Morphological, Cytological and Molecular Studies and Feeding and Defecation Pattern of Hybrids from Experimental Crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphological Studies in Scanning Electron Microscopy
2.3. Cytogenetic Analysis
2.4. Molecular Analysis
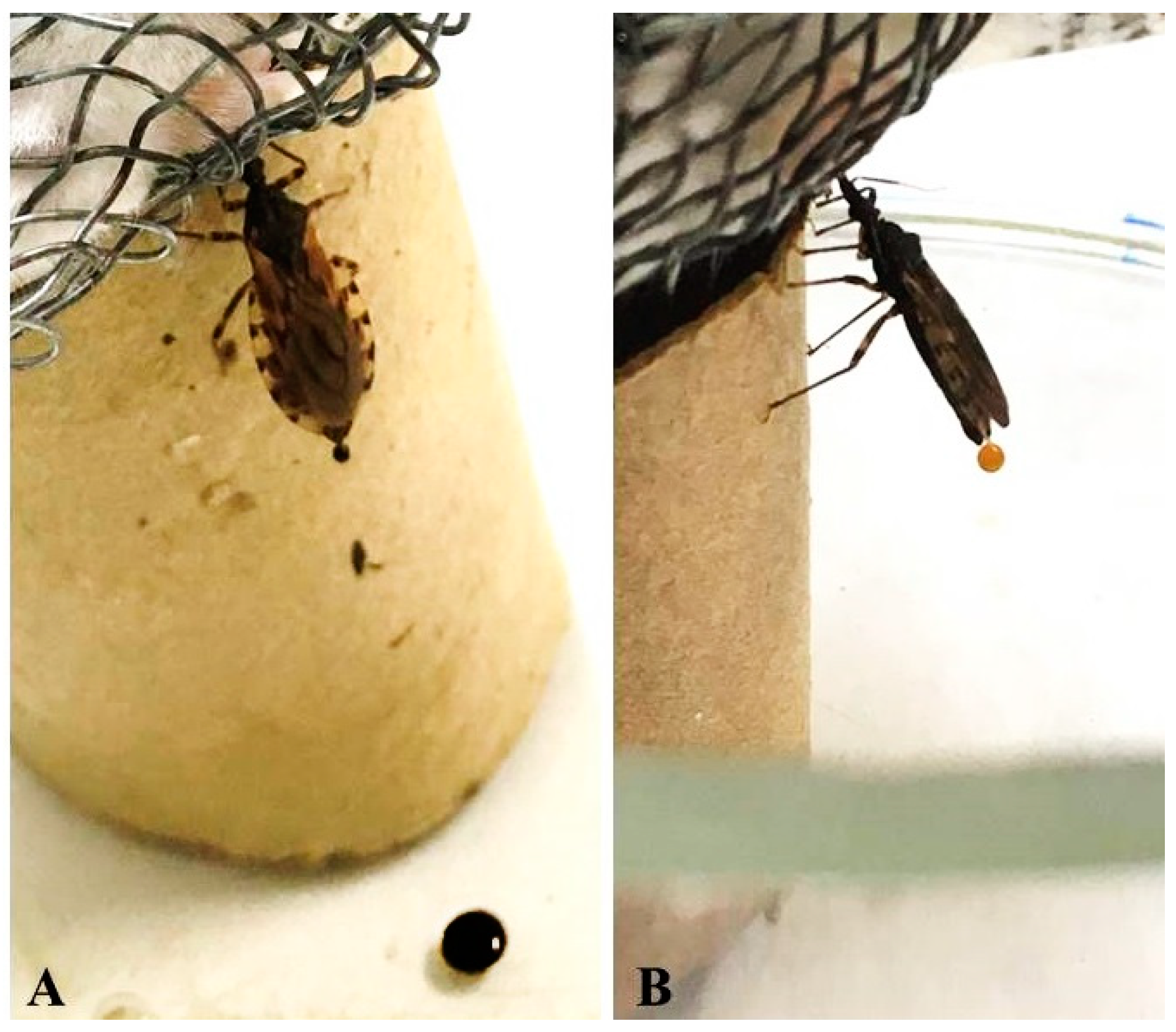
2.5. Feeding and Defecation Behavior
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 23 March 2022).
