Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective
Abstract
1. Introduction
2. Bat Trypanosomes
2.1. Diversity, Taxonomy, Distribution and Host Range
2.2. Vectors
2.3. Life-Cycle and Pathogenesis
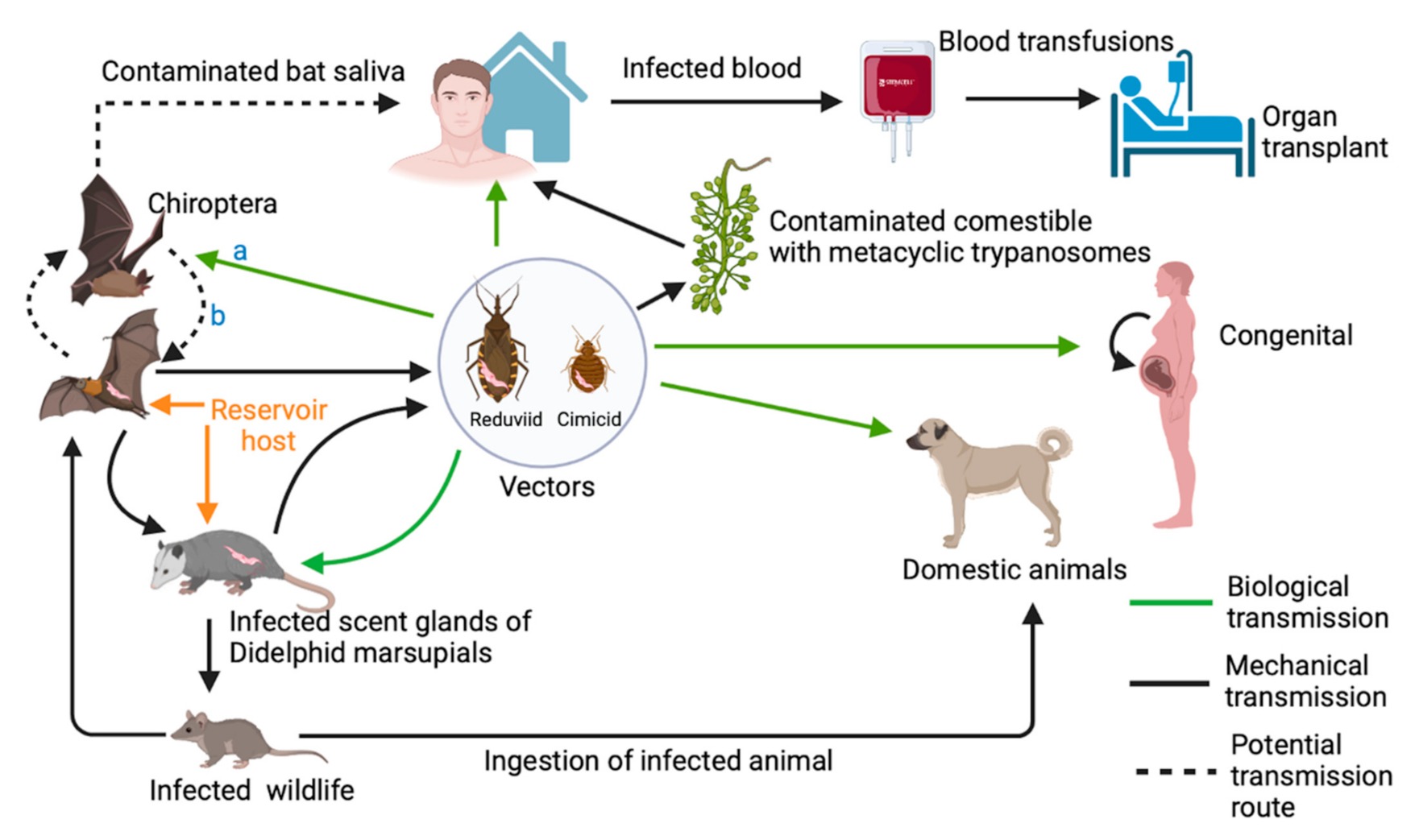
2.4. Alternative Transmission Routes
2.5. Atypical Cases of Animal Trypanosomiasis in Humans
2.6. Parasite Detection Methods
3. Epidemiology and Evolution of Bat Trypanosomes in a One Health Framework
3.1. Role of Bats in the Evolution of Trypanosoma cruzi: The Bat Seeding-Hypothesis
3.2. The Complex Diversity and Epidemiology of Trypanosoma cruzi of Bats
3.2.1. The Repertoire of Trypanosoma cruzi Genotypes in Bats
3.2.2. Bats as Reservoirs of Trypanosoma cruzi in the Domestic and Sylvatic Cycles
3.3. Epidemiological Role of Bats as Reservoirs and Vectors of Trypanosoma evansi
4. Biosecurity Concerns
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of Chagas disease. Parasit. Vectors 2014, 7, 317. [Google Scholar] [CrossRef]
- Wang, P.G.; Kudelko, M.; Kwok, K.T.; Bruzzone, R.; Nal, B. Molecular dissection of dengue virus egress: Involvement of the class II ARF small GTPase. Hong Kong Med. J. 2016, 22 (Suppl. 7), 43–45. [Google Scholar]
- Hoare, C.A. The Trypanosomes of Mammals: A Zoological Monograph; Blackwell Scientific Publishing: Oxford, England, 1972. [Google Scholar]
- Aregawi, W.G.; Agga, G.E.; Abdi, R.D.; Buscher, P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasit. Vectors 2019, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Coll, O.; Juncosa, T.; Verges, M.; del Pino, M.; Fumado, V.; Bosch, J.; Posada, E.J.; Hernandez, S.; Fisa, R.; et al. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin. Infect. Dis. 2009, 48, 1736–1740. [Google Scholar] [CrossRef]
- Nobrega, A.A.; Garcia, M.H.; Tatto, E.; Obara, M.T.; Costa, E.; Sobel, J.; Araujo, W.N. Oral transmission of Chagas disease by consumption of acai palm fruit, Brazil. Emerg. Infect. Dis. 2009, 15, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; Ciosi, M.; Hamilton, A.; Scullion, K.M.; Dede, P.; Igbinosa, I.B.; Nmorsi, O.P.; Masiga, D.; Turner, C.M. Molecular identification of different trypanosome species and subspecies in tsetse flies of northern Nigeria. Parasit. Vectors 2016, 9, 301. [Google Scholar] [CrossRef]
- Reid, S.A. Trypanosoma evansi control and containment in Australasia. Trends Parasitol. 2002, 18, 219–224. [Google Scholar] [CrossRef]
- Sulkin, S.E.; Allen, R. Virus infections in bats. Monogr. Virol. 1974, 8, 1–103. [Google Scholar]
- Turmelle, A.S.; Olival, K.J. Correlates of viral richness in bats (order Chiroptera). Ecohealth 2009, 6, 522–539. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T.; Bowen, R.A.; Cryan, P.M.; McCracken, G.F.; O’Shea, T.J.; Peel, A.J.; Gilbert, A.; Webb, C.T.; Wood, J.L. Ecology of zoonotic infectious diseases in bats: Current knowledge and future directions. Zoonoses Public Health 2013, 60, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.A.; Lisboa, C.V.; Costa, L.M.; Moratelli, R.; Nascimento, M.P.; Costa, L.P.; Leite, Y.L.R.; Llewellyn, M.S.; Xavier, S.; Roque, A.L.R.; et al. High Trypanosoma spp. diversity is maintained by bats and triatomines in Espirito Santo state, Brazil. PLoS ONE 2017, 12, e0188412. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.A.; Pavan, M.G.; Rodrigues, M.S.; Lisboa, C.V.; Kluyber, D.; Desbiez, A.L.J.; Herrera, H.M.; Roque, A.L.R.; Lima, L.; Teixeira, M.M.G.; et al. Trypanosoma rangeli Genetic, Mammalian Hosts, and Geographical Diversity from Five Brazilian Biomes. Pathogens 2021, 10, 736. [Google Scholar] [CrossRef]
