Prevalence of bacteria in the conjunctival sac and on the eyelid margin of clinically normal cats. J. Small Anim. Pract. 1996, 37, 364–366. [Google Scholar] [CrossRef]
- Takahashi, T.; Kaneko, M.; Mori, Y.; Tsuji, M.; Kikuchi, N.; Hiramune, T. Phylogenetic analyses of Staphylococcus based on the 16S rDNA sequence and assignment of clinical isolates from animals. J. Vet. Med. Sci. 1997, 59, 775–783. [Google Scholar] [CrossRef][Green Version]
- Dolieslager, S.M.; Riggio, M.P.; Lennon, A.; Lappin, D.F.; Johnston, N.; Taylor, D.; Bennett, D. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. J. Vet. Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef]
- Freshwater, A. Why your housecat’s trite little bite could cause you quite a fright: A study of domestic felines on the occurrence and antibiotic susceptibility of Pasteurella multocida. Zoonoses Public Health 2008, 55, 507–513. [Google Scholar] [CrossRef]
- Barrs, V.R.; Allan, G.S.; Martin, P.; Beatty, J.A.; Malik, R. Feline pyothorax: A retrospective study of 27 cases in Australia. J. Feline Med. Surg. 2005, 7, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ottenjann, M.; Luebke-Becker, A.; Linzmann, H.; Brunnberg, L.; Kohn, B. Pyothorax in 26 cats: Clinical signs, laboratory results and therapy (2000–2007). Berl. Munch. Tierarztl. Wochenschr. 2008, 121, 365–373. [Google Scholar] [PubMed]
- Roy, J.; Messier, S.; Labrecque, O.; Cox, W.R. Clinical and in vitro efficacy of amoxicillin against bacteria associated with feline skin wounds and abscesses. Can. Vet. J. 2007, 48, 607. [Google Scholar]
- Kimura, R.; Hayashi, Y.; Takeuchi, T.; Shimizu, M.; Iwata, M.; Tanahashi, J.; Ito, M. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J. Infect. Chemother. 2004, 10, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Layton, C.T. Pasteurella multocida meningitis and septic arthritis secondary to a cat bite. J. Emerg. Med. 1999, 17, 445–448. [Google Scholar] [CrossRef]
- Westling, K.; Bygdeman, S.; Engkvist, O.; Jorup-Rönström, C. Pasteurella multocida infection following cat bites in humans. J. Infect. 2000, 40, 97–98. [Google Scholar] [CrossRef]
- Foster, S.F.; Martin, P. Lower respiratory tract infections in cats: Reaching beyond empirical therapy. J. Feline Med. Surg. 2011, 13, 313–332. [Google Scholar] [CrossRef]
- Schulz, B.; Wolf, G.; Hartmann, K. Bacteriological and antibiotic sensitivity test results in 271 cats with respiratory tract infections. Vet. Rec. Open 2006, 158, 269. [Google Scholar] [CrossRef]
- Veir, J.K.; Ruch-Gallie, R.; Spindel, M.E.; Lappin, M.R. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J. Feline Med. Surg. 2008, 10, 551–557. [Google Scholar] [CrossRef]
- Egberink, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H. Bordetella bronchiseptica infection in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 610–614. [Google Scholar] [CrossRef]
- Speakman, A.; Dawson, S.; Binns, S.; Gaskell, C.; Hart, C.; Gaskell, R. Bordetella bronchiseptica infection in the cat. J. Small Anim. Pract. 1999, 40, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Adler, K.; Radeloff, I.; Stephan, B.; Greife, H.; Hellmann, K. Bacteriological and virological status in upper respiratory tract infections of cats (cat common cold complex). Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 120–125. [Google Scholar] [PubMed]
- Overgaauw, P.A.M.; Vinke, C.M.; Hagen, M.A.E.v.; Lipman, L.J.A. A One Health Perspective on the Human-Companion Animal Relationship with Emphasis on Zoonotic Aspects. Int. J. Environ. Res. Public Health 2020, 17, 3789. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).