Mycobacterium tuberculosis Transmission in High-Incidence Settings—New Paradigms and Insights
Abstract
:1. Introduction
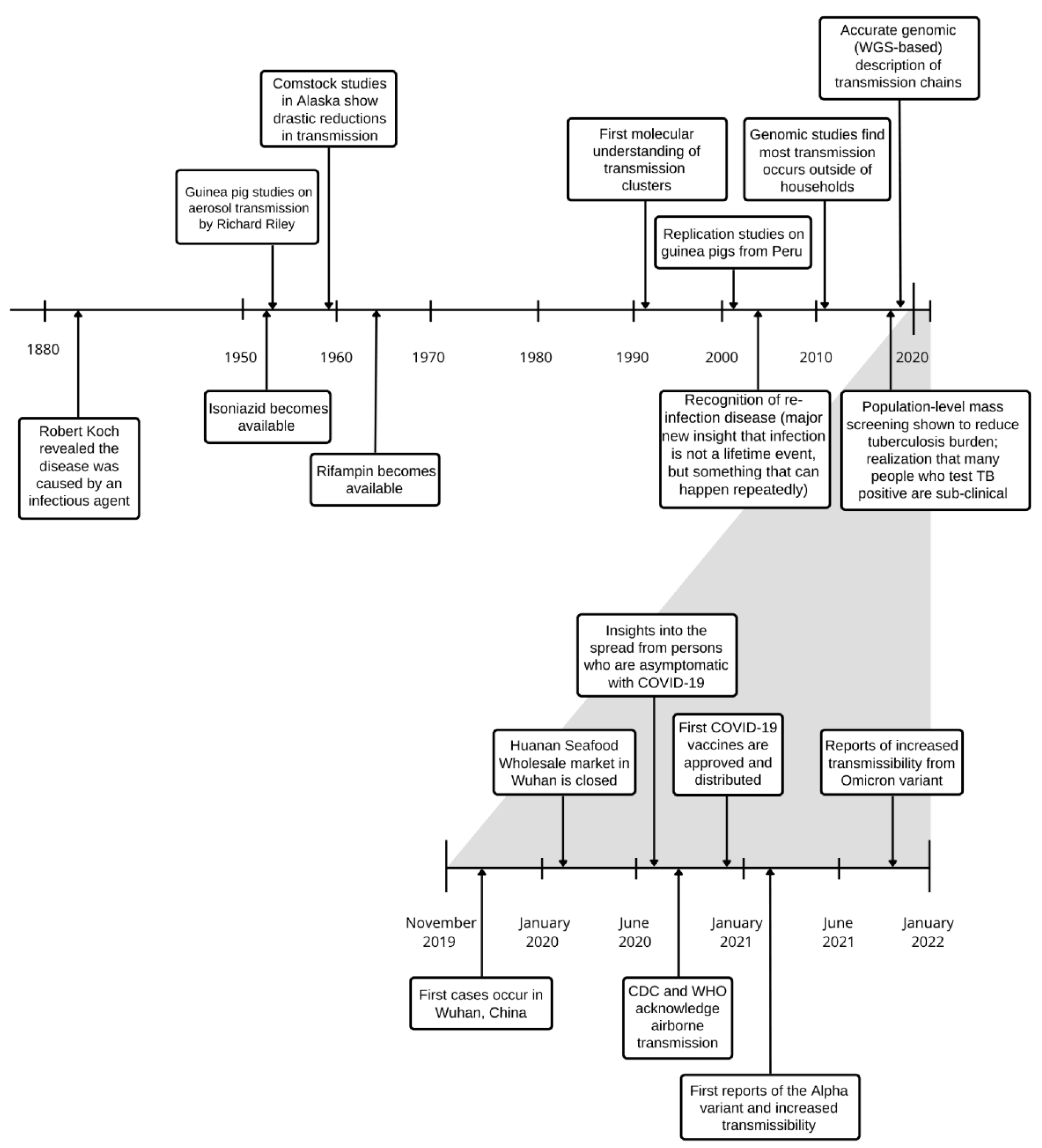
2. Historical Insights
2.1. Principles of Aerosol Transmission
2.2. Spread amongst Close Contacts
2.3. Host-Related Factors in Transmission
2.4. Transmission of Drug-Resistant Tuberculosis
2.5. Unusual Routes of Transmission
3. New Paradigms of Airborne Transmission
3.1. Continuum of Droplet-Aerosol Spread
3.2. Transmission from Asymptomatic Individuals & Subclinical Tuberculosis
3.3. Individual-Level Transmission Heterogeneity and ‘Super-Spreaders’
3.4. New Aerosol Transmission Insights from the COVID-19 Experience
4. New Paradigms and Tools from Tuberculosis Research
4.1. Reducing Community Transmission
4.2. Measuring Aerosol Transmission
4.3. Reducing Drug-Resistant Tuberculosis Spread
4.4. Genomic Transmission Tracking
Author Contributions
Funding
Conflicts of Interest
References
- Chisholm, R.H.; Trauer, J.M.; Curnoe, D.; Tanaka, M.M. Controlled Fire Use in Early Humans Might Have Triggered the Evolutionary Emergence of Tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 9051–9056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The History of Tuberculosis: From the First Historical Records to the Isolation of Koch’s Bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar] [PubMed]
- Hermans, S.; Horsburgh, C.R., Jr.; Wood, R. A Century of Tuberculosis Epidemiology in the Northern and Southern Hemisphere: The Differential Impact of Control Interventions. PLoS ONE 2015, 10, e0135179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issarow, C.M.; Mulder, N.; Wood, R. Modelling the Risk of Airborne Infectious Disease Using Exhaled Air. J. Theor. Biol. 2015, 372, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathema, B.; Andrews, J.R.; Cohen, T.; Borgdorff, M.W.; Behr, M.; Glynn, J.R.; Rustomjee, R.; Silk, B.J.; Wood, R. Drivers of Tuberculosis Transmission. J. Infect. Dis. 2017, 216, S644–S653. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.; Morrow, C.; Ginsberg, S.; Piccoli, E.; Kalil, D.; Sassi, A.; Walensky, R.P.; Andrews, J.R. Quantification of Shared Air: A Social and Environmental Determinant of Airborne Disease Transmission. PLoS ONE 2014, 9, e106622. [Google Scholar] [CrossRef]
- Andrews, J.R.; Morrow, C.; Walensky, R.P.; Wood, R. Integrating Social Contact and Environmental Data in Evaluating Tuberculosis Transmission in a South African Township. J. Infect. Dis. 2014, 210, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Verver, S.; Warren, R.M.; Munch, Z.; Richardson, M.; van der Spuy, G.D.; Borgdorff, M.W.; Behr, M.A.; Beyers, N.; van Helden, P.D. Proportion of Tuberculosis Transmission That Takes Place in Households in a High-Incidence Area. Lancet 2004, 363, 212–214. [Google Scholar] [CrossRef]
- Martinez, L.; Shen, Y.; Mupere, E.; Kizza, A.; Hill, P.C.; Whalen, C.C. Transmission of Mycobacterium Tuberculosis in Households and the Community: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Glynn, J.R.; Guerra-Assunção, J.A.; Houben, R.M.G.J.; Sichali, L.; Mzembe, T.; Mwaungulu, L.K.; Mwaungulu, J.N.; McNerney, R.; Khan, P.; Parkhill, J.; et al. Whole Genome Sequencing Shows a Low Proportion of Tuberculosis Disease Is Attributable to Known Close Contacts in Rural Malawi. PLoS ONE 2015, 10, e0132840. [Google Scholar] [CrossRef]
- Middelkoop, K.; Mathema, B.; Myer, L.; Shashkina, E.; Whitelaw, A.; Kaplan, G.; Kreiswirth, B.; Wood, R.; Bekker, L.-G. Transmission of Tuberculosis in a South African Community With a High Prevalence of HIV Infection. J. Infect. Dis. 2015, 211, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Dowdy, D.W.; Behr, M.A. Are We Underestimating the Annual Risk of Infection with Mycobacterium Tuberculosis in High-Burden Settings? Lancet Infect. Dis. 2022, 22, e271–e278. [Google Scholar] [CrossRef]
- Cords, O.; Martinez, L.; Warren, J.L.; O’Marr, J.M.; Walter, K.S.; Cohen, T.; Zheng, J.; Ko, A.I.; Croda, J.; Andrews, J.R. Incidence and Prevalence of Tuberculosis in Incarcerated Populations: A Systematic Review and Meta-Analysis. Lancet Public Health 2021, 6, e300–e308. [Google Scholar] [CrossRef]