- Dario, M.A.; Rodrigues, M.S.; Barros, J.H.D.; Xavier, S.C.D.; D’Andrea, P.S.; Roque, A.L.R.; Jansen, A.M. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espirito Santo state, Brazil). Parasite Vector 2016, 9, 477. [Google Scholar] [CrossRef]
- Dean, L.M.; Sugay, W. Trypanosoma possoai n. sp. In vampire bats Desmodus rotundus from the state of San Paulo, Brazil. Rev. Inst. Med. St. Paulo 1963, 5, 165. [Google Scholar]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. Biomed. Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Schofeild, C.J. Control of Triatominae; CABI Publishing: Wallingford, UK, 2004; pp. 547–563. [Google Scholar]
- Dos Santos, F.C.B.; Lisboa, C.V.; Xavier, S.C.C.; Dario, M.A.; Verde, R.S.; Calouro, A.M.; Roque, A.L.R.; Jansen, A.M. Trypanosoma sp. diversity in Amazonian bats (Chiroptera; Mammalia) from Acre State, Brazil. Parasitology 2018, 145, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.L.; Taylor, C.L.; Austen, J.M.; Banks, P.B.; Ahlstrom, L.A.; Ryan, U.M.; Irwin, P.J.; Oskam, C.L. Molecular identification of the Trypanosoma (Herpetosoma) lewisi clade in black rats (Rattus rattus) from Australia. Parasitol Res. 2020, 119, 1691–1696. [Google Scholar] [CrossRef]
- El-Rahman, A.R.; Monib Mel, S.; Hassan, A.A.; Ghanam, M.E.; Shataat, M.A.; el-Damarany, M. Studies on the Megatrypanum trypanosomes of the Egyptian bat (Pipistrellum kuhli) from Sohag Governorate, Egypt. J. Egypt Soc. Parasitol. 2001, 31, 87–93. [Google Scholar]
- Ellis, J.; Barratt, J.; Kaufer, A.; Pearn, L.; Armstrong, B.; Johnson, M.; Park, Y.; Downey, L.; Cao, M.; Neill, L.; et al. A new subspecies of Trypanosoma cyclops found in the Australian terrestrial leech Chtonobdella bilineata. Parasitology 2021, 148, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Alvarez, O.; Ortiz, P.A.; Lima, L.; Costa-Martins, A.G.; Serrano, M.G.; Herder, S.; Buck, G.A.; Camargo, E.P.; Hamilton, P.B.; Stevens, J.R.; et al. Trypanosoma rangeli is phylogenetically closer to Old World trypanosomes than to Trypanosoma cruzi. Int. J. Parasitol. 2018, 48, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Ewers, W.H. Trypanosoma aunawa sp. n. from an insectivorous bat, Miniopterus tristris, in New Guinea, which may be transmitted by a leech. J. Parasitol. 1974, 60, 172–178. [Google Scholar] [CrossRef]
- Eybpoosh, S.; Haghdoost, A.A.; Mostafavi, E.; Bahrampour, A.; Azadmanesh, K.; Zolala, F. Molecular epidemiology of infectious diseases. Electron. Physician 2017, 9, 5149–5158. [Google Scholar] [CrossRef]
- Ferreira Lde, L.; Pereira, M.H.; Guarneri, A.A. Revisiting Trypanosoma rangeli Transmission Involving Susceptible and Non-Susceptible Hosts. PLoS ONE 2015, 10, e0140575. [Google Scholar] [CrossRef][Green Version]
