The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Collection and Dissection
2.2. Identification of Insects
2.3. DNA Isolation, PCR, and Sequencing
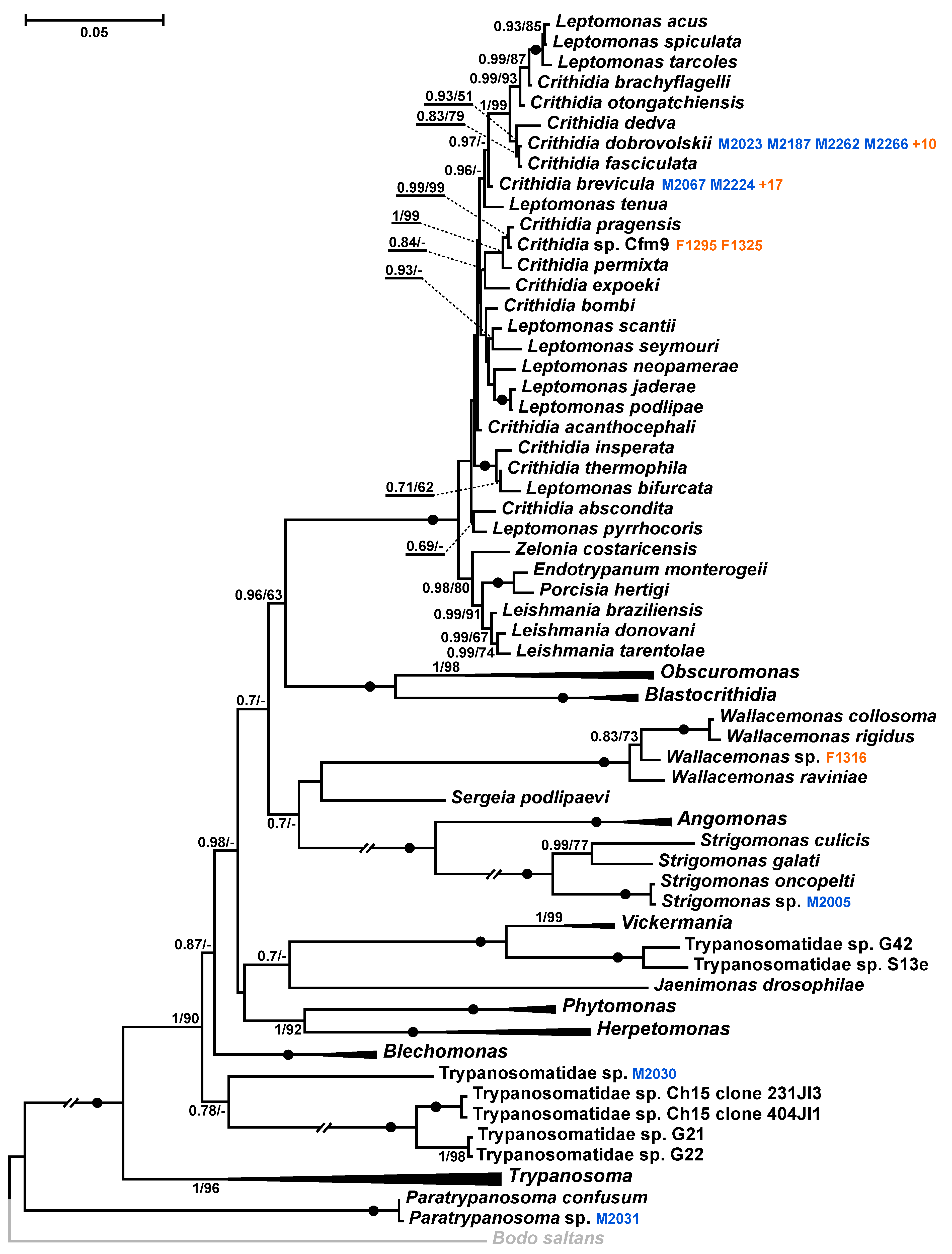
2.4. Phylogenetic Analysis
2.5. Experimental Infections
2.5.1. Larvae
2.5.2. Imagines
3. Results
3.1. Natural Trypanosomatid Infections in Overwintering Mosquitoes
3.2. Natural Trypanosomatid Prevalence in Adult Mosquitoes at the End of the Warm Season
3.3. Natural Trypanosomatid Prevalence in Mosquitoe Larvae
3.4. Experimental Infections
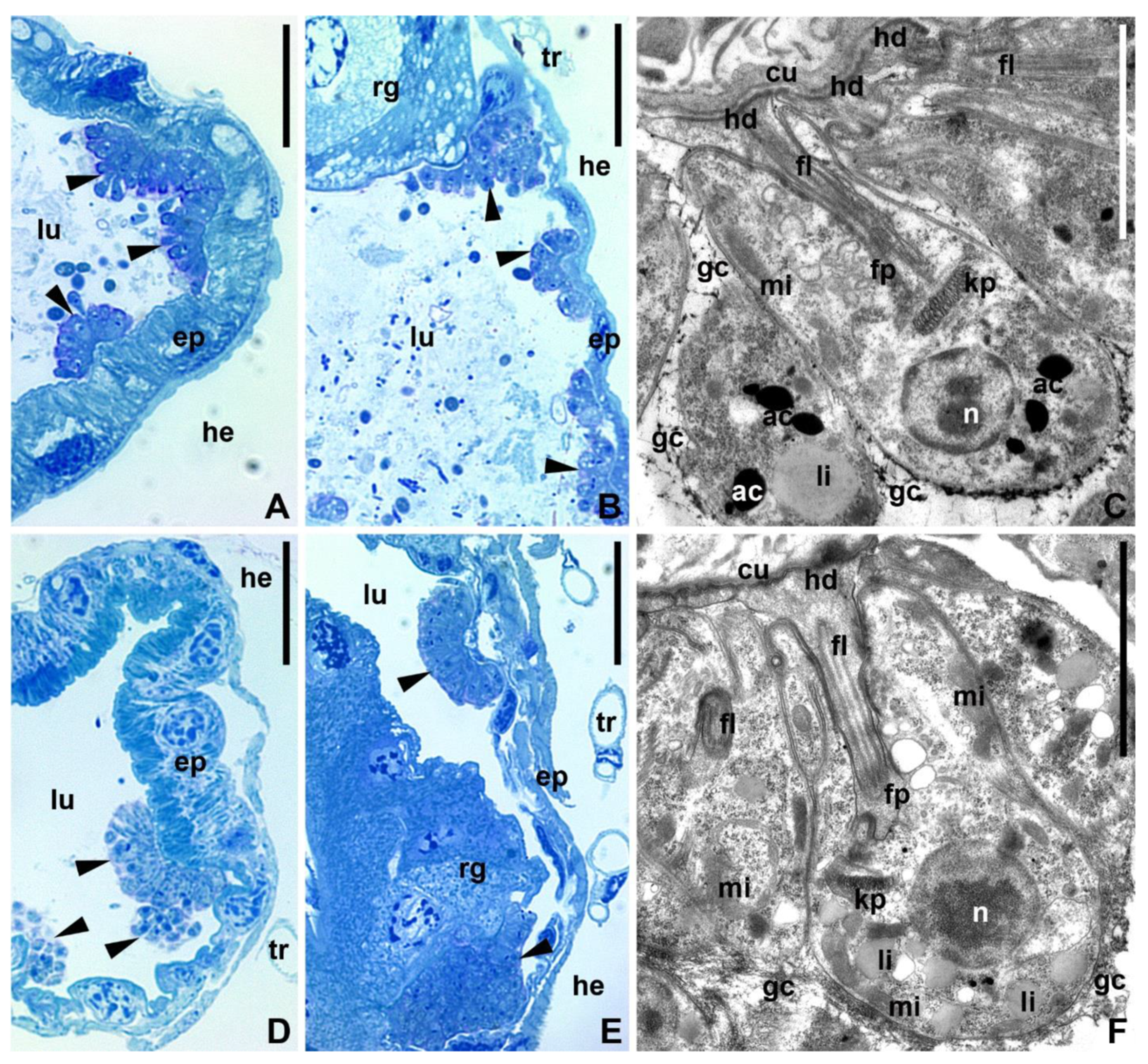
3.5. Trypanosomatid Development in the Gut
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maslov, D.A.; Opperdoes, F.R.; Kostygov, A.Y.; Hashimi, H.; Lukeš, J.; Yurchenko, V. Recent advances in trypanosomatid research: Genome organization, expression, metabolism, taxonomy and evolution. Parasitology 2019, 146, 1–27. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Karnkowska, A.; Votýpka, J.; Tashyreva, D.; Maciszewski, K.; Yurchenko, V.; Lukeš, J. Euglenozoa: Taxonomy, diversity and ecology, symbioses and viruses. Open Biol. 2021, 11, 200407. [Google Scholar] [CrossRef]
- Frolov, A.O.; Kostygov, A.Y.; Yurchenko, V. Development of monoxenous trypanosomatids and phytomonads in insects. Trends Parasitol. 2021, 37, 538–551. [Google Scholar] [CrossRef]
- Sergent, E.; Sergent, E. Sur un flagellé nouveau de l’intestin des Culex et des Stegomyia, Herpetomonas algeriense. Sur un autre flagellé et sur des spirochaetae de l’intestin des larves de moustiques. C R. Soc. Biol. 1906, 60, 291–293. [Google Scholar]
- Léger, L. Sur un flagellé parasite de l’Anopheles maculipennis. C R. Seances Soc. Biol. 1902, 54, 354–356. [Google Scholar]
- Novy, F.G.; Macneal, W.J.; Torrey, H.N. Mosquito trypanosomes. J. Hyg. 1906, 6, 110. [Google Scholar] [CrossRef]
- Podlipaev, S.A. [Catalogue of World Fauna of Trypanosomatidae (Protozoa)]; Zoologicheskii Institut AN SSSR: Leningrad, Russia, 1990; Volume 144, p. 178. (In Russian) [Google Scholar]
- Wallace, F.G. The trypanosomatid parasites of insects and arachnids. Exp. Parasitol 1966, 18, 124–193. [Google Scholar] [CrossRef]
- McGhee, R.B.; Cosgrove, W.B. Biology and physiology of the lower Trypanosomatidae. Microbiol. Rev. 1980, 44, 140–173. [Google Scholar] [CrossRef]
- Blacklock, D.B.; Lourie, E.M. The demonstration of viable Leishmania in the faeces of experimentally infected bed-bugs. Ann. Trop Med. Parasitol. 1931, 25, 359–368. [Google Scholar] [CrossRef]
