Mycobacterium bovis Strain Ravenel Is Attenuated in Cattle
Abstract
:1. Introduction
2. Methods
2.1. Mycobacterium Tuberculosis Variant Bovis Challenge Strains
2.2. Cattle Studies: Treatment Groups and Aerosol MBO Challenge Procedures
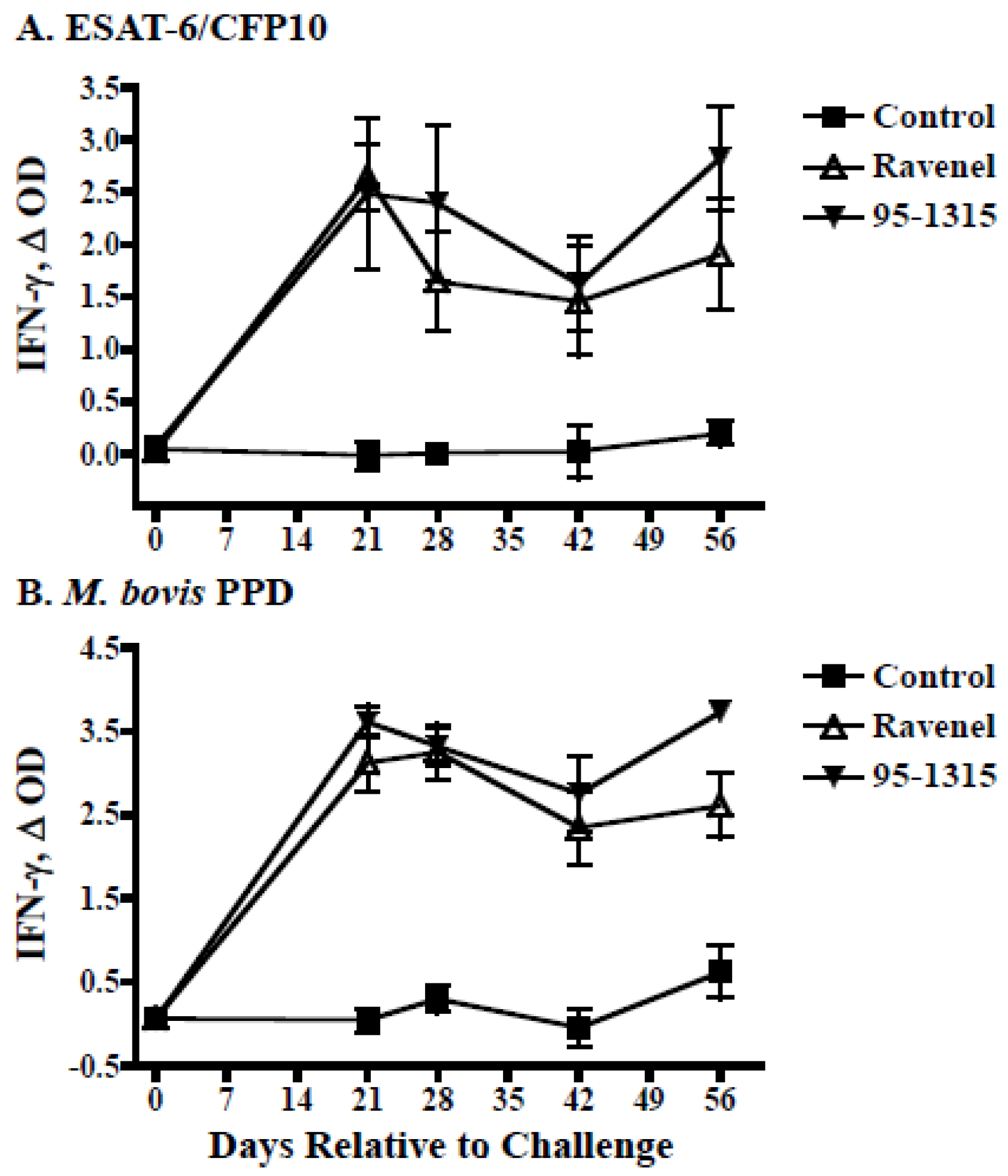
2.3. IFN-γ Whole Blood Assays
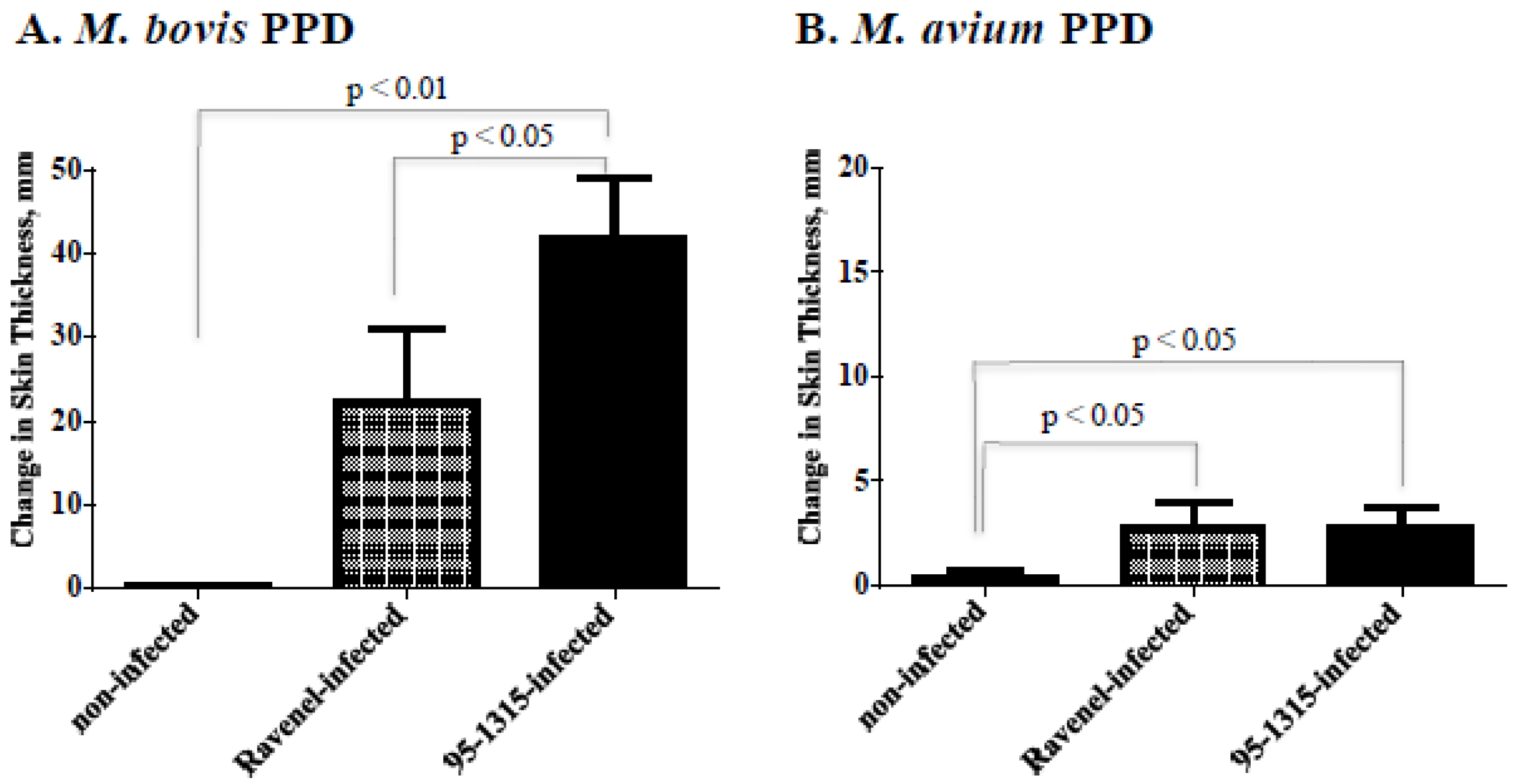
2.4. Delayed Type Hypersensitivity (DTH) Responses (Skin Test Procedures)
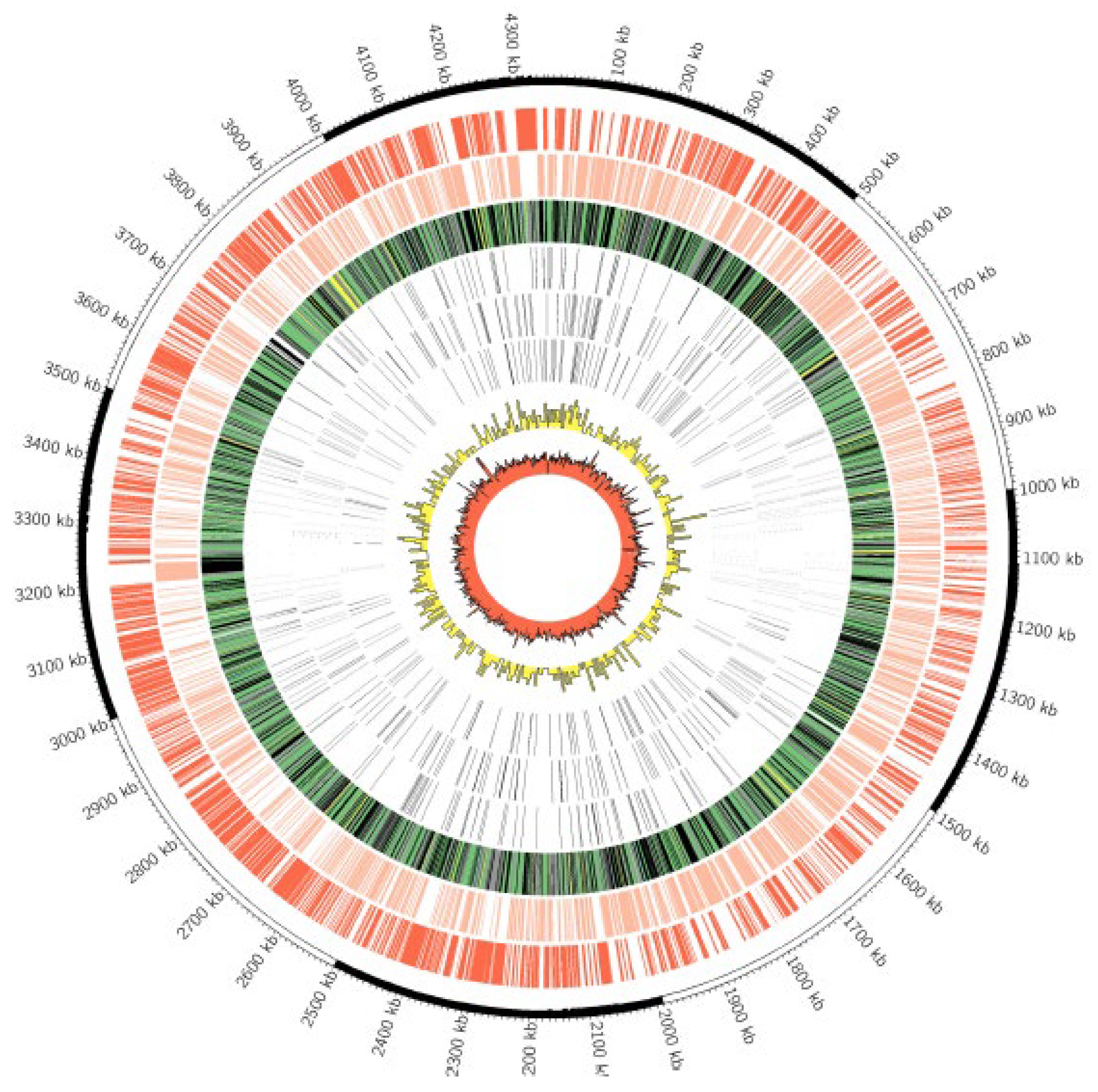
2.5. Whole Genome Sequencing of MBO Ravenel
2.6. Genome Comparisons
2.6.1. Identification of Single Nucleotide Polymorphisms (SNPs) in MBO Ravenel
2.6.2. Region of Difference (RD) and Gene Presence/Absence Analysis
3. Results
3.1. Experimental Infection of Cattle
3.2. Whole Genome Sequencing
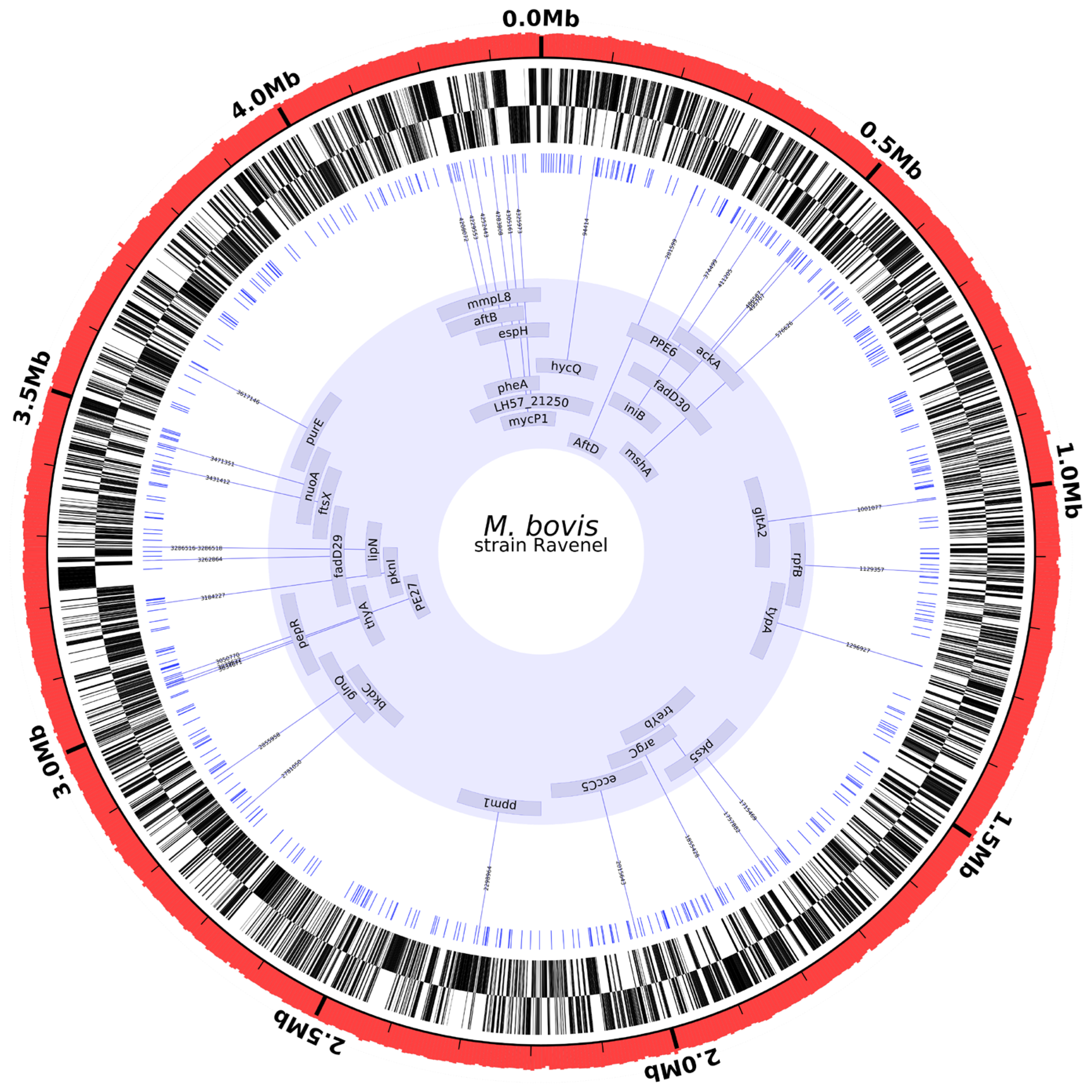
3.3. Genome Comparisons
SNPs from MBO Ravenel versus Virulent MBO Strains
4. Discussion
4.1. Ravenel Elicits Robust Cell-Mediated Immune Response but Is Attenuated
4.2. Ravenel Carries SNPs in Genes Encoding Cell Wall Integrity
4.3. Ravenel Carries SNPs in Genes Associated with Respiration or Lipid Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleeberg, H.H. Human tuberculosis of bovine origin in relation to public health. Rev. Sci. Tech. Off. Int. Epizoot. 1984, 3, 23–27. [Google Scholar]