- Franzen, O.; Talavera-Lopez, C.; Ochaya, S.; Butler, C.E.; Messenger, L.A.; Lewis, M.D.; Llewellyn, M.S.; Marinkelle, C.J.; Tyler, K.M.; Miles, M.A.; et al. Comparative genomic analysis of human infective Trypanosoma cruzi lineages with the bat-restricted subspecies T. cruzi marinkellei. BMC Genom. 2012, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.S.; Castro, D.P.; Figueiredo, M.B.; Azambuja, P. Parasite-mediated interactions within the insect vector: Trypanosoma rangeli strategies. Parasit. Vectors 2012, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.A.; Molyneux, D.H. Trypanosoma (Megatrypanum) incertum from Pipistrellus pipistrellus: Development and transmission by cimicid bugs. Parasitology 1988, 96 Pt 3, 433–447. [Google Scholar] [CrossRef]
- Gibson, W.; Bingle, L.; Blendeman, W.; Brown, J.; Wood, J.; Stevens, J. Structure and sequence variation of the trypanosome spliced leader transcript. Mol. Biochem. Parasitol. 2000, 107, 269–277. [Google Scholar] [CrossRef]
- Grisard, E.C.; Sturm, N.R.; Campbell, D.A. A new species of trypanosome, Trypanosoma desterrensis sp. n., isolated from South American bats. Parasitology 2003, 127, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Cruickshank, C.; Stevens, J.R.; Teixeira, M.M.; Mathews, F. Parasites reveal movement of bats between the New and Old Worlds. Mol. Phylogenet. Evol. 2012, 63, 521–526. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Gibson, W.C.; Stevens, J.R. Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Mol. Phylogenet. Evol. 2007, 44, 15–25. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Stevens, J.R. 15—Classification and Phylogeny of Trypanosoma Cruzi. In American Trypanosomiasis Chagas Disease, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hamilton, P.B.; Stevens, J.R.; Gaunt, M.W.; Gidley, J.; Gibson, W.C. Trypanosomes are monophyletic: Evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int. J. Parasitol. 2004, 34, 1393–1404. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Stevens, J.R.; Gidley, J.; Holz, P.; Gibson, W.C. A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae). Int. J. Parasitol. 2005, 35, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Teixeira, M.M.; Stevens, J.R. The evolution of Trypanosoma cruzi: The ‘bat seeding’ hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Schofeild, C.J. Elimination of Rhodnius prolixus in Central America. Parasites Vectors 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoare, C.A. Vampire bats as vectors and hosts of equine and bovine trypanosomes. Acta Trop. 1965, 22, 204–216. [Google Scholar]
- Hodo, C.L.; Hamer, S.A. Toward an Ecological Framework for Assessing Reservoirs of Vector-Borne Pathogens: Wildlife Reservoirs of Trypanosoma cruzi across the Southern United States. ILAR J. 2017, 58, 379–392. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Austen, J.M.; O’Dea, M.; Jackson, B.; Ryan, U. High prevalence of Trypanosoma vegrandis in bats from Western Australia. Vet. Parasitol. 2015, 214, 342–347. [Google Scholar] [CrossRef][Green Version]