- Hanson, W.L.; McGhee, R.B. Experimental infection of the hemipteron Oncopeltus fasciatus with Trypanosomatidae isolated from other hosts. J. Protozool. 1963, 10, 233–238. [Google Scholar] [CrossRef]
- Podlipaev, S.A. Insect trypanosomatids: The need to know more. Mem. Inst. Oswaldo Cruz. 2000, 95, 517–522. [Google Scholar] [CrossRef]
- Wallace, F.G.; Camargo, E.P.; McGhee, R.B.; Roitman, I. Guidelines for the description of new species of lower trypanosomatids. J. Protozool. 1983, 30, 308–313. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Borghesan, T.C.; Ferreira, R.C.; Santos, M.A.; Takata, C.S.; Campaner, M.; Nunes, V.L.; Milder, R.V.; de Souza, W.; Camargo, E.P. Phylogenetic validation of the genera Angomonas and Strigomonas of trypanosomatids harboring bacterial endosymbionts with the description of new species of trypanosomatids and of proteobacterial symbionts. Protist 2011, 162, 503–524. [Google Scholar] [CrossRef]
- Flegontov, P.; Votýpka, J.; Skalický, T.; Logacheva, M.D.; Penin, A.A.; Tanifuji, G.; Onodera, N.T.; Kondrashov, A.S.; Volf, P.; Archibald, J.M.; et al. Paratrypanosoma is a novel early-branching trypanosomatid. Curr. Biol. 2013, 23, 1787–1793. [Google Scholar] [CrossRef]
- Ishemgulova, A.; Butenko, A.; Kortisova, L.; Boucinha, C.; Grybchuk-Ieremenko, A.; Morelli, K.A.; Tesarova, M.; Kraeva, N.; Grybchuk, D.; Panek, T.; et al. Molecular mechanisms of thermal resistance of the insect trypanosomatid Crithidia thermophila. PLoS ONE 2017, 12, e0174165. [Google Scholar] [CrossRef]
- Van Dyken, M.; Bolling, B.G.; Moore, C.G.; Blair, C.D.; Beaty, B.J.; Black, W.C.t.; Foy, B.D. Molecular evidence for trypanosomatids in Culex mosquitoes collected during a West Nile virus survey. Int. J. Parasitol. 2006, 36, 1015–1023. [Google Scholar] [CrossRef]
- Schoener, E.R.; Harl, J.; Himmel, T.; Fragner, K.; Weissenbock, H.; Fuehrer, H.P. Protozoan parasites in Culex pipiens mosquitoes in Vienna. Parasitol. Res. 2019, 118, 1261–1269. [Google Scholar] [CrossRef]
- Schoener, E.; Uebleis, S.S.; Cuk, C.; Nawratil, M.; Obwaller, A.G.; Zechmeister, T.; Lebl, K.; Radrová, J.; Zittra, C.; Votýpka, J.; et al. Trypanosomatid parasites in Austrian mosquitoes. PLoS ONE 2018, 13, e0196052. [Google Scholar] [CrossRef]
- Svobodová, M.; Volf, P.; Votýpka, J. Trypanosomatids in ornithophilic bloodsucking Diptera. Med. Vet. Entomol. 2015, 29, 444–447. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Grybchuk-Ieremenko, A.; Malysheva, M.N.; Frolov, A.O.; Yurchenko, V. Molecular revision of the genus Wallaceina. Protist 2014, 165, 594–604. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Malysheva, M.N.; Frolov, A.O. [Investigation of causes of the conflict between taxonomy and molecular phylogeny of trypanosomatids by the example of Leptomonas nabiculae Podlipaev, 1987]. Parazitologiia 2011, 45, 409–424. (In Russian) [Google Scholar]