- Scriba, T.J.; Fiore-Gartland, A.; Penn-Nicholson, A.; Mulenga, H.; Kimbung Mbandi, S.; Borate, B.; Mendelsohn, S.C.; Hadley, K.; Hikuam, C.; Kaskar, M.; et al. Biomarker-Guided Tuberculosis Preventive Therapy (CORTIS): A Randomised Controlled Trial. Lancet Infect. Dis. 2021, 21, 354–365. [Google Scholar] [CrossRef]
- Churchyard, G.J.; Fielding, K.L.; Lewis, J.J.; Coetzee, L.; Corbett, E.L.; Godfrey-Faussett, P.; Hayes, R.J.; Chaisson, R.E.; Grant, A.D. A Trial of Mass Isoniazid Preventive Therapy for Tuberculosis Control. N. Engl. J. Med. 2014, 370, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Pai, M.; Kasaeva, T.; Swaminathan, S. COVID-19’s Devastating Effect on Tuberculosis Care—A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, P.; Shen, Y.; Li, C.; Wang, J.; Zhu, L.; Lu, W.; Martinez, L. Collateral Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Tuberculosis Control in Jiangsu Province, China. Clin. Infect. Dis. 2021, 73, 542–544. [Google Scholar] [CrossRef]
- McQuaid, C.F.; Vassall, A.; Cohen, T.; Fiekert, K.; White, R.G. The Impact of COVID-19 on TB: A Review of the Data. Int. J. Tuberc. Lung Dis. 2021, 25, 436–446. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Jones-López, E.C.; Ayakaka, I.; Kim, S.; Menyha, H.; Kirenga, B.; Muchwa, C.; Joloba, M.; Dryden-Peterson, S.; Reilly, N.; et al. Variability of Infectious Aerosols Produced during Coughing by Patients with Pulmonary Tuberculosis. Am. J. Respir. Crit. Care Med. 2012, 186, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Dinkele, R.; Gessner, S.; McKerry, A.; Leonard, B.; Leukes, J.; Seldon, R.; Warner, D.F.; Wood, R. Aerosolization of Mycobacterium Tuberculosis by Tidal Breathing. Am. J. Respir. Crit. Care Med. 2022, 206, 206–216. [Google Scholar] [CrossRef]
- Chengalroyen, M.D.; Beukes, G.M.; Gordhan, B.G.; Streicher, E.M.; Churchyard, G.; Hafner, R.; Warren, R.; Otwombe, K.; Martinson, N.; Kana, B.D. Detection and Quantification of Differentially Culturable Tubercle Bacteria in Sputum from Patients with Tuberculosis. Am. J. Respir. Crit. Care Med. 2016, 194, 1532–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukamolova, G.V.; Turapov, O.; Malkin, J.; Woltmann, G.; Barer, M.R. Resuscitation-Promoting Factors Reveal an Occult Population of Tubercle Bacilli in Sputum. Am. J. Respir. Crit. Care Med. 2010, 181, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Sherman, J.M.; Tovar, M.A.; Bravard, M.A.; Valencia, T.; Montoya, R.; Quino, W.; D’Arcy, N.; Ramos, E.S.; Gilman, R.H.; et al. Sputum Microscopy With Fluorescein Diacetate Predicts Tuberculosis Infectiousness. J. Infect. Dis. 2017, 216, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennelly, K.P. Particle Sizes of Infectious Aerosols: Implications for Infection Control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Morrison, J.; Pai, M.; Hopewell, P.C. Tuberculosis and Latent Tuberculosis Infection in Close Contacts of People with Pulmonary Tuberculosis in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2008, 8, 359–368. [Google Scholar] [CrossRef]
- Fox, G.J.; Nhung, N.V.; Sy, D.N.; Hoa, N.L.; Anh, L.T.; Anh, N.T.; Hoa, N.B.; Dung, N.H.; Buu, T.N.; Loi, N.T. Household-Contact Investigation for Detection of Tuberculosis in Vietnam. N. Engl. J. Med. 2018, 378, 221–229. [Google Scholar] [CrossRef]
- Kim, S.; Wu, X.; Hughes, M.D.; Upton, C.; Narunsky, K.; Mendoza-Ticona, A.; Khajenoori, S.; Gonzales, P.; Badal-Faesen, S.; Shenje, J.; et al. ACTG A5300/IMPAACT 2003/PHOENix Feasibility Study Team. High Prevalence of Tuberculosis Infection and Disease in Child Household Contacts of Adults With Rifampin-resistant Tuberculosis. Pediatr. Infect. Dis. J. 2022, 41, e194–e202. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R.; Acuna-Villaorduna, C.; Desai Ahuja, S.; Altet, N.; Augusto, O.; Baliashvili, D.; Basu, S.; et al. The Risk of Tuberculosis in Children after Close Exposure: A Systematic Review and Individual-Participant Meta-Analysis. Lancet 2020, 395, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Mandalakas, A.M.; Hesseling, A.C.; Kay, A.; Du Preez, K.; Martinez, L.; Ronge, L.; DiNardo, A.; Lange, C.; Kirchner, H.L. Tuberculosis Prevention in Children: A Prospective Community-Based Study in South Africa. Eur. Respir. J. 2021, 57, 2003028. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Tuberculosis Preventive Treatment; World Health Organization: Geneva, Switzerland, 2020; ISBN 92-4-000150-6.