- Austen, J.M.; Van Kampen, E.; Egan, S.L.; O’Dea, M.A.; Jackson, B.; Ryan, U.M.; Irwin, P.J.; Prada, D. First report of Trypanosoma dionisii (Trypanosomatidae) identified in Australia. Parasitology 2020, 147, 1801–1809. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Mackie, J.T.; Stenner, R.; Gillett, A.; Irwin, P.; Ryan, U. Trypanosoma teixeirae: A new species belonging to the T. cruzi clade causing trypanosomosis in an Australian little red flying fox (Pteropus scapulatus). Vet. Parasitol. 2016, 223, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Beltz, L.A. Kinetoplastids and bats. In Bats and Human Health; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 285–301. [Google Scholar]
- Lima, L.; Silva, F.M.; Neves, L.; Attias, M.; Takata, C.S.; Campaner, M.; de Souza, W.; Hamilton, P.B.; Teixeira, M.M. Evolutionary insights from bat trypanosomes: Morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) erneyi sp. nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Protist 2012, 163, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.T.; Hawkins, J.D. Trypanosomes and experimental trypanosomaisis in East African bats. Acta Trop. 1975, 32, 57–64. [Google Scholar]
- Lima, L.; Espinosa-Alvarez, O.; Hamilton, P.B.; Neves, L.; Takata, C.S.; Campaner, M.; Attias, M.; de Souza, W.; Camargo, E.P.; Teixeira, M.M. Trypanosoma livingstonei: A new species from African bats supports the bat seeding hypothesis for the Trypanosoma cruzi clade. Parasit. Vectors 2013, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H. Trypanosomes of bats. In Parasitic Protozoa; Kreier, J.P., Baker, J.R., Eds.; Academic Press: New York, NY, USA, 1991; pp. 95–223. [Google Scholar]
- Cavazzana, M., Jr.; Marcili, A.; Lima, L.; da Silva, F.M.; Junqueira, A.C.; Veludo, H.H.; Viola, L.B.; Campaner, M.; Nunes, V.L.; Paiva, F.; et al. Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int. J. Parasitol. 2010, 40, 345–355. [Google Scholar] [CrossRef]
- Lima, L.; Espinosa-Alvarez, O.; Pinto, C.M.; Cavazzana, M., Jr.; Pavan, A.C.; Carranza, J.C.; Lim, B.K.; Campaner, M.; Takata, C.S.; Camargo, E.P.; et al. New insights into the evolution of the Trypanosoma cruzi clade provided by a new trypanosome species tightly linked to Neotropical Pteronotus bats and related to an Australian lineage of trypanosomes. Parasit. Vectors 2015, 8, 657. [Google Scholar] [CrossRef]
- Villena, F.E.; Gomez-Puerta, L.A.; Jhonston, E.J.; Del Alcazar, O.M.; Maguina, J.L.; Albujar, C.; Laguna-Torres, V.A.; Recuenco, S.E.; Ballard, S.B.; Ampuero, J.S. First Report of Trypanosoma cruzi Infection in Salivary Gland of Bats from the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2018, 99, 723–728. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Karnkowska, A.; Votypka, J.; Tashyreva, D.; Maciszewski, K.; Yurchenko, V.; Lukes, J. Euglenozoa: Taxonomy, diversity and ecology, symbioses and viruses. Open Biol. 2021, 11, 200407. [Google Scholar] [CrossRef]
- Mafie, E.; Rupa, F.H.; Takano, A.; Suzuki, K.; Maeda, K.; Sato, H. First record of Trypanosoma dionisii of the T. cruzi clade from the Eastern bent-winged bat (Miniopterus fuliginosus) in the Far East. Parasitol. Res. 2018, 117, 673–680. [Google Scholar] [CrossRef]
- Stevens, J.R.; Teixeira, M.M.; Bingle, L.E.; Gibson, W.C. The taxonomic position and evolutionary relationships of Trypanosoma rangeli. Int. J. Parasitol. 1999, 29, 749–757. [Google Scholar] [CrossRef]