- Pan American Health Organization. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 23 March 2022).
- Alevi, K.C.C.; Oliveira, J.; Rocha, D.S.; Galvão, C. Trends in taxonomy of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to integrative taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Correia, J.P.S.O.; Gil-Santana, H.R.; Dale, C.; Galvão, C. Triatoma guazu Lent and Wygodzinsky is a junior synonym of Triatoma williami Galvão, Souza and Lima. Insects 2022, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Gil-Santana, H.R.; Chavez, T.; Pita, S.; Panzera, F.; Galvão, C. Panstrongylus noireaui, a remarkable new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 2022, 1104, 203–225. [Google Scholar] [CrossRef]
- Costa, J.; Dale, C.; Galvão, C.; Almeida, C.E.; Dujardin, J.P. Do the new triatomine species pose new challenges or strategies for monitoring Chagas disease? An overview from 1979-2021. Mem. Inst. Oswaldo Cruz 2021, 116, e210015. [Google Scholar] [CrossRef] [PubMed]
- Alevi, K.C.C.; Oliveira, J.; Garcia, A.C.C.; Cristal, D.C.; Delgado, L.M.G.; Bittinelli, I.F.; Reis, Y.V.; Ravazi, A.; Oliveira, A.B.B.; Galvão, C.; et al. Triatoma rosai sp. nov. (Hemiptera, Triatominae): A new species of Argentinian Chagas disease vector described based on integrative taxonomy. Insects 2020, 11, 830. [Google Scholar] [CrossRef]
- Ravazi, A.; Oliveira, J.; Campos, F.F.; Madeira, F.F.; Reis, Y.V.; Oliveira, A.B.B.; Azeredo-Oliveira, M.T.V.; Rosa, J.A.; Galvão, C.; Alevi, K.C.C. Trends in evolution of the Rhodniini tribe (Hemiptera, Triatominae): Experimental crosses between Psammolestes tertius Lent & Jurberg, 1965 and P. coreodes Bergroth, 1911 and analysis of the reproductive isolating mechanisms. Parasit. Vec. 2021, 14, 350. [Google Scholar]
- Alevi, K.C.C.; Pinotti, H.; Azeredo-Oliveira, M.T.V.; Araújo, R.F.; Mendonça, V.J.; Rosa, J.A. Hybrid collapse confirms the specific status of Triatoma bahiensis Sherlock and Serafim, 1967 (Hemiptera, Triatominae), an endemic species in Brazil. Am. J. Trop. Med. Hyg. 2018, 98, 475–477. [Google Scholar] [CrossRef]
- Nascimento, J.D.; Rosa, J.A.; Salgado-Roa, F.C.; Hernández, C.; Pardo-Diaz, C.; Alevi, K.C.C.; Ravazi, A.; Oliveira, J.; Azeredo-Oliveira, M.T.V.; Salazar, C.; et al. Taxonomical over splitting in the Rhodnius prolixus (Insecta: Hemiptera: Reduviidae) clade: Are R. taquarussuensis (da Rosa et al., 2017) and R. neglectus (Lent, 1954) the same species? PLoS ONE 2019, 14, e0213043. [Google Scholar] [CrossRef]
- Mayr, E. Animal Species and Evolution; Harvard University Press: Cambridge, MA, USA, 1963; 797p. [Google Scholar]
- Mayr, E. Populations, Species, and Evolution; Harvard University Press: Cambridge, MA, USA, 1970; 453 p. [Google Scholar]
- Dobzhansky, T. Genetics of the Evolutionary Process; Columbia University Press: New York, NY, USA, 1972; 505p. [Google Scholar]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Hannah, L.; Midgley, G.F.; Hughes, G.; Bomhard, B. The view from the Cape: Extinction risk, protected areas and climate change. BioScience 2005, 55, 231–242. [Google Scholar] [CrossRef]
- Martínez-Ibarra, J.A.; Nogueda-Torres, B.; Salazar-Schettino, P.M.; Cabrera-Bravo, M.; Vences-Blanco, M.O.; Rocha-Chávez, G. Transmission capacity of Trypanosoma cruzi (Trypanosomatida: Trypanosomatidae) by three subspecies of Meccus phyllosomus (Heteroptera: Reduviidae) and their hybrids. J. Med. Entomol. 