- Middelkoop, K.; Bekker, L.-G.; Morrow, C.; Lee, N.; Wood, R. Decreasing Household Contribution to TB Transmission with Age: A Retrospective Geographic Analysis of Young People in a South African Township. BMC Infect. Dis. 2014, 14, 221. [Google Scholar] [CrossRef]
- Crampin, A.C.; Glynn, J.R.; Traore, H.; Yates, M.D.; Mwaungulu, L.; Mwenebabu, M.; Chaguluka, S.D.; Floyd, S.; Drobniewski, F.; Fine, P.E.M. Tuberculosis Transmission Attributable to Close Contacts and HIV Status, Malawi. Emerg. Infect. Dis. 2006, 12, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.S.; Auld, S.C.; Brust, J.C.M.; Mathema, B.; Ismail, N.; Moodley, P.; Mlisana, K.; Allana, S.; Campbell, A.; Mthiyane, T.; et al. Transmission of Extensively Drug-Resistant Tuberculosis in South Africa. N. Engl. J. Med. 2017, 376, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Martinez, L.; le Roux, D.M.; Barnett, W.; Stadler, A.; Nicol, M.P.; Zar, H.J. Tuberculin Skin Test Conversion and Primary Progressive Tuberculosis Disease in the First 5 Years of Life: A Birth Cohort Study from Cape Town, South Africa. Lancet Child Adolesc. Health 2018, 2, 46–55. [Google Scholar] [CrossRef]
- Martinez, L.; Lo, N.C.; Cords, O.; Hill, P.C.; Khan, P.; Hatherill, M.; Mandalakas, A.; Kay, A.; Croda, J.; Horsburgh, C.R. Paediatric Tuberculosis Transmission Outside the Household: Challenging Historical Paradigms to Inform Future Public Health Strategies. Lancet Respir. Med. 2019, 7, 544–552. [Google Scholar] [CrossRef]
- Marquez, C.; Atukunda, M.; Balzer, L.B.; Chamie, G.; Kironde, J.; Ssemmondo, E.; Ruel, T.D.; Mwangwa, F.; Tram, K.H.; Clark, T.D. The Age-Specific Burden and Household and School-Based Predictors of Child and Adolescent Tuberculosis Infection in Rural Uganda. PLoS ONE 2020, 15, e0228102. [Google Scholar] [CrossRef]
- Martinez, L.; Verma, R.; Croda, J.; Horsburgh, C.R.; Walter, K.S.; Degner, N.; Middelkoop, K.; Koch, A.; Hermans, S.; Warner, D.F.; et al. Detection, Survival and Infectious Potential of Mycobacterium Tuberculosis in the Environment: A Review of the Evidence and Epidemiological Implications. Eur. Respir. J. 2019, 53, 1802302. [Google Scholar] [CrossRef]
- McCreesh, N.; White, R.G. An Explanation for the Low Proportion of Tuberculosis That Results from Transmission between Household and Known Social Contacts. Sci. Rep. 2018, 8, 5382. [Google Scholar] [CrossRef] [Green Version]
- Coleman, M.; Hill, J.; Timeon, E.; Tonganibeia, A.; Eromanga, B.; Islam, T.; Trauer, J.M.; Chambers, S.T.; Christensen, A.; Fox, G.J. Population-Wide Active Case Finding and Prevention for Tuberculosis and Leprosy Elimination in Kiribati: The PEARL Study Protocol. BMJ Open 2022, 12, e055295. [Google Scholar] [CrossRef]
- Trauer, J.M.; Dodd, P.J.; Gomes, M.G.M.; Gomez, G.B.; Houben, R.M.G.J.; McBryde, E.S.; Melsew, Y.A.; Menzies, N.A.; Arinaminpathy, N.; Shrestha, S.; et al. The Importance of Heterogeneity to the Epidemiology of Tuberculosis. Clin. Infect. Dis. 2019, 69, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Marais, B.J.; Obihara, C.C.; Warren, R.M.; Schaaf, H.S.; Gie, R.P.; Donald, P.R. The Burden of Childhood Tuberculosis: A Public Health Perspective. Int. J. Tuberc. Lung Dis. 2005, 9, 1305–1313. [Google Scholar]
- Jimenez-Corona, M.-E. Gender Differentials of Pulmonary Tuberculosis Transmission and Reactivation in an Endemic Area. Thorax 2006, 61, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Lienhardt, C.; Fielding, K.; Sillah, J.; Bah, B.; Gustafson, P.; Warndorff, D.; Palayew, M.; Lisse, I.; Donkor, S.; Diallo, S.; et al. Investigation of the Risk Factors for Tuberculosis: A Case–Control Study in Three Countries in West Africa. Int. J. Epidemiol. 2005, 34, 914–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, B.E.; Thiruvengadam, K.; Kadam, D.; Ovung, S.; Sivakumar, S.; Bala, Y.; Shivakumar, S.V.; Paradkar, M.; Gupte, N.; Suryavanshi, N.; et al. Smoking, Alcohol Use Disorder and Tuberculosis Treatment Outcomes: A Dual Co-Morbidity Burden That Cannot Be Ignored. PLoS ONE 2019, 14, e0220507. [Google Scholar] [CrossRef] [Green Version]
- de Gijsel, D.; von Reyn, C.F. A Breath of Fresh Air: BCG Prevents Adult Pulmonary Tuberculosis. Int. J. Infect. Dis. 2019, 80, S6–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Rakshit, S.; Adiga, V.; Dias, M.; Dwarkanath, P.; D’Souza, G.; Vyakarnam, A. A Century of BCG: Impact on Tuberculosis Control and Beyond. Immunol. Rev. 2021, 301, 98–121. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.-C.; Croda, J.; Hill, P.C. Infant BCG Vaccination and Risk of Pulmonary and Extrapulmonary Tuberculosis throughout the Life Course: A Systematic Review and Individual Participant Data Meta-Analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- Gebhart, J.; Anselm, A.; Heyder, J.; Stahlhofen, W. The Human Lung as Aerosol Particle Generator. J. Aerosol Med. 1988, 1, 196–197. [Google Scholar]
- Martinez, L.; Sekandi, J.N.; Castellanos, M.E.; Zalwango, S.; Whalen, C.C. Infectiousness of HIV-Seropositive Patients with Tuberculosis in a High-Burden African Setting. Am. J. Respir. Crit. Care Med. 2016, 194, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Theron, G.; Limberis, J.; Venter, R.; Smith, L.; Pietersen, E.; Esmail, A.; Calligaro, G.; te Riele, J.; de Kock, M.; van Helden, P.; et al. Bacterial and Host Determinants of Cough Aerosol Culture-Positivity in Patients with Drug-Resistant versus Drug-Susceptible Tuberculosis. Nat. Med. 2020, 26, 1435–1443. [Google Scholar] [CrossRef]
- Small, P.M.; Hopewell, P.C.; Singh, S.P.; Paz, A.; Parsonnet, J.; Ruston, D.C.; Schecter, G.F.; Daley, C.L.; Schoolnik, G.K. The Epidemiology of Tuberculosis in San Francisco—A Population-Based Study Using Conventional and Molecular Methods. N. Engl. J. Med. 1994, 330, 1703–1709. [Google Scholar] [CrossRef]
- Dinkele, R.; Gessner, S.; McKerry, A.; Leonard, B.; Seldon, R.; Koch, A.S.; Morrow, C.; Gqada, M.; Kamariza, M.; Bertozzi, C.R.; et al. Capture and Visualization of Live Mycobacterium Tuberculosis Bacilli from Tuberculosis Patient Bioaerosols. PLoS Pathog. 2021, 17, e1009262. [Google Scholar] [CrossRef]