- Týč, J.; Votýpka, J.; Klepetková, H.; Šuláková, H.; Jirků, M.; Lukeš, J. Growing diversity of trypanosomatid parasites of flies (Diptera: Brachcera): Frequent cosmopolitism and moderate host specificity. Mol. Phylogenet. Evol. 2013, 69, 255–264. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Frolov, A.O.; Malysheva, M.N.; Ganyukova, A.I.; Drachko, D.; Yurchenko, V.; Agasoi, V.V. Development of two species of the Trypanosoma theileri complex in tabanids. Parasit. Vectors 2022, 15, 95. [Google Scholar] [CrossRef]
- Frolov, A.O.; Malysheva, M.N.; Ganyukova, A.I.; Spodareva, V.V.; Králová, J.; Yurchenko, V.; Kostygov, A.Y. If host is refractory, insistent parasite goes berserk: Trypanosomatid Blastocrithidia raabei in the dock bug Coreus marginatus. PLoS ONE 2020, 15, e0227832. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar]
- Razygraev, A.V.; Sulesco, T.M. The use of the Bayes factor for identification of Culex pipiens and C. torrentium (Diptera: Culicidae) based on morphometric wing characters. Entomol. Rev. 2020, 100, 220–227. [Google Scholar] [CrossRef]
- Börstler, J.; Lühken, R.; Rudolf, M.; Steinke, S.; Melaun, C.; Becker, S.; Garms, R.; Krüger, A. The use of morphometric wing characters to discriminate female Culex pipiens and Culex torrentium. J. Vector Ecol. 2014, 39, 204–212. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Frolov, A.O. [Leptomonas jaculum (Leger, 1902) Woodcock 1914: A leptomonas or a blastocrithidia?]. Parazitologiia 2007, 41, 126–136. (In Russian) [Google Scholar]
- Maslov, D.A.; Lukeš, J.; Jirků, M.; Simpson, L. Phylogeny of trypanosomes as inferred from the small and large subunit rRNAs: Implications for the evolution of parasitism in the trypanosomatid protozoa. Mol. Biochem. Parasitol. 1996, 75, 197–205. [Google Scholar] [CrossRef]
- Gerasimov, E.S.; Kostygov, A.Y.; Yan, S.; Kolesnikov, A.A. From cryptogene to gene? ND8 editing domain reduction in insect trypanosomatids. Eur. J. Protistol. 2012, 48, 185–193. [Google Scholar] [CrossRef]
- Malysheva, M.N.; Mamkaeva, M.A.; Kostygov, A.Y.; Frolov, A.O.; Karpov, S.A. Culture collection of parasitic protists at the Zoological Institute RAS (CCPP ZIN RAS). Protistology 2016, 10, 26–42. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Simpson, A.M.; Simpson, L. Labeling of Crithidia fasciculata DNA with [3H]thymidine. J. Protozool. 1974, 21, 379–382. [Google Scholar] [CrossRef]