2016, 53, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, J.A.; Nogueda-Torres, B.; García-Lin, J.C.; Arroyo-Reys, D.; Salazar- Montano, L.F.; Hernandez-Navarro, J.A.; Díaz-Snches, C.G.; Toro-Arreola, E.S.; Rocha-Chavez, G. Importance of hybrids of Meccus phyllosomus mazzottii, and M. p. pallidipennis, and M. p. phyllosomus to the transmission of Trypanosoma cruzi in Mexico. Jpn. J. Infect. Dis. 2016, 69, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, J.A.; Nogueda-Torres, B.; Salazar-Montaño, L.F.; García-Lino, J.C.; Arroyo-Reyes, D.; Hernández-Navarro, J.A. Comparison of biological fitness in crosses between subspecies of Meccus phyllosomus (Hemiptera: Reduviidae: Triatominae) in southern Mexico. Insect. Sci. 2017, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Medina, T.; Nogueda-Torres, B.; Ceballos-Rodríguez, R.S.; Godínez-Aceves, K.C.; Martínez-Ibarra, J.A. Enhancing fitness in offspring of crosses between two triatomine species. J. Vector Ecol. 2019, 44, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, J.A.; Cárdenas-Sosa, M.A.; Montañez-Valdez, O.D.; Michel-Parra, J.G.; Nogueda-Torres, B. Biological parameters and estimation of the vectorial capacity of two subspecies of Triatoma protracta (Uhler) and their laboratory hybrids in Mexico. J. Vector Ecol. 2021, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.E.; Wisnievsky-Colli, C. Triatoma sordida Stål 1859 (Hemiptera, Reduviidae: Triatominae) in palms of northeastern Argentina. Mem. Inst. Oswaldo Cruz 2001, 96, 895–899. [Google Scholar] [CrossRef]
- Maffey, L.; Cardinal, M.V.; Ordonez-Krasnowski, P.C.; Lanati, L.A.; Lauricella, M.A.; Schijman, A.G.; Gürtler, R.E. Direct molecular identification of Trypanosoma cruzi discrete typing units in domestic and peridomestic Triatoma infestans, and Triatoma sordida from the Argentine Chaco. Parasitology 2012, 139, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.B.; Machado, E.M.M.; Borges, E.C.; Lorosa, E.S.; Andrade, R.E.; Diotaiuti, L.; Azeredo, B.V.M. Trypanosoma cruzi peridomiciliar transmission by Triatoma sordida in the municipality of Patis, Minas Gerais State, Brazil. Mem. Inst. Oswaldo Cruz 1997, 92, 434. [Google Scholar]
- Lorosa, E.S.; Andrade, R.E.; Santos, S.M.; Pereira, C.A. Estudo da infecção natural e da fonte alimentar do Triatoma sordida (Stal, 1859), (Hemiptera, Reduviidae) na região norte de Minas Gerais, Brazil, através da reação de precipitina. Entomol. Vectores 1998, 5, 13–22. [Google Scholar]
- Rossi, J.C.N.; Duarte, E.C.; Gurgel-Gonçalves, R. Factors associated with the occurrence of Triatoma sordida (Hemiptera: Reduviidae) in rural localities of Central-West Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 192–200. [Google Scholar] [CrossRef][Green Version]
- Rosa, J.A.; Rocha, C.S.; Gardim, S.; Pinto, M.C.; Mendonça, V.J.; Ferreira-Filho, J.C.R.; Carvalho, E.O.C.; Camargo, L.M.A.; Oliveira, J.; Nascimento, J.D.; et al. Description of Rhodnius montenegrensis n. sp. (Hemiptera, Reduviidae: Triatominae) from the state of Rondônia, Brazil. Zootaxa 2012, 3478, 62–76. [Google Scholar] [CrossRef]
- Alevi, K.C.C.; Mendonca, P.P.; Pereira, N.P.; Rosa, J.A.; Azeredo-Oliveira, M.T.V. Karyotype of Triatoma melanocephala Neiva & Pinto (1923). Does this species fit in the Brasiliensis subcomplex? Infect. Genet. Evol. 2012, 12, 1652–1653. [Google Scholar] [PubMed]
- De Vaio, E.S.; Grucci, B.; Castagnino, A.M.; Franca, M.E.; Martinez, M.E. Meiotic differences between three triatomine species (Hemiptera: Reduviidae). Genetica 1985, 67, 185–191. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61. 2019. Available online: http://www.mesquiteproject.org (accessed on 30 March 2022).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: E cient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Diotaiuti, D.; Penido, C.M.; Pires, H.H.R.; Dias, J.C.P. Dinâmica da alimentação e dejeção do Triatoma sordida. Rev. Soc. Bras. Med. Trop. 1995, 28, 195–198. [Google Scholar] [CrossRef][Green Version]
- JASP Team (2022). JASP (Version 0.16.2) [Computer Software]. Available online: https://jasp-stats.org/ (accessed on 20 October 2022).