- Williams, C.M.; Abdulwhhab, M.; Birring, S.S.; De Kock, E.; Garton, N.J.; Townsend, E.; Pareek, M.; Al-Taie, A.; Pan, J.; Ganatra, R.; et al. Exhaled Mycobacterium Tuberculosis Output and Detection of Subclinical Disease by Face-Mask Sampling: Prospective Observational Studies. Lancet Infect. Dis. 2020, 20, 607–617. [Google Scholar] [CrossRef]
- Middelkoop, K.; Koch, A.S.; Hoosen, Z.; Bryden, W.; Call, C.; Seldon, R.; Warner, D.F.; Wood, R.; Andrews, J.R. Environmental Air Sampling for Detection and Quantification of Mycobacterium Tuberculosis in Clinical Settings: Proof of Concept. Infect. Control Hosp. Epidemiol. 2022, 1–6. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the Effect of Individual Variation on Disease Emergence. Nature 2005, 438, 355–359. [Google Scholar] [CrossRef]
- Sultan, L.; Nyka, W.; Mills, C.; O’grady, F.; Wells, W.; Riley, R. Tuberculosis Disseminators: A Study of the Variability of Aerial Infectivity of Tuberculous Patients. Am. Rev. Respir. Dis. 1960, 82, 358–369. [Google Scholar]
- Turner, R.D.; Birring, S.S.; Darmalingam, M.; Hooper, R.L.; Kunst, H.; Matos, S.; Bothamley, G.H. Daily Cough Frequency in Tuberculosis and Association with Household Infection. Int. J. Tuberc. Lung Dis. 2018, 22, 863–870. [Google Scholar] [CrossRef]
- Patterson, B.; Wood, R. Is Cough Really Necessary for TB Transmission? Tuberc. Edinb. Scotl. 2019, 117, 31–35. [Google Scholar] [CrossRef]
- Kendall, E.A.; Fofana, M.O.; Dowdy, D.W. The Burden of Transmitted Multi-Drug Resistance among Epidemics of Tuberculosis: A Transmission Model. Lancet Respir. Med. 2015, 3, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Knight, G.M.; Colijn, C.; Shrestha, S.; Fofana, M.; Cobelens, F.; White, R.G.; Dowdy, D.W.; Cohen, T. The Distribution of Fitness Costs of Resistance-Conferring Mutations Is a Key Determinant for the Future Burden of Drug-Resistant Tuberculosis: A Model-Based Analysis. Clin. Infect. Dis. 2015, 61, S147–S154. [Google Scholar] [CrossRef] [Green Version]
- Merker, M.; Rasigade, J.-P.; Barbier, M.; Cox, H.; Feuerriegel, S.; Kohl, T.A.; Shitikov, E.; Klaos, K.; Gaudin, C.; Antoine, R. Transcontinental Spread and Evolution of Mycobacterium Tuberculosis W148 European/Russian Clade toward Extensively Drug Resistant Tuberculosis. Nat. Commun. 2022, 13, 5105. [Google Scholar] [CrossRef]
- Grandjean, L.; Gilman, R.H.; Martin, L.; Soto, E.; Castro, B.; Lopez, S.; Coronel, J.; Castillo, E.; Alarcon, V.; Lopez, V.; et al. Transmission of Multidrug-Resistant and Drug-Susceptible Tuberculosis within Households: A Prospective Cohort Study. PLoS Med. 2015, 12, e1001843. [Google Scholar] [CrossRef] [PubMed]
- Streicher, E.M.; Warren, R.M.; Kewley, C.; Simpson, J.; Rastogi, N.; Sola, C.; van der Spuy, G.D.; van Helden, P.D.; Victor, T.C. Genotypic and Phenotypic Characterization of Drug-Resistant Mycobacterium Tuberculosis Isolates from Rural Districts of the Western Cape Province of South Africa. J. Clin. Microbiol. 2004, 42, 891–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streicher, E.M.; Müller, B.; Chihota, V.; Mlambo, C.; Tait, M.; Pillay, M.; Trollip, A.; Hoek, K.G.P.; Sirgel, F.A.; Gey van Pittius, N.C.; et al. Emergence and Treatment of Multidrug Resistant (MDR) and Extensively Drug-Resistant (XDR) Tuberculosis in South Africa. Infect. Genet. Evol. 2012, 12, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Murray, M. Modeling Epidemics of Multidrug-Resistant M. Tuberculosis of Heterogeneous Fitness. Nat. Med. 2004, 10, 1117–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrell, S.; Gagneux, S. Infectiousness, Reproductive FItness and Evolution of Drug-Resistant Mycobacterium Tuberculosis. Int. J. Tuberc. Lung Dis. 2009, 13, 1456–1466. [Google Scholar]
- Kadura, S.; King, N.; Nakhoul, M.; Zhu, H.; Theron, G.; Köser, C.U.; Farhat, M. Systematic Review of Mutations Associated with Resistance to the New and Repurposed Mycobacterium Tuberculosis Drugs Bedaquiline, Clofazimine, Linezolid, Delamanid and Pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. [Google Scholar] [CrossRef]
- Omar, S.V.; Ismail, F.; Ndjeka, N.; Kaniga, K.; Ismail, N.A. Bedaquiline-Resistant Tuberculosis Associated with Rv0678 Mutations. N. Engl. J. Med. 2022, 386, 93–94. [Google Scholar] [CrossRef]
- Harouna Hamidou, Z.; Mamadou, S.; Saad, J. Molecular Detection of Mycobacterium Tuberculosis Sensu Stricto in the Soil of Niger. New Microbes New Infect. 2021, 44, 100939. [Google Scholar] [CrossRef]
- Velayati, A.A.; Farnia, P.; Mozafari, M.; Malekshahian, D.; Farahbod, A.M.; Seif, S.; Rahideh, S.; Mirsaeidi, M. Identification and Genotyping of Mycobacterium Tuberculosis Isolated From Water and Soil Samples of a Metropolitan City. Chest 2015, 147, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. The Source and Fate of Mycobacterium Tuberculosis Complex in Wastewater and Possible Routes of Transmission. BMC Public Health 2022, 22, 145. [Google Scholar] [CrossRef]
- Straus, I.; Dubarry, A. Recherches Sur La Duree de La Vie Des Microbes Pathogenes Dans I’eau. [Research on the Duration of Life of Pathogenic Microbes in Water]. Arch. Med. Exp. Anat. Pathol. 1889, 1, 5. [Google Scholar]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial Communities in Full-Scale Wastewater Treatment Systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [Green Version]
- Manoj, N.; Rigzin, K.; Deepali, J. Screening Paper Currency for Mycobacterium Tuberculosis Using Loop-Mediated Isothermal Amplification. J. Appl. Biol. Biotechnol. 2022, 10, 221–225. [Google Scholar] [CrossRef]
- Donald, P.R.; Diacon, A.H.; Lange, C.; Demers, A.-M.; Nardell, E. Droplets, Dust and Guinea Pigs: An Historical Review of Tuberculosis Transmission Research, 1878–1940. Int. J. Tuberc. Lung Dis. 2018, 22, 972–982. [Google Scholar] [CrossRef]
- Sterling, T.R.; Pope, D.S.; Bishai, W.R.; Harrington, S.; Gershon, R.R.; Chaisson, R.E. Transmission of Mycobacterium Tuberculosis from a Cadaver to an Embalmer. N. Engl. J. Med. 2000, 342, 246–248. [Google Scholar] [CrossRef]
- Galagan, J.E. Genomic Insights into Tuberculosis. Nat. Rev. Genet. 2014, 15, 307–320. [Google Scholar] [CrossRef]
- Travis, E.R.; Hung, Y.; Porter, D.; Paul, G.; James, R.; Roug, A.; Kato-Maeda, M.; Kazwala, R.; Smith, W.A.; Hopewell, P.; et al. Environmental Reservoirs of Mycobacterium Bovis and Mycobacterium Tuberculosis in the Ruaha Region, Tanzania; Microbiology. bioRxiv 2019, 790824. [Google Scholar]