- Frolov, A.O.; Malysheva, M.N. [Description of Crithidia allae sp.n. and Crithidia brevicula sp.n. (Protozoa, Trypanosomatidae) from the predator bug Nabis brevis Scholtz (Hemiptera, Miridae)]. Rus. J. Zool. 1989, 68, 5–10. (In Russian) [Google Scholar]
- Razygraev, A.V. On longevity of adult Chaoborids (Diptera, Chaoboridae) under sugar feeding conditions. Entomol. Rev. 2022, 102, 279–285. [Google Scholar] [CrossRef]
- Campbell, I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]
- Králová, J.; Grybchuk-Ieremenko, A.; Votýpka, J.; Novotný, V.; Kment, P.; Lukeš, J.; Yurchenko, V.; Kostygov, A.Y. Insect trypanosomatids in Papua New Guinea: High endemism and diversity. Int. J. Parasitol. 2019, 49, 1075–1086. [Google Scholar] [CrossRef]
- Malysheva, M.N.; Kostygov, A.Y.; Frolov, A.O. Niche partitioning within an insect host: Trypanosomatids Wallacemonas raviniae and Trypanosoma (Megatrypanum) sp. in the horsefly Hybomitra solstitialis. Protistology 2022, 16, 87–97. [Google Scholar] [CrossRef]
- Yurchenko, V.; Lukeš, J.; Jirků, M.; Maslov, D.A. Selective recovery of the cultivation-prone components from mixed trypanosomatid infections: A case of several novel species isolated from Neotropical Heteroptera. Int. J. Syst. Evol. Microbiol. 2009, 59, 893–909. [Google Scholar] [CrossRef]
- Westenberger, S.J.; Sturm, N.R.; Yanega, D.; Podlipaev, S.A.; Zeledon, R.; Campbell, D.A.; Maslov, D.A. Trypanosomatid biodiversity in Costa Rica: Genotyping of parasites from Heteroptera using the spliced leader RNA gene. Parasitology 2004, 129, 537–547. [Google Scholar] [CrossRef]
- Maslov, D.A.; Westenberger, S.J.; Xu, X.; Campbell, D.A.; Sturm, N.R. Discovery and barcoding by analysis of spliced leader RNA gene sequences of new isolates of Trypanosomatidae from Heteroptera in Costa Rica and Ecuador. J. Eukaryot. Microbiol. 2007, 54, 57–65. [Google Scholar] [CrossRef]
- Votýpka, J.; Klepetková, H.; Jirků, M.; Kment, P.; Lukeš, J. Phylogenetic relationships of trypanosomatids parasitising true bugs (Insecta: Heteroptera) in sub-Saharan Africa. Int. J. Parasitol. 2012, 42, 489–500. [Google Scholar] [CrossRef]
- Ganyukova, A.I.; Malysheva, M.N.; Smirnov, P.A.; Frolov, A.O. Crithidia dobrovolskii sp. n. (Kinetoplastida: Trypanosomatidae) from parasitoid fly Lypha dubia (Diptera: Tachinidae): Morphology and phylogenetic position. Protistology 2019, 13, 206–214. [Google Scholar] [CrossRef]
- Podlipaev, S.A.; Rokitskaya, T.A. [Classification of isolates of insect’s trypanosomatids: Isoenzyme analysis]. Parazitologiya 1999, 33, 350–357. (In Russian) [Google Scholar]