- Mendonça, V.J.; Alevi, K.C.C.; Medeiros, L.M.; Nascimento, J.D.; Azeredo-Oliveira, M.T.V.; Rosa, J.A. Cytogenetic and morphologic approaches of hybrids from experimental crosses between Triatoma lenti Sherlock & Serafim, 1967 and T. sherlocki Papa et al., 2002 (Hemiptera: Reduviidae). Infect. Genet. Evol. 2014, 26, 123–131. [Google Scholar] [PubMed]
- Davila-Barboza, J.A.; Saucedo-Montalvo, M.C.; Favela-Lara, S.; Ponce-Garcia, G.; Fernandez-Salas, I.; Quiroz-Martinez, H.; Flores, A.E. Morphological and genetic relation in hybrids of Triatomines (Hemiptera: Reduviidae) of the phyllosoma Complex from Mexico. Ann. Entomol. Soc. Am. 2020, 113, 398–406. [Google Scholar] [CrossRef]
- Pinotti, H.; Alevi, K.C.C.; Oliveira, J.; Ravazi, A.; Madeira, F.F.; Reis, Y.V.; Oliveira, A.B.B.; Azeredo-Oliveira, M.T.V.; Rosa, J.A. Segregation of phenotypic characteristics in hybrids of Triatoma brasiliensis species complex (Hemiptera, Reduviidae, Triatominae). Infect. Genet. Evol. 2014, 91, 104798. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, H.; Oliveira, J.; Ravazi, A.; Madeira, F.F.; Reis, Y.V.; Oliveira, A.B.B.; Azeredo-Oliveira, M.T.V.; Rosa, J.A.; Alevi, K.C.C. Revisiting the hybridization processes in the Triatoma brasiliensis complex (Hemiptera, Triatominae): Interspecific genomic compatibility point to a possible recent diversification of the species grouped in this monophyletic complex. PLoS ONE 2021, 16, e0257992. [Google Scholar] [CrossRef]
- Almeida, C.E.; Oliveira, H.L.; Correia, N.; Dornak, L.L.; Gumiel, M.; Neiva, V.L.; Harry, M.; Mendonça, V.J.; Costa, J.; Galvão, C. Dispersion capacity of Triatoma sherlocki, Triatoma juazeirensis and laboratory-bred hybrids. Acta Trop. 2012, 122, 71–79. [Google Scholar] [CrossRef]
- Usinger, R.L.; Wygodzinsky, P.; Ryckman, E.R. The biosystematics of Triatominae. Annu. Rev. Entomol. 1966, 11, 309–329. [Google Scholar] [CrossRef]
- Neves, S.J.M.; Sousa, P.S.; Oliveira, J.; Ravazi, A.; Madeira, F.F.; Reis, Y.V.; de Oliveira, A.B.B.; Pinotti, H.; de Azeredo-Oliveira, M.T.V.; da Rosa, J.A.; et al. Prezygotic isolation confirms the exclusion of Triatoma melanocephala, T. vitticeps and T. tibiamaculata of the T. brasiliensis subcomplex (Hemiptera, Triatominae). Infect. Genet. Evol. 2020, 79, 104149. [Google Scholar] [CrossRef]
- Cesaretto, N.R.; Oliveira, J.; Ravazi, A.; Madeira, F.F.; Reis, Y.V.; Oliveira, A.B.B.; Vicente, R.D.; Cristal, D.C.; Galvão, C.; Azeredo-Oliveira, M.T.V.; et al. Trends in taxonomy of Triatomini (Hemiptera, Reduviidae, Triatominae): Reproductive compatibility reinforces the synonymization of Meccus Stål, 1859 with Triatoma Laporte, 1832. Parasit. Vect. 2021, 14, 340. [Google Scholar] [CrossRef]
- Riley, R. The secondary pairing of bivalents with genetically similar chromosomes. Nature 1966, 185, 751–752. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta. 2013, 1833, 1979–1984. [Google Scholar] [CrossRef]