- Wilkins, M.J.; Meyerson, J.; Bartlett, P.C.; Spieldenner, S.L.; Berry, D.E.; Mosher, L.B.; Kaneene, J.B.; Robinson-dunn, B.; Stobierski, M.G.; Boulton, M.L. Human Mycobacterium bovis infection and bovine tuberculosis outbreak, Michigan, 1994–2007. Emerg. Infect. Dis. 2008, 14, 657–660. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dannenberg, A.M. Pathogenesis of pulmonary Mycobacterium bovis infection: Basic principles established by the rabbit model. Tuberculosis 2001, 81, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.L.; North, R.J. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect. Immun. 1995, 63, 3428–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, H.J.; Dannenberg, A.M.; Lurie, M.B. Phagocytosis of Tubercle Bacilli by Rabbit Pulmonary Alveolar Macrophages and Its Relation to Native Resistance to Tuberculosis. J. Immunol. 1963, 91, 553–556. [Google Scholar]
- Nedeltchev, G.G.; Raghunand, T.R.; Jassal, M.S.; Lun, S.; Cheng, Q.J.; Bishai, W.R. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect. Immun. 2009, 77, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Harboe, M.; Nagai, S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am. Rev. Respir. Dis. 1984, 129, 444–452. [Google Scholar]
- Tsenova, L.; Sokol, K.; Freedman, V.H.; Kaplan, G. A Combination of Thalidomide Plus Antibiotics Protects Rabbits from Mycobacterial Meningitis-Associated Death. J. Infect. Dis. 1998, 177, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Yamamoto, K.; Okuyama, H.; Kimura, T. Microbicidal activity and morphological characteristics of lung macrophages in Mycobacterium bovis BCG cell wall-induced lung granuloma in mice. Infect. Immun. 