- D’Agata, E.M.; Wise, S.; Stewart, A.; Lefkowitz, L.B. Nosocomial Transmission of Mycobacterium Tuberculosis from an Extrapulmonary Site. Infect. Control Hosp. Epidemiol. 2001, 22, 10–12. [Google Scholar] [CrossRef]
- Gaustad, V. Tuberculosis Primary Infection in Three Children Occurring in Connection with Fall into Highly Contaminated River Water. Pub. Health Engr. Abst. 1947, 29, 524–537. [Google Scholar]
- Senecal, P. Primary Pulmonary Tuberculosis in Two Children in Association with a Fall into Sewage Contaminated Water. Acta Tuberc. Scand. 1950, 24, 357–364. [Google Scholar]
- Michalak, K.; Austin, C.; Diesel, S.; Bacon, M.J.; Zimmerman, P.; Maslow, J.N. Mycobacterium Tuberculosis Infection as a Zoonotic Disease: Transmission between Humans and Elephants. Emerg. Infect. Dis. 1998, 4, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Stephens, N.; Vogelnest, L.; Lowbridge, C.; Christensen, A.; Marks, G.B.; Sintchenko, V.; Mcanulty, J. Transmission of Mycobacterium Tuberculosis from an Asian Elephant ( Elephas Maximus ) to a Chimpanzee ( Pan Troglodytes ) and Humans in an Australian Zoo. Epidemiol. Infect. 2013, 141, 1488–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartoris, G.; Seddon, J.A.; Rabie, H.; Nel, E.D.; Schaaf, H.S. Abdominal Tuberculosis in Children: Challenges, Uncertainty, and Confusion. J. Pediatric Infect. Dis. Soc. 2020, 9, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, B.; Banda, L.; Mzembe, T.; Crampin, A.C.; Glynn, J.R.; Clark, T.G. Bayesian Reconstruction of Mycobacterium Tuberculosis Transmission Networks in a High Incidence Area over Two Decades in Malawi Reveals Associated Risk Factors and Genomic Variants. Microb. Genom. 2020, 6, e000361. [Google Scholar] [CrossRef] [PubMed]
- Classen, C.N.; Warren, R.; Richardson, M.; Hauman, J.H.; Gie, R.P.; Ellis, J.H.P.; van Helden, P.D.; Beyers, N. Impact of Social Interactions in the Community on the Transmission of Tuberculosis in a High Incidence Area. Thorax 1999, 54, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Mathema, B.; Hu, Y.; Zhao, Q.; Weili, J.; Xu, B. Role of Casual Contacts in the Recent Transmission of Tuberculosis in Settings with High Disease Burden. Clin. Microbiol. Infect. Dis. 2014, 20, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Hella, J.; Morrow, C.; Mhimbira, F.; Ginsberg, S.; Chitnis, N.; Gagneux, S.; Mutayoba, B.; Wood, R.; Fenner, L. Tuberculosis Transmission in Public Locations in Tanzania: A Novel Approach to Studying Airborne Disease Transmission. J. Infect. 2017, 75, 191–197. [Google Scholar] [CrossRef]
- Comstock, G.W.; Ferebee, S.H.; Hammes, L.M. A Controlled Trial of Community-Wide Isoniazid Prophylaxis in Alaska. Am. Rev. Respir. Dis. 1967, 95, 935–943. [Google Scholar]
- Kaplan, G.J.; Fraser, R.I.; Comstock, G.W. Tuberculosis in Alaska, 1970: The Continued Decline of the Tuberculosis Epidemic. Am. Rev. Respir. Dis. 1972, 105, 920–926. [Google Scholar]
- Marks, G.B.; Nguyen, N.V.; Nguyen, P.T.B.; Nguyen, T.-A.; Nguyen, H.B.; Tran, K.H.; Nguyen, S.V.; Luu, K.B.; Tran, D.T.T.; Vo, Q.T.N.; et al. Community-Wide Screening for Tuberculosis in a High-Prevalence Setting. N. Engl. J. Med. 2019, 381, 1347–1357. [Google Scholar] [CrossRef]
- Wilson, N.; Corbett, S.; Tovey, E. Airborne Transmission of COVID-19. BMJ 2020, 370, m3206. [Google Scholar] [CrossRef]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and Its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef]
- Goldman, E. Exaggerated Risk of Transmission of COVID-19 by Fomites. Lancet Infect. Dis. 2020, 20, 892–893. [Google Scholar] [CrossRef]
- Rocha, A.L.S.; Pinheiro, J.R.; Nakamura, T.C.; da Silva, J.D.S.; Rocha, B.G.S.; Klein, R.C.; Birbrair, A.; Amorim, J.H. Fomites and the Environment Did Not Have an Important Role in COVID-19 Transmission in a Brazilian Mid-Sized City. Sci. Rep. 2021, 11, 15960. [Google Scholar] [CrossRef]
- Cheng, P.; Luo, K.; Xiao, S.; Yang, H.; Hang, J.; Ou, C.; Cowling, B.J.; Yen, H.-L.; Hui, D.S.; Hu, S.; et al. Predominant Airborne Transmission and Insignificant Fomite Transmission of SARS-CoV-2 in a Two-Bus COVID-19 Outbreak Originating from the Same Pre-Symptomatic Index Case. J. Hazard. Mater. 2022, 425, 128051. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol Emission and Superemission during Human Speech Increase with Voice Loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.R.; Morawska, L. The Mechanism of Breath Aerosol Formation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Patterson, B.; Morrow, C.; Singh, V.; Moosa, A.; Gqada, M.; Woodward, J.; Mizrahi, V.; Bryden, W.; Call, C.; Patel, S. Detection of Mycobacterium Tuberculosis Bacilli in Bio-Aerosols from Untreated TB Patients. Gates Open Res. 2017, 1, 11. [Google Scholar] [CrossRef]
- Darquenne, C. Deposition Mechanisms. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef]
- Bourouiba, L.; Dehandschoewercker, E.; Bush, J.W.M. Violent Expiratory Events: On Coughing and Sneezing. J. Fluid Mech. 2014, 745, 537–563. [Google Scholar] [CrossRef]
- Hershkovitz, I.; Donoghue, H.D.; Minnikin, D.E.; Besra, G.S.; Lee, O.Y.-C.; Gernaey, A.M.; Galili, E.; Eshed, V.; Greenblatt, C.L.; Lemma, E.; et al. Detection and Molecular Characterization of 9000-Year-Old Mycobacterium Tuberculosis from a Neolithic Settlement in the Eastern Mediterranean. PLoS ONE 2008, 3, e3426. [Google Scholar] [CrossRef] [PubMed]
- Frascella, B.; Richards, A.S.; Sossen, B.; Emery, J.C.; Odone, A.; Law, I.; Onozaki, I.; Esmail, H.; Houben, R.M.G.J. Subclinical Tuberculosis Disease—A Review and Analysis of Prevalence Surveys to Inform Definitions, Burden, Associations, and Screening Methodology. Clin. Infect. Dis. 2021, 73, e830–e841. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The Definition of Tuberculosis Infection Based on the Spectrum of Tuberculosis Disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.C.; Dodd, P.J.; Banu, S.; Frascella, B.; Garden, F.L.; Horton, K.C.; Hossain, S.; Law, I.; van Leth, F.; Marks, G.B.; et al. Estimating the Contribution of Subclinical Tuberculosis Disease to Transmission—An Individual Patient Data Analysis from Prevalence Surveys. MedRxiv 2022. [Google Scholar] [CrossRef]