- Ganyukova, A.I.; Zolotarev, A.V.; Frolov, A.O. Geographical distribution and host range of monoxenous trypanosomatid Crithidia brevicula (Frolov et Malysheva, 1989) in the northern regions of Eurasia. Protistology 2020, 14, 70–78. [Google Scholar] [CrossRef]
- Lukeš, J.; Skalický, T.; Týč, J.; Votýpka, J.; Yurchenko, V. Evolution of parasitism in kinetoplastid flagellates. Mol. Biochem Parasitol 2014, 195, 115–122. [Google Scholar] [CrossRef]
- Strickman, D.; Linton, Y.; Wilkerson, R.C. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2021; Volume 1, p. 599. [Google Scholar]
- Clark, T.B.; Kellen, W.R.; Lindegren, J.E.; Smith, T.A. The transmission of Crithidia fasciculata Leger 1902 in Culiseta Incidens (Thomson). J. Protozool. 1964, 11, 400–402. [Google Scholar] [CrossRef]
- Wallace, F.G. Flagellate parasites of mosquitoes with special reference to Crithidia fasciculata Leger, 1902. J. Parasitol. 1943, 29, 196–205. [Google Scholar] [CrossRef]
- Patton, W.S. Preliminary note on the life cycle of a species of Herpetomonas found in Culex pipiens. Br. Med. J. 1907, 2, 78–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patton, W.S. Studies on the Flagellates of the Genera Herpetomonas, Crithidia and Rhynchoidomonas: No. 1. The Morphology and Life History of Herpetomonas Culicis, Novy, MacNeal, and Torrey; Superintendent Government Printing: Calcutta, India, 1912; Volume 58, p. 21. [Google Scholar]
- Votýpka, J.; Petrželková, K.J.; Brzoňová, J.; Jirků, M.; Modrý, D.; Lukeš, J. How monoxenous trypanosomatids revealed hidden feeding habits of their tsetse fly hosts. Folia Parasitol. 2021, 68, 019. [Google Scholar] [CrossRef]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; p. 828. [Google Scholar]
- Carvajal, M.A.; Jimenez, N.; Faundez, E.I. A predation record of Nabis paranensis (Hemiptera: Heteroptera) over Aedes albifasciatus. J. Am. Mosq. Control. Assoc. 2019, 35, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; Kment, P.; Yurchenko, V.; Lukeš, J. Endangered monoxenous trypanosomatid parasites: A lesson from island biogeography. Biodivers Conserv. 2020, 29, 3635–3667. (In English) [Google Scholar] [CrossRef]
- Podlipaev, S.A.; Malysheva, M.N.; Kolesnikov, A.A. Leptomonas rigidus sp. n. (Trypanosomatidae)—A parasite of Salda littoralis L (Hemiptera, Heteroptera). Acta Protozool. 1991, 30, 121–127. [Google Scholar]
- Wallace, F.G.; Clark, T.B.; Dyer, M.I.; Collins, T. Two new species of flagellates cultivated from insects of the genus Gerris. J. Protozool. 1960, 7, 390–392. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Metagenomic shotgun sequencing reveals host species as an important driver of virome composition in mosquitoes. Sci. Rep. 2021, 11, 8448. (In English) [Google Scholar] [CrossRef]