1984, 45, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Khare, S.; Hondalus, M.K.; Nunes, J.; Bloom, B.R.; Garry Adams, L. Mycobacterium bovis ΔleuD auxotroph-induced protective immunity against tissue colonization, burden and distribution in cattle intranasally challenged with Mycobacterium bovis Ravenel S. Vaccine 2007, 25, 1743–1755. [Google Scholar] [CrossRef]
- Converse, P.J.; Dannenberg, A.M.; Shigenaga, T.; Mcmurray, D.N.; Phalen, S.W.; Stanford, J.L.; Rook, G.A.W.; Koru-Sengul, T.; Abbey, H.; Estep, J.E.; et al. Pulmonary bovine-type tuberculosis in rabbits: Bacillary virulence, inhaled dose effects, tuberculin sensitivity, and Mycobacterium vaccae immunotherapy. Clin. Diagn. Lab. Immunol. 1998, 5, 871–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Via, L.E.; Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Seok, Y.E.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008, 76, 2333–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, R.J.; Ryan, L.; LaCource, R.; Mogues, T.; Goodrich, M.E. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect. Immun. 1999, 67, 5483–5485. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Palmer, M.V.; Nonnecke, B.J.; Thacker, T.C.; Scherer, C.F.C.; Estes, D.M.; Hewinson, R.G.; Vordermeier, H.M.; Barnes, S.W.; Federe, G.C.; et al. Efficacy and immunogenicity of Mycobacterium bovis ΔRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 2009, 27, 1201–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, M.V.; Waters, W.R.; Whipple, D.L. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 2003, 82, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Buddle, B.M.; Skinner, M.A.; Wedlock, D.N.; De Lisle, G.W.; Vordermeier, H.M.; Hewinson, R.G. Cattle as a model for development of vaccines against human tuberculosis. Tuberculosis 2005, 85, 19–24. [Google Scholar] [CrossRef]
- Johnson, L.; Dean, G.; Rhodes, S.; Hewinson, G.; Vordermeier, M.; Wangoo, A. Low-dose Mycobacterium bovis infection in cattle results in pathology indistinguishable from that of high-dose infection. Tuberculosis 2007, 87, 71–76. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Connery, N.L.; McNair, J.; Welsh, M.D.; Skuce, R.A.; Bryson, D.G.; McMurray, D.N.; Pollock, J.M. Experimental exposure of cattle to a precise aerosolised challenge of Mycobacterium bovis: A novel model to study bovine tuberculosis. Tuberculosis 2007, 87, 405–414. [Google Scholar] [CrossRef]
- Schmitt, S.M.; Fitzgerald, S.D.; Cooley, T.M.; Bruning-Fann, C.S.; Sullivan, L.; Berry, D.; Carlson, T.; Minnis, R.B.; Payeur, J.B.; Sikarskie, J. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 1997, 33, 749–758. [Google Scholar] [CrossRef]
- Larsen, M.H.; Biermann, K.; Jacobs, W.R. Laboratory Maintenance of Mycobacterium tuberculosis. Curr. Protoc. Microbiol. 2007, 6, 10A.1.1–10A.1.8. [Google Scholar] [CrossRef]