- Stuck, L.; van Haaster, A.-C.; Kapata-Chanda, P.; Klinkenberg, E.; Kapata, N.; Cobelens, F. How “Subclinical” Is Subclinical Tuberculosis? An Analysis of National Prevalence Survey Data from Zambia. Clin. Infect. Dis. 2022, 75, 842–848. [Google Scholar] [CrossRef]
- Bajema, K.L.; Bassett, I.V.; Coleman, S.M.; Ross, D.; Freedberg, K.A.; Wald, A.; Drain, P.K. Subclinical Tuberculosis among Adults with HIV: Clinical Features and Outcomes in a South African Cohort. BMC Infect. Dis. 2019, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Chung, C.; Jung, S.S.; Park, H.K.; Lee, S.-S.; Lee, K.M. Clinical Profiles of Subclinical Disease among Pulmonary Tuberculosis Patients: A Prospective Cohort Study in South Korea. BMC Pulm. Med. 2020, 20, 316. [Google Scholar] [CrossRef]
- Onozaki, I.; Law, I.; Sismanidis, C.; Zignol, M.; Glaziou, P.; Floyd, K. National Tuberculosis Prevalence Surveys in Asia, 1990–2012: An Overview of Results and Lessons Learned. Trop. Med. Int. Health 2015, 20, 1128–1145. [Google Scholar] [CrossRef]
- Wood, R.; Morrow, C.; Barry, C.E.; Bryden, W.A.; Call, C.J.; Hickey, A.J.; Rodes, C.E.; Scriba, T.J.; Blackburn, J.; Issarow, C.; et al. Real-Time Investigation of Tuberculosis Transmission: Developing the Respiratory Aerosol Sampling Chamber (RASC). PLoS ONE 2016, 11, e0146658. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Oeltmann, J.E.; Hill, A.N.; Tobias, J.L.; Boyd, R.; Click, E.S.; Finlay, A.; Mondongo, C.; Zetola, N.M.; Moonan, P.K. Characterizing tuberculosis transmission dynamics in high-burden urban and rural settings. Sci. Rep. 2022, 12, 6780. [Google Scholar] [CrossRef]
- Riley, R.L.; Mills, C.; O’grady, F.; Sultan, L.; Wittstadt, F.; Shivpuri, D. Infectiousness of Air from a Tuberculosis Ward: Ultraviolet Irradiation of Infected Air: Comparative Infectiousness of Different Patients. Am. Rev. Respir. Dis. 1962, 85, 511–525. [Google Scholar]
- Riley, R.L.; Mills, C.; Nyka, W.; Weinstock, N.; Storey, P.; Sultan, L.; Riley, M.; Wells, W. Aerial Dissemination of Pulmonary Tuberculosis. A Two-Year Study of Contagion in a Tuberculosis Ward. Am. J. Hyg. 1959, 70, 185–196. [Google Scholar]
- Escombe, A.R.; Moore, D.A.J.; Gilman, R.H.; Pan, W.; Navincopa, M.; Ticona, E.; Martínez, C.; Caviedes, L.; Sheen, P.; Gonzalez, A.; et al. The Infectiousness of Tuberculosis Patients Coinfected with HIV. PLoS Med. 2008, 5, e188. [Google Scholar] [CrossRef] [Green Version]
- Escombe, A.R.; Moore, D.A.J.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R.; et al. Upper-Room Ultraviolet Light and Negative Air Ionization to Prevent Tuberculosis Transmission. PLoS Med. 2009, 6, e1000043. [Google Scholar] [CrossRef] [Green Version]
- Handel, A.; Martinez, L.; Sekandi, J.N.; Bellan, S.E.; Zhu, L.; Chen, C.; Liu, Q.; Donkor, S.; Sutherland, J.; Hill, P.C.; et al. Evidence for Supercoughers in an Analysis of Six Tuberculosis Cohorts from China, Peru, The Gambia and Uganda. Int. J. Tuberc. Lung Dis. 2019, 23, 1286–1292. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M.; Sun, F.; Jit, M.; Munday, J.D.; et al. Early Dynamics of Transmission and Control of COVID-19: A Mathematical Modelling Study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.L.; Harding, N.; Zachreson, C.; Cliff, O.M.; Prokopenko, M. Modelling Transmission and Control of the COVID-19 Pandemic in Australia. Nat. Commun. 2020, 11, 5710. [Google Scholar] [CrossRef]
- Badr, H.S.; Du, H.; Marshall, M.; Dong, E.; Squire, M.M.; Gardner, L.M. Association between Mobility Patterns and COVID-19 Transmission in the USA: A Mathematical Modelling Study. Lancet Infect. Dis. 2020, 20, 1247–1254. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; et al. Physical Distancing, Face Masks, and Eye Protection to Prevent Person-to-Person Transmission of SARS-CoV-2 and COVID-19: A Systematic Review and Meta-Analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Driessche, K.V.; Mahlobo, P.Z.; Venter, R.; Caldwell, J.; Jennings, K.; Diacon, A.H.; Cotton, M.F.; de Groot, R.; Hens, N.; Marx, F.M.; et al. Face Masks in the Post-COVID-19 Era: A Silver Lining for the Damaged Tuberculosis Public Health Response? Lancet Respir. Med. 2021, 9, 340–342. [Google Scholar] [CrossRef]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.; Yen, H.-L.; Li, Y.; Ip, D.K.; Peiris, J. Respiratory Virus Shedding in Exhaled Breath and Efficacy of Face Masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clase, C.M.; Fu, E.L.; Ashur, A.; Beale, R.C.L.; Clase, I.A.; Dolovich, M.B.; Jardine, M.J.; Joseph, M.; Kansiime, G.; Mann, J.F.E.; et al. Forgotten Technology in the COVID-19 Pandemic: Filtration Properties of Cloth and Cloth Masks—A Narrative Review. Mayo Clin. Proc. 2020, 95, 2204–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Seong, D.; Li, H.; Chung, S.K.; Park, Y.; Lee, M.; Lee, S.W.; Yon, D.K.; Kim, J.H.; Lee, K.H.; et al. Comparative Effectiveness of N95, Surgical or Medical, and Non-medical Facemasks in Protection against Respiratory Virus Infection: A Systematic Review and Network Meta-analysis. Rev. Med. Virol. 2022, 32, e2336. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis Associated with Infliximab, a Tumor Necrosis Factor α–Neutralizing Agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, C.R.; O’Donnell, M.; Chamblee, S.; Moreland, J.L.; Johnson, J.; Marsh, B.J.; Narita, M.; Johnson, L.S.; von Reyn, C.F. Revisiting Rates of Reactivation Tuberculosis: A Population-Based Approach. Am. J. Respir. Crit. Care Med. 2010, 182, 420–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.R.; Morrow, C.; Wood, R. Modeling the Role of Public Transportation in Sustaining Tuberculosis Transmission in South Africa. Am. J. Epidemiol. 2013, 177, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Murray, E.J.; Marais, B.J.; Mans, G.; Beyers, N.; Ayles, H.; Godfrey-Faussett, P.; Wallman, S.; Bond, V. A Multidisciplinary Method to Map Potential Tuberculosis Transmission ‘Hot Spots’ in High-Burden Communities. Int. J. Tuberc. Lung Dis. 2009, 13, 767–774. [Google Scholar]