- Noguchi, H.; Tilden, E.B. Comparative studies of herpetomonads and leishmanias. I. Cultivation of herpetomonads from insects and plants. J. Exp. Med. 1926, 44, 307–325. [Google Scholar] [CrossRef]
- Mcghee, R.B.; Hanson, W.L. Growth and reproduction of Leptomonas oncopelti in milkweed bug, Oncopeltus fasciatus. J. Protozool. 1962, 9, 488–493. [Google Scholar] [CrossRef]
- Noguchi, H. Comparative studies of herpetomonads and leishmanias. II. Differentiation of the organisms by serological reactions and fermentation tests. J. Exp. Med. 1926, 44, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.P. Phytomonas and other trypanosomatid parasites of plants and fruit. Adv. Parasitol. 1999, 42, 29–112. [Google Scholar] [PubMed]
- Kozminsky, E.; Kraeva, N.; Ishemgulova, A.; Dobakova, E.; Lukeš, J.; Kment, P.; Yurchenko, V.; Votýpka, J.; Maslov, D.A. Host-specificity of monoxenous trypanosomatids: Statistical analysis of the distribution and transmission patterns of the parasites from Neotropical Heteroptera. Protist 2015, 166, 551–568. [Google Scholar] [CrossRef]
- Maslov, D.A.; Votýpka, J.; Yurchenko, V.; Lukeš, J. Diversity and phylogeny of insect trypanosomatids: All that is hidden shall be revealed. Trends Parasitol. 2013, 29, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; Maslov, D.A.; Yurchenko, V.; Jirků, M.; Kment, P.; Lun, Z.R.; Lukeš, J. Probing into the diversity of trypanosomatid flagellates parasitizing insect hosts in South-West China reveals both endemism and global dispersal. Mol. Phylogenet. Evol. 2010, 54, 243–253. [Google Scholar] [CrossRef]
- Votýpka, J.; Kment, P.; Kriegová, E.; Vermeij, M.J.A.; Keeling, P.J.; Yurchenko, V.; Lukeš, J. High prevalence and endemism of trypanosomatids on a small Caribbean island. J. Eukaryot. Microbiol. 2019, 66, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.O.; Malysheva, M.N.; Ganyukova, A.I.; Yurchenko, V.; Kostygov, A.Y. Obligate development of Blastocrithidia papi (Trypanosomatidae) in the Malpighian tubules of Pyrrhocoris apterus (Hemiptera) and coordination of host-parasite life cycles. PLoS ONE 2018, 13, e0204467. [Google Scholar]


| Collection Date | Cave | Culex p. pipiens | Culex torrentium | Culiseta annulata | Overall |
|---|---|---|---|---|---|
| 26.01.22 | Zhemchuzhnaya | 3/55 (5.5%) | 1/22 (4.5%) | 1/18 (5.6%) | 5/95 (5.3%) |
| 11.03.22 | Santa-Maria | 0/32 (0%) | 0/40 (0%) | 0/2 (0%) | 0/74 (0%) |