- Wangoo, A.; Johnson, L.; Gough, J.; Ackbar, R.; Inglut, S.; Hicks, D.; Spencer, Y.; Hewinson, G.; Vordermeier, M. Advanced granulomatous lesions in Mycobacterium bovis-infected cattle are associated with increased expression of type I procollagen, gammadelta (WC1+) T cells and CD 68+ cells. J. Comp. Pathol. 2005, 133, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Palmer, M.V.; Nonnecke, B.J.; Thacker, T.C.; Scherer, C.F.C.; Estes, D.M.; Jacobs, W.R.; Glatman-Freedman, A.; Larsen, M.H. Failure of a Mycobacterium tuberculosis ΔRD1 ΔpanCD double deletion mutant in a neonatal calf aerosol M. bovis challenge model: Comparisons to responses elicited by M. bovis bacille Calmette Guerin. Vaccine 2007, 25, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Alonge, M.; Soyk, S.; Ramakrishnan, S.; Wang, X.; Goodwin, S.; Sedlazeck, F.J.; Lippman, Z.B.; Schatz, M.C. RaGOO: Fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 2019, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A.; STeven, J.J.; Marra, M.A.; et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Wattam, A.R.; Abraham, D.; Dalay, O.; Disz, T.L.; Driscoll, T.; Gabbard, J.L.; Gillespie, J.J.; Gough, R.; Hix, D.; Kenyon, R.; et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014, 42, D581–D591. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Snippy: Rapid Haploid Variant Calling and Core SNP Phylogeny. GitHub. 2015. Available online: https://github.com/tseemann/snippy (accessed on 11 November 2020).