- Gandhi, N.R.; Weissman, D.; Moodley, P.; Ramathal, M.; Elson, I.; Kreiswirth, B.N.; Mathema, B.; Shashkina, E.; Rothenberg, R.; Moll, A.P.; et al. Nosocomial Transmission of Extensively Drug-Resistant Tuberculosis in a Rural Hospital in South Africa. J. Infect. Dis. 2013, 207, 9–17. [Google Scholar] [CrossRef]
- Nardell, E.; McInnis, B.; Thomas, B.; Weidhaas, S. Exogenous Reinfection with Tuberculosis in a Shelter for the Homeless. N. Engl. J. Med. 1986, 315, 1570–1575. [Google Scholar] [CrossRef]
- Valway, S.E.; Greifinger, R.B.; Papania, M.; Kilburn, J.O.; Woodley, C.; DiFerdinando, G.T.; Dooley, S.W. Multidrug-Resistant Tuberculosis in the New York State Prison System, 1990–1991. J. Infect. Dis. 1994, 170, 151–156. [Google Scholar] [CrossRef]
- Murray, E.J.; Dodd, P.J.; Marais, B.; Ayles, H.; Shanaube, K.; Schaap, A.; White, R.G.; Bond, V. Sociological Variety and the Transmission Efficiency of Mycobacterium Tuberculosis: A Secondary Analysis of Qualitative and Quantitative Data from 15 Communities in Zambia. BMJ Open 2021, 11, e047136. [Google Scholar] [CrossRef]
- Richardson, E.T.; Morrow, C.D.; Kalil, D.B.; Bekker, L.G.; Wood, R. Shared air: A renewed focus on ventilation for the prevention of tuberculosis transmission. PLoS ONE 2014, 9, e96334. [Google Scholar] [CrossRef] [Green Version]
- Somsen, G.A.; van Rijn, C.; Kooij, S.; Bem, R.A.; Bonn, D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir. Med. 2020, 8, 658–659. [Google Scholar] [CrossRef]
- Lau, M.S.; Grenfell, B.; Thomas, M.; Bryan, M.; Nelson, K.; Lopman, B. Characterizing superspreading events and age-specific infectiousness of SARS-CoV-2 transmission in Georgia, USA. Proc. Natl. Acad. Sci. USA 2020, 117, 22430–22435. [Google Scholar] [CrossRef]
- Bracken, H.M. Tuberculosis in Prisons. J. Am. Med. Assoc. 1904, 42, 255. [Google Scholar] [CrossRef] [Green Version]
- Bunyasi, E.W.; Middelkoop, K.; Koch, A.; Hoosen, Z.; Mulenga, H.; Luabeya, A.K.K.; Shenje, J.; Mendelsohn, S.C.; Tameris, M.; Scriba, T.J.; et al. Molecular Detection of Airborne Mycobacterium Tuberculosis in South African High Schools. Am. J. Respir. Crit. Care Med. 2022, 205, 350–356. [Google Scholar] [CrossRef]
- Chaumont, F.S.B.F.D. I. On the Theory of Ventilation: An Attempt to Establish a Positive Basis for the Calculation of the Amount of Fresh Air Required for an Inhabited Air-Space. Proc. R. Soc. Lond. 1875, 23, 187–201. [Google Scholar]
- Rudnick, S.N.; Milton, D.K. Risk of Indoor Airborne Infection Transmission Estimated from Carbon Dioxide Concentration: Indoor Airborne Transmission of Infectious Diseases. Indoor Air 2003, 13, 237–245. [Google Scholar] [CrossRef]
- Erdmann, C.A.; Steiner, K.C.; Apte, M.G. Indoor Carbon Dioxide Concentrations and Sick Building Syndrome Symptoms in the BASE Study Revisited: Analyses of the 100 Building Dataset. eScholarship 2002. [Google Scholar]
- Nathavitharana, R.R.; Mishra, H.; Sullivan, A.; Hurwitz, S.; Lederer, P.; Meintjes, J.; Nardell, E.; Theron, G. Predicting Airborne Infection Risk: Association between Personal Ambient Carbon Dioxide Level Monitoring and Incidence of Tuberculosis Infection in South African Health Workers. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Roy, C.J.; Milton, D.K. Airborne Transmission of Communicable Infection—The Elusive Pathway. N. Engl. J. Med. 2004, 350, 1710–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaussé, P. Nouvelles Recherches Sur La Contagion de La Tuberculose Par l’air Expire Pendant La Toux. Ann. Inst. Pasteur 1916, 30, 613–641. [Google Scholar]
- Patterson, B.; Dinkele, R.; Gessner, S.; Morrow, C.; Kamariza, M.; Bertozzi, C.R.; Kamholz, A.; Bryden, W.; Call, C.; Warner, D.F.; et al. Sensitivity Optimisation of Tuberculosis Bioaerosol Sampling. PLoS ONE 2020, 15, e0238193. [Google Scholar] [CrossRef] [PubMed]
- Johnstone-Robertson, S.P.; Mark, D.; Morrow, C.; Middelkoop, K.; Chiswell, M.; Aquino, L.D.; Bekker, L.-G.; Wood, R. Social Mixing Patterns within a South African Township Community: Implications for Respiratory Disease Transmission and Control. Am. J. Epidemiol. 2011, 174, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Patterson, B.; Morrow, C.D.; Kohls, D.; Deignan, C.; Ginsburg, S.; Wood, R. Mapping Sites of High TB Transmission Risk: Integrating the Shared Air and Social Behaviour of TB Cases and Adolescents in a South African Township. Sci. Total Environ. 2017, 583, 97–103. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Mphahlele, M.; Venter, K.; Stoltz, A.; Mathebula, R.; Masotla, T.; van der Walt, M.; Pagano, M.; Jensen, P.; Nardell, E. Rapid Impact of Effective Treatment on Transmission of Multidrug Resistant Tuberculosis. Int. J. Tuberc. Lung Dis. 2014, 18, 1019–1025. [Google Scholar] [CrossRef]
- Menzies, D. Effect of Treatment on Contagiousness of Patients with Active Pulmonary Tuberculosis. Infect. Control Hosp. Epidemiol. 1997, 18, 582–586. [Google Scholar] [CrossRef]
- Fennelly, K.; Poynton, M.; Sivasubramani, S. Rapid Killing of Mycobacteria in Aerosols by Exposure to Isoniazid. In A46. Treatment of Mycobacterial Infections; American Thoracic Society: San Diego, CA, USA, 2009; p. 1678. ISBN 1073-449X. [Google Scholar]
- Loudon, R.G.; Bumgarner, L.R.; Lacy, J.; Coffman, G.K. Aerial Transmission of Mycobacteria. Am. Rev. Respir. Dis. 1969, 100, 165–171. [Google Scholar]
- Pillay, S.; Steingart, K.R.; Davies, G.R.; Chaplin, M.; De Vos, M.; Schumacher, S.G.; Warren, R.; Theron, G. Xpert MTB/XDR for Detection of Pulmonary Tuberculosis and Resistance to Isoniazid, Fluoroquinolones, Ethionamide, and Amikacin. Cochrane Database Syst. Rev. 2022, 2022, CD014841. [Google Scholar] [CrossRef]
- Stoltz, A.C.; De Kock, E.J.; Nathavitharana, R.R.; Lederer, P.; Kruger, J.A.; Keulder, S.; Jensen, P.; Nardell, E. Multi-Drug Resistant TB Treatment Regimen, Including Bedaquiline and Linezolid, Failed to Reduce Transmission over 14 Days. In A26. Diagnosis and Treatment of Tuberculosis; American Thoracic Society: San Diego, CA, USA, 2017; p. A1187. ISBN 1073-449X. [Google Scholar]
- Pečerska, J.; Kühnert, D.; Meehan, C.J.; Coscollá, M.; de Jong, B.C.; Gagneux, S.; Stadler, T. Quantifying Transmission Fitness Costs of Multi-Drug Resistant Tuberculosis. Epidemics 2021, 36, 100471. [Google Scholar] [CrossRef]