- Wang, L.J.; Han, H.J.; Zhao, M.; Liu, J.W.; Luo, L.M.; Wen, H.L.; Qin, X.R.; Zhou, C.M.; Qi, R.; Yu, H.; et al. Trypanosoma dionisii in insectivorous bats from northern China. Acta Trop. 2019, 193, 124–128. [Google Scholar] [CrossRef]
- Clement, L.; Dietrich, M.; Markotter, W.; Fasel, N.J.; Monadjem, A.; Lopez-Baucells, A.; Scaravelli, D.; Theou, P.; Pigeault, R.; Ruedi, M.; et al. Out of Africa: The origins of the protozoan blood parasites of the Trypanosoma cruzi clade found in bats from Africa. Mol. Phylogenet. Evol. 2020, 145, 106705. [Google Scholar] [CrossRef] [PubMed]
- Cottontail, V.M.; Kalko, E.K.; Cottontail, I.; Wellinghausen, N.; Tschapka, M.; Perkins, S.L.; Pinto, C.M. High local diversity of Trypanosoma in a common bat species, and implications for the biogeography and taxonomy of the T. cruzi clade. PLoS ONE 2014, 9, e108603. [Google Scholar] [CrossRef]
- Stevens, J.R.; Gibson, W. The molecular evolution of trypanosomes. Parasitol. Today 1999, 15, 432–437. [Google Scholar] [CrossRef]
- Da Silva, F.M.; Noyes, H.; Campaner, M.; Junqueira, A.C.; Coura, J.R.; Anez, N.; Shaw, J.J.; Stevens, J.R.; Teixeira, M.M. Phylogeny, taxonomy and grouping of Trypanosoma rangeli isolates from man, triatomines and sylvatic mammals from widespread geographical origin based on SSU and ITS ribosomal sequences. Parasitology 2004, 129, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Baker, J.R.; Miles, M.A.; Godfrey, D.G.; Barrett, T.V. Biochemical characterization of some species of Trypanosoma (Schizotrypanum) from Bats (Microchiroptera). Am. J. Trop. Med. Hyg. 1978, 27, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.; Austen, J.; Gillett, A.; Warren, K.; Paparini, A.; Irwin, P.; Ryan, U. First report of Trypanosoma vegrandis in koalas (Phascolarctos cinereus). Parasitol. Int. 2016, 65, 316–318. [Google Scholar] [CrossRef]
- Bradwell, K.R.; Koparde, V.N.; Matveyev, A.V.; Serrano, M.G.; Alves, J.M.P.; Parikh, H.; Huang, B.; Lee, V.; Espinosa-Alvarez, O.; Ortiz, P.A.; et al. Genomic comparison of Trypanosoma conorhini and Trypanosoma rangeli to Trypanosoma cruzi strains of high and low virulence. BMC Genom. 2018, 19, 770. [Google Scholar] [CrossRef]
- Botero, A.; Cooper, C.; Thompson, C.K.; Clode, P.L.; Rose, K.; Thompson, R.C. Morphological and Phylogenetic Description of Trypanosoma noyesi sp. nov.: An Australian Wildlife Trypanosome within the T. cruzi Clade. Protist 2016, 167, 425–439. [Google Scholar] [CrossRef]
- Barros, J.H.S.; Lima, L.; Schubach, A.O.; Teixeira, M.M.G. Trypanosoma madeirae sp. n.: A species of the clade T. cruzi associated with the neotropical common vampire bat Desmodus rotundus. Int. J. Parasitol. Parasites Wildl. 2019, 8, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.D.; Tapia-Calle, G.; Munoz-Cruz, G.; Poveda, C.; Rendon, L.M.; Hincapie, E.; Guhl, F. Trypanosome species in neo-tropical bats: Biological, evolutionary and epidemiological implications. Infect. Genet. Evol. 2014, 22, 250–256. [Google Scholar] [CrossRef]