- Faksri, K.; Xia, E.; Tan, J.H.; Teo, Y.Y.; Ong, R.T. In silico region of difference (RD) analysis of Mycobacterium tuberculosis complex from sequence reads using RD-Analyzer. BMC Genom. 2016, 17, 847. [Google Scholar] [CrossRef] [Green Version]
- Kapopoulou, A.; Lew, J.M.; Cole, S.T. The MycoBrowser portal: A comprehensive and manually annotated resource for mycobacterial genomes. Tuberculosis 2011, 91, 8–13. [Google Scholar] [CrossRef]
- Converse, S.E.; Mougous, J.D.; Leavell, M.D.; Leary, J.A.; Bertozzi, C.R.; Cox, J.S. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA 2003, 100, 6121–6126. [Google Scholar] [CrossRef] [Green Version]
- Domenech, P.; Reed, M.B.; Barry, C.E. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect. Immun. 2005, 73, 3492–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, G.; Tyagi, S.; Bishai, W.R. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect. Immun. 2005, 73, 2533–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeliger, J.C.; Holsclaw, C.M.; Schelle, M.W.; Botyanszki, Z.; Gilmore, S.A.; Tully, S.E. Elucidation and chemical modulation of sulfolipid-1 biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2012, 287, 7990–8000. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Belardinelli, J.M.; Pasca, M.R.; De Rossi, E.; Riccardi, G.; Chiarelli, L.R. Promiscuous Targets for Antitubercular Drug Discovery: The Paradigm of DprE1 and MmpL3. Appl. Sci. 2020, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.T.; Haiderer, E.R.; Coulson, G.B.; Conner, K.N.; Ellsworth, E.; Chen, C.; Alvarez-Cabrera, N.; Li, W.; Jackson, M.; Dick, T.; et al. Identification of new MMPL3 inhibitors by untargeted and targeted mutant screens defines MMPL3 domains with differential resistance. Antimicrob. Agents Chemother. 2019, 63, e00547-19. [Google Scholar] [CrossRef]
- Jankute, M.; Alderwick, L.J.; Noack, S.; Veerapen, N.; Nigou, J.; Besra, G.S. Disruption of mycobacterial aftB results in complete loss of terminal β(1 → 2) arabinofuranose residues of Lipoarabinomannan. ACS Chem. Biol. 2017, 12, 183–190. [Google Scholar] [CrossRef]
- Raad, R.B.; Méniche, X.; De Sousa-D’Auria, C.; Chami, M.; Salmeron, C.; Tropis, M.; Labarre, C.; Daffé, M.; Houssin, C.; Bayan, N. A deficiency in arabinogalactan biosynthesis affects Corynebacterium glutamicum mycolate outer membrane stability. J. Bacteriol. 2010, 192, 2691–2700. [Google Scholar] [CrossRef] [Green Version]
- Hett, E.C.; Chao, M.C.; Deng, L.L.; Rubin, E.J. A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog. 2008, 4, e1000001. [Google Scholar] [CrossRef] [Green Version]
- Hett, E.C.; Chao, M.C.; Steyn, A.J.; Fortune, S.M.; Deng, L.L.; Rubin, E.J. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol. 2007, 66, 658–668. [Google Scholar] [CrossRef]
- Kana, B.D.; Gordhan, B.G.; Downing, K.J.; Sung, N.; Vostroktunova, G.; Machowski, E.E.; Tsenova, L.; Young, M.; Kaprelyants, A.; Kaplan, G.; et al. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth In Vitro. Mol. Microbiol. 2008, 67, 672–684. [Google Scholar] [CrossRef] [Green Version]