- Petrov, D.A.; Schutzman, J.L.; Hartl, D.L.; Lozovskaya, E.R. Diverse transposable elements are mobilized in hybrid dysgenesis in Drosophila virilis. Proc. Nat. Acad. Sci. USA 1995, 92, 8050–8054. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; O’Neill, M.; Graves, J. Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature 1998, 393, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Galvão, C. Vetores da doença de Chagas no Brasil, 1st ed.; Sociedade Brasileira de Zoologia: Curitiba, Brazil, 2014; 289p. [Google Scholar]
- López, A.G.; Cardozo, M.; Oscherev, E.B.; Crocco, L.B. Dynamics of feeding and defecation behavior of Triatoma infestans hybrids. Parasitol. Res. 2020, 119, 2775–2781. [Google Scholar] [CrossRef] [PubMed]





| Species | Cyt B | ITS-1 |
|---|---|---|
| T. sordida | MH054940 | * |
| T. rosai | * | * |
| Hybrid 1 | * | * |
| Hybrid 2 | * | * |
| Feeding | Defecation | |
|---|---|---|
| T. sordida ♀ | 30:29 | 18:47 |
| T. sordida ♂ | 32:56 | 23:09 |
| T. rosai ♀ | 31:49 | 22:02 |
| T. rosai ♂ | 34:27 | 25:11 |
| Hybrid 1 ♀ | 32:00 | 19:14 |
| Hybrid 1 ♂ | 35:46 | 24:18 |
| Hybrid 2 ♀ | 31:17 | 21:01 |
| Hybrid 2 ♂ | 36:12 | 23:15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, R.D.; Madeira, F.F.; Borsatto, K.C.; Garcia, A.C.C.; Cristal, D.C.; Delgado, L.M.G.; Bittinelli, I.d.F.; De Mello, D.V.; Dos Reis, Y.V.; Ravazi, A.; et al. Morphological, Cytological and Molecular Studies and Feeding and Defecation Pattern of Hybrids from Experimental Crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae). Pathogens 2022, 11, 1302. https://doi.org/10.3390/pathogens11111302
Vicente RD, Madeira FF, Borsatto KC, Garcia ACC, Cristal DC, Delgado LMG, Bittinelli IdF, De Mello DV, Dos Reis YV, Ravazi A, et al. Morphological, Cytological and Molecular Studies and Feeding and Defecation Pattern of Hybrids from Experimental Crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae). Pathogens. 2022; 11(11):1302. https://doi.org/10.3390/pathogens11111302
Chicago/Turabian StyleVicente, Roberto Dezan, Fernanda Fernandez Madeira, Kelly Cristine Borsatto, Ariane Cristina Caris Garcia, Daniel Cesaretto Cristal, Luiza Maria Grzyb Delgado, Isadora de Freitas Bittinelli, Denis Vinicius De Mello, Yago Visinho Dos Reis, Amanda Ravazi, and et al. 2022. "Morphological, Cytological and Molecular Studies and Feeding and Defecation Pattern of Hybrids from Experimental Crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae)" Pathogens 11, no. 11: 1302. https://doi.org/10.3390/pathogens11111302
APA StyleVicente, R. D., Madeira, F. F., Borsatto, K. C., Garcia, A. C. C., Cristal, D. C., Delgado, L. M. G., Bittinelli, I. d. F., De Mello, D. V., Dos Reis, Y. V., Ravazi, A., Galvão, C., De Azeredo-Oliveira, M. T. V., Da Rosa, J. A., De Oliveira, J., & Alevi, K. C. C. (2022). Morphological, Cytological and Molecular Studies and Feeding and Defecation Pattern of Hybrids from Experimental Crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae). Pathogens, 11(11), 1302. https://doi.org/10.3390/pathogens11111302