| 13.04.22 | Zhemchuzhnaya | 2/111 (1.8%) | 2/37 (5.4%) | 0/0 | 4/148 (2.7%) |
| Location | Prevalence | |
|---|---|---|
| Total | Monoxenous 1 | |
| Novgorod Region | 14/19 (74%) | 13/19 (68%) or 10/19 (53%) 2 |
| Pskov Region | 7/11 (64%) | 6/11 (55%) |
| Leningrad Region | 16/17 (94%) | 15/17 (88%) |
| Species | Month | Assessment | Prevalence |
|---|---|---|---|
| Aedes riparius | Late April | Microscopy or PCR | 0/41 |
| A. punctor | May | Microscopy or PCR | 0/54 |
| A. communis | May | Microscopy or PCR | 0/53 |
| Culex p. pipiens | August | Microscopy | 0/13 |
| Cx. torrentium | July–August | Microscopy or PCR | 0/210 |
| Host | Parasite | Control | 2 h | 24 h | 4 d | Pupae | Imagines | Died 3 |
|---|---|---|---|---|---|---|---|---|
| Aedes cantans 1 | Crithidia brevicula M1183 | 0/3 | 8/11 | 4/7 | 0/9 | - | - | 0 |
| Aedes punctor 2 | Crithidia fasciculata | 0/58 | 10/10 | 0/15 | - | 0/17 | 0/9 | 7 |
| Aedes communis 2 | Crithidia fasciculata | 0/30 | 7/7 | 0/7 | - | 0/10 | 0/6 | 0 |
| Culex torrentium 2 | Crithidia fasciculata | 0/70 | 10/10 | 0/30 | - | 0/20 | 0/8 | 2 |
| Culex torrentium 2 | Crithidia dobrovolskii | 0/60 | 10/10 | 0/30 | - | 0/14 | 0/5 | 1 |
| Parasite | 1 d | 2 d | 4 d | Control |
|---|---|---|---|---|
| Crithidia dobrovolskii | 4/9 (44%) | 3/4 (75%) | 5/5 (100%) | 0/18 (0%) |
| Crithidia brevicula M1183 | 2/3 (67%) | 18/38 (47%) | 16/29 (55%) | |
| Crithidia brevicula Nbr | - | - | 2/2 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostygov, A.Y.; Malysheva, M.N.; Ganyukova, A.I.; Razygraev, A.V.; Drachko, D.O.; Yurchenko, V.; Agasoi, V.V.; Frolov, A.O. The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates. Pathogens 2022, 11, 1326. https://doi.org/10.3390/pathogens11111326
Kostygov AY, Malysheva MN, Ganyukova AI, Razygraev AV, Drachko DO, Yurchenko V, Agasoi VV, Frolov AO. The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates. Pathogens. 2022; 11(11):1326. https://doi.org/10.3390/pathogens11111326
Chicago/Turabian StyleKostygov, Alexei Y., Marina N. Malysheva, Anna I. Ganyukova, Alexey V. Razygraev, Daria O. Drachko, Vyacheslav Yurchenko, Vera V. Agasoi, and Alexander O. Frolov. 2022. "The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates" Pathogens 11, no. 11: 1326. https://doi.org/10.3390/pathogens11111326
APA StyleKostygov, A. Y., Malysheva, M. N., Ganyukova, A. I., Razygraev, A. V., Drachko, D. O., Yurchenko, V., Agasoi, V. V., & Frolov, A. O. (2022). The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates. Pathogens, 11(11), 1326. https://doi.org/10.3390/pathogens11111326