- Stevens, J.R.; Noyes, H.A.; Schofield, C.J.; Gibson, W. The molecular evolution of Trypanosomatidae. Adv. Parasitol. 2001, 48, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Maslov, D.A.; Opperdoes, F.R.; Kostygov, A.Y.; Hashimi, H.; Lukes, J.; Yurchenko, V. Recent advances in trypanosomatid research: Genome organization, expression, metabolism, taxonomy and evolution. Parasitology 2019, 146, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Votypka, J.; d’Avila-Levy, C.M.; Grellier, P.; Maslov, D.A.; Lukes, J.; Yurchenko, V. New approaches to systematics of Trypanosomatidae: Criteria for taxonomic (re)description. Trends Parasitol. 2015, 31, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Botero, A.; Wayne, A.F.; Godfrey, S.S.; Lymbery, A.J.; Thompson, R.C. Morphological polymorphism of Trypanosoma copemani and description of the genetically diverse T. vegrandis sp. nov. from the critically endangered Australian potoroid, the brush-tailed bettong (Bettongia penicillata (Gray, 1837)). Parasit. Vectors 2013, 6, 121. [Google Scholar] [CrossRef]
- Austen, J.M.; Jefferies, R.; Friend, J.A.; Ryan, U.; Adams, P.; Reid, S.A. Morphological and molecular characterization of Trypanosoma copemani n. sp. (Trypanosomatidae) isolated from Gilbert’s potoroo (Potorous gilbertii) and quokka (Setonix brachyurus). Parasitology 2009, 136, 783–792. [Google Scholar] [CrossRef] [PubMed]
- McInnes, L.M.; Hanger, J.; Simmons, G.; Reid, S.A.; Ryan, U.M. Novel trypanosome Trypanosoma gilletti sp. (Euglenozoa: Trypanosomatidae) and the extension of the host range of Trypanosoma copemani to include the koala (Phascolarctos cinereus). Parasitology 2011, 138, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Breinl, A. Parasite protozoa encountered in the blood of Australian native animals. Aust. Inst. Trop. Med. 1913, 30–38. [Google Scholar]
- Mackerras, M.J. The haematozoa of Australian mammals. Aust. J. Zool. 1959, 7, 105–135. [Google Scholar] [CrossRef]
- Bower, S.M.; Woo, P.T.K. Development of Trypanosoma (Schizotrypanum) hedricki in Cimex brevis (Hemiptera: Cimicidae). Can. J. Zool. 1981, 59, 530–545. [Google Scholar] [CrossRef]
- Paterson, W.B.; Woo, P.T.K. An ultrastructural study of the culture forms of Trypanosoma (Schizotrypanum) myoti. Can. J. Zool. 1983, 61, 2807–2815. [Google Scholar] [CrossRef]
- Rodhain, J. Trypanosome d’un chéiroptère insectivore Nycteris hispida Schreber au Congo Beige. Bull. Soc. Pathol. Exot. 1923, 16, 659–663. [Google Scholar]
- Rodhain, J. Trypanosoma leleupi n. sp. parasite de Hipposideros caffer au Katanga. Annls. Parasit. Hum. Comp. 1951, 26, 133–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeledon, R.; Rosabal, R. Trypanosoma leonidasdeanei sp. n. in insectivorous bats of Costa Rica. Ann. Trop. Med. Parasit 1969, 63, 221–228. [Google Scholar] [CrossRef]
- Miltgen, F.; Landau, I. Trypanosoma (Megatrypanum) lizae n.sp. un trypanosome ayant des formes geantes chez les microchiropteres Hipposideros cyclops au Gabo. Ann. Parasitolo Gie Hum. Comp. 1979, 54, 163–169. [Google Scholar] [CrossRef]
- Wenyon, C.M. Report of a Travelling Pathologist and Protozoologist; Wellcome Research Laboratory: Beckenham, UK, 1909; p. 121. [Google Scholar]
- Marinkelle, C.J. Trypanosoma (Megatrypanum) megachiropterum sp.n. from the flying fox. Pteropus tonganus Quoy and Galmard. J. Protozool. 1979, 26, 352–353. [Google Scholar]
- Leger, M.; Baury, A. Trypanosome de la chauve-souris du Senegal Hipposideros