- Kana, B.D.; Mizrahi, V. Resuscitation-promoting factors as lytic enzymes for bacterial growth and signaling. FEMS Immunol. Med. Microbiol. 2010, 58, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell-Goldman, E.; Xu, J.; Wang, X.; Chan, J.; Tufariello, J.A.M. A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect. Immun. 2008, 76, 4269–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Gonsaud, M.; Barthe, P.; Bagnéris, C.; Henderson, B.; Ward, J.; Roumestand, C.; Keep, N.H. The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes. Nat. Struct. Mol. Biol. 2005, 12, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Romano, M.; Ruggiero, A.; Vitagliano, L.; De Simone, A.; Berisio, R. Carbohydrate recognition by RpfB from Mycobacterium tuberculosis unveiled by crystallographic and molecular dynamics analyses. Biophys. J. 2013, 104, 2530–2539. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544, Erratum in Nature 1998, 396, 190. [Google Scholar] [CrossRef] [Green Version]
- Lima, P.; Sidders, B.; Morici, L.; Reader, R.; Senaratne, R.; Casali, N.; Riley, L.W. Enhanced mortality despite control of lung infection in mice aerogenically infected with a Mycobacterium tuberculosis mce1 operon mutant. Microbes Infect. 2007, 9, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Tekaia, F.; Gordon, S.V.; Garnier, T.; Brosch, R.; Barrell, B.G.; Cole, S.T. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 1999, 79, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Siméone, R.; Léger, M.; Constant, P.; Malaga, W.; Marrakchi, H.; Daffé, M.; Guilhot, C.; Chalut, C. Delineation of the roles of FadD22, FadD26 and FadD29 in the biosynthesis of phthiocerol dimycocerosates and related compounds in Mycobacterium tuberculosis. FEBS J. 2010, 277, 2715–2725. [Google Scholar] [CrossRef]
- Trivedi, O.A.; Arora, P.; Sridharan, V.; Tickoo, R.; Mohanty, D.; Gokhale, R.S. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 2004, 428, 441–445. [Google Scholar] [CrossRef]
- Gilbert, S.; Hood, L.C.; Seah, S.Y.K. Characterization of an aldolase involved in cholesterol side chain degradation in Mycobacterium tuberculosis. J. Bacteriol. 2018, 200, e00512-17. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.T.; VanderVen, B.C.; Sherman, D.R.; Russell, D.G.; Sampson, N.S. Pathway profiling in Mycobacterium tuberculosis: Elucidation of cholesterol-derived catabolite and enzymes that catalyze its metabolism. J. Biol. Chem. 2011, 286, 43668–43678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, E.A.; Janagama, H.K.; Ribeiro-lima, J.; Vulchanova, L.; Seth, M.; Yang, M.; Kurmi, K.; Waters, W.R. Circulating Mycobacterium bovis Peptides and Host Response Proteins as Biomarkers for Unambiguous Detection of Subclinical Infection. J. Clin. Microbiol. 2014, 52, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne, G.; Malaga, W.; Laval, F.; Lemassu, A.; Guilhot, C.; Daffé, M. Identification of the polyketide synthase involved in the biosynthesis of the surface-exposed lipooligosaccharides in mycobacteria. J. Bacteriol. 2009, 191, 2613–2621. [Google Scholar] [CrossRef]
- Rousseau, C.; Sirakova, T.; Dubey, V.; Bordat, Y.; Kolattukudy, P.; Gicquel, B.; Jackson, M. Virulence attenuation of two Mas-like polyketide synthase mutants of Mycobacterium tuberculosis. Microbiology 2003, 149, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, F.; Brosch, R. ESX/Type VII Secretion Systems—An Important Way Out for Mycobacterial Proteins. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef]