- Kizny Gordon, A.; Marais, B.; Walker, T.M.; Sintchenko, V. Clinical and Public Health Utility of Mycobacterium Tuberculosis Whole Genome Sequencing. Int. J. Infect. Dis. 2021, 113, S40–S42. [Google Scholar] [CrossRef]
- Arnott, A.; Draper, J.; Rockett, R.J.; Lam, C.; Sadsad, R.; Gall, M.; Martinez, E.; Byun, R.; Musto, J.; Marais, B.; et al. Documenting Elimination of Co-Circulating COVID-19 Clusters Using Genomics in New South Wales, Australia. BMC Res. Notes 2021, 14, 415. [Google Scholar] [CrossRef]
- Guthrie, J.L.; Kong, C.; Roth, D.; Jorgensen, D.; Rodrigues, M.; Hoang, L.; Tang, P.; Cook, V.; Johnston, J.; Gardy, J.L. Molecular Epidemiology of Tuberculosis in British Columbia, Canada: A 10-Year Retrospective Study. Clin. Infect. Dis. 2018, 66, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, J.L.; Marchand-Austin, A.; Cronin, K.; Lam, K.; Pyskir, D.; Kong, C.; Jorgensen, D.; Rodrigues, M.; Roth, D.; Tang, P.; et al. Universal Genotyping Reveals Province-Level Differences in the Molecular Epidemiology of Tuberculosis. PLoS ONE 2019, 14, e0214870. [Google Scholar] [CrossRef] [Green Version]
- Gurjav, U.; Outhred, A.C.; Jelfs, P.; McCallum, N.; Wang, Q.; Hill-Cawthorne, G.A.; Marais, B.J.; Sintchenko, V. Whole Genome Sequencing Demonstrates Limited Transmission within Identified Mycobacterium Tuberculosis Clusters in New South Wales, Australia. PLoS ONE 2016, 11, e0163612. [Google Scholar] [CrossRef] [Green Version]
- Marais, B.J.; Walker, T.M.; Cirillo, D.M.; Raviglione, M.; Abubakar, I.; van der Werf, M.J.; Boehme, C.; Niemann, S.; Castro, K.G.; Zumla, A. Aiming for Zero Tuberculosis Transmission in Low-Burden Countries. Lancet Respir. Med. 2017, 5, 846–848. [Google Scholar] [CrossRef]
- Outhred, A.C.; Holmes, N.; Sadsad, R.; Martinez, E.; Jelfs, P.; Hill-Cawthorne, G.A.; Gilbert, G.L.; Marais, B.J.; Sintchenko, V. Identifying Likely Transmission Pathways within a 10-Year Community Outbreak of Tuberculosis by High-Depth Whole Genome Sequencing. PLoS ONE 2016, 11, e0150550. [Google Scholar] [CrossRef]
- Bainomugisa, A.; Lavu, E.; Pandey, S.; Majumdar, S.; Banamu, J.; Coulter, C.; Marais, B.; Coin, L.; Graham, S.M.; du Cros, P. Evolution and Spread of a Highly Drug Resistant Strain of Mycobacterium Tuberculosis in Papua New Guinea. BMC Infect. Dis. 2022, 22, 437. [Google Scholar] [CrossRef]
- Bainomugisa, A.; Lavu, E.; Hiashiri, S.; Majumdar, S.; Honjepari, A.; Moke, R.; Dakulala, P.; Hill-Cawthorne, G.A.; Pandey, S.; Marais, B.J. Multi-Clonal Evolution of Multi-Drug-Resistant/Extensively Drug-Resistant Mycobacterium Tuberculosis in a High-Prevalence Setting of Papua New Guinea for over Three Decades. Microb. Genom. 2018, 4, e000147. [Google Scholar] [CrossRef]
- World Health Organization. Accelerating Access to Genomics for Global Health: Promotion, Implementation, Collaboration, and Ethical, Legal, and Social Issues: A Report of the WHO Science Council; World Health Organization: Geneva, Switzerland, 2022.
- Meehan, C.J.; Goig, G.A.; Kohl, T.A.; Verboven, L.; Dippenaar, A.; Ezewudo, M.; Farhat, M.R.; Guthrie, J.L.; Laukens, K.; Miotto, P. Whole Genome Sequencing of Mycobacterium Tuberculosis: Current Standards and Open Issues. Nat. Rev. Microbiol. 2019, 17, 533–545. [Google Scholar] [CrossRef]

| Unanswered Questions | Insight from SARS-CoV-2 Research |
|---|---|
| Are asymptomatic and/or subclinical tuberculosis cases infectious? Furthermore, if so, how infectious are they compared to those with clinically apparent disease and what is the combined contribution to epidemic spread? | Yes; people with asymptomatic SARS-CoV-2 infection shown to be infectious, albeit less infectious than symptomatic cases. However, symptomatic cases contribute a large proportion of population-level transmission |
| Are there strain related variability in transmission? | Yes; major differences demonstrated in different Variants of Concern (VoCs) |
| Is there a transmission fitness cost to tuberculosis drug-resistance? | No; little research on drug-resistance, less relevant. Some level of ‘vaccine escape’ associated with VoCs |
| In which community locations does transmission most commonly occur? | Yes; the vast majority of transmission shown to occur in crowded indoor settings with poor ventilation, including households, pubs and clubs, public transportation (e.g., buses and trains), hospitals, and elderly care settings. |
| What are the key variables associated with transmission heterogeneity, to close contacts and at population-level? | Yes; wide transmission heterogeneity was demonstrated, with consideration that asymptomatic spread makes a major contribution to population-level spread. |
| How frequently does superspreading occur and what factors are associated with superspreading? | Yes, a variety of factors have been described-mainly related to the infectiousness of the source case, their participation in large congregate settings and general mobility within the population. |
| To what extent do potential institutional amplifiers (e.g., prisons, mines, hospitals, churches, schools, etc.) contribute to community-wide tuberculosis transmission? | Not well characterized, but less relevant with extensive population spread. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, M.; Martinez, L.; Theron, G.; Wood, R.; Marais, B. Mycobacterium tuberculosis Transmission in High-Incidence Settings—New Paradigms and Insights. Pathogens 2022, 11, 1228. https://doi.org/10.3390/pathogens11111228
Coleman M, Martinez L, Theron G, Wood R, Marais B. Mycobacterium tuberculosis Transmission in High-Incidence Settings—New Paradigms and Insights. Pathogens. 2022; 11(11):1228. https://doi.org/10.3390/pathogens11111228
Chicago/Turabian StyleColeman, Mikaela, Leonardo Martinez, Grant Theron, Robin Wood, and Ben Marais. 2022. "Mycobacterium tuberculosis Transmission in High-Incidence Settings—New Paradigms and Insights" Pathogens 11, no. 11: 1228. https://doi.org/10.3390/pathogens11111228
APA StyleColeman, M., Martinez, L., Theron, G., Wood, R., & Marais, B. (2022). Mycobacterium tuberculosis Transmission in High-Incidence Settings—New Paradigms and Insights. Pathogens, 11(11), 1228. https://doi.org/10.3390/pathogens11111228