- Ohol, Y.M.; Goetz, D.H.; Chan, K.; Shiloh, M.U.; Craik, C.S.; Cox, J.S. Mycobacterium tuberculosis MycP1 Protease Plays a Dual Role in Regulation of ESX-1 Secretion and Virulence. Cell Host Microbe 2010, 7, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, N.; Siddiqui, I.; Sharma, P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis 2008, 88, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Pym, A.S.; Brodin, P.; Brosch, R.; Huerre, M.; Cole, S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002, 46, 709–717. [Google Scholar] [CrossRef]
- Santos, C.L.; Correia-Neves, M.; Moradas-Ferreira, P.; Mendes, M.V. A walk into the LuxR regulators of Actinobacteria: Phylogenomic distribution and functional diversity. PLoS ONE 2012, 7, e46758. [Google Scholar] [CrossRef]

| Treatment Group | Mean Disease Score a | ||
|---|---|---|---|
| Tracheobronchial Lymph Nodes | Mediastinal Lymph Nodes | Lung | |
| Non-infected | 0 ± 0 (0/5) | 0 ± 0 (0/5) | 0 ± 0 (0/5) |
| MBO strain Ravenel infected | 0 ± 0 (0/5) | 0 ± 0 (0/5) | 0.04 ± 0.04 (1/5) |
| MBO strain 95-1315 infected | 1.4 ± 0.2 * (5/5) | 1.4 ± 0.2 * (5/5) | 1.2 ± 0.3 * (5/5) |
| Treatment Group | Quantitative Culture (CFU/g) a | Qualitative Culture b | ||
|---|---|---|---|---|
| Tracheobronchial Lymph Node | Mediastinal Lymph Node | Lung | ||
| Non-infected | 0 ± 0 | 0/5 | 0/5 | 0/5 |
| MBO Ravenel infected | 427 ± 1246 | 4/5 | 1/5 | 0/5 |
| MBO 95-1315 infected | 28,141 ± 9063 * | 5/5 | 4/5 | 3/5 |
| Position | Gene Name | Gene Identifier | Mycobrowser Classification | Functional Annotation | DNA Change (Protein Change) |
|---|---|---|---|---|---|
| 4305161 | mycP1 | MB3913C | Intermediary metabolism and respiration | membrane-anchored mycosin mycp1 (serine protease) (subtilisin-like protease) (subtilase-like) (mycosin-1) | C374T (T125I) |
| 4283808 | espH | MB3897 | Cell wall and cell processes | esx-1 secretion-associated protein | G307A (A103T) |
| 4229553 | mmpL8 | MB3853C | Cell wall and cell processes | conserved integral membrane transport protein | G2662A (V888I) |
| 4208072 | aftB | MB3835C | Cell wall and cell processes | possible arabinofuranosyltransferase | C1450T (H484Y) |
| 2015643 | eccC5 | MB1812 | Cell wall and cell processes | esx conserved component eccc5. esx-5 type vii secretion system protein | C1520T (T507M) |
| 1129357 | rpfB | MB1036 | Cell wall and cell processes | Probable resuscitation-promoting factor | A788G (E263G) |
| 1715469 | pks5 | MB1554C | Lipid metabolism | Probable polyketide synthase | G1363A (G455S) |
| 3262864 | fadD29 | MB2974C | Lipid metabolism | fatty-acid-amp ligase fadd29 (fatty-acid-amp synthetase) (fatty-acid-amp synthase) | A692G (N231S) |
| 3929690 | fadE29 | MB3573C | Lipid metabolism | Probable Acyl-CoA dehydrogenase | T1079G (V360G) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, S.A.; Brenner, E.P.; Palmer, M.V.; Waters, W.R.; Thacker, T.C.; Vilchèze, C.; Larsen, M.H.; Jacobs, W.R., Jr.; Sreevatsan, S. Mycobacterium bovis Strain Ravenel Is Attenuated in Cattle. Pathogens 2022, 11, 1330. https://doi.org/10.3390/pathogens11111330
Hadi SA, Brenner EP, Palmer MV, Waters WR, Thacker TC, Vilchèze C, Larsen MH, Jacobs WR Jr., Sreevatsan S. Mycobacterium bovis Strain Ravenel Is Attenuated in Cattle. Pathogens. 2022; 11(11):1330. https://doi.org/10.3390/pathogens11111330
Chicago/Turabian StyleHadi, Syeda A., Evan P. Brenner, Mitchell V. Palmer, W. Ray Waters, Tyler C. Thacker, Catherine Vilchèze, Michelle H. Larsen, William R. Jacobs, Jr., and Srinand Sreevatsan. 2022. "Mycobacterium bovis Strain Ravenel Is Attenuated in Cattle" Pathogens 11, no. 11: 1330. https://doi.org/10.3390/pathogens11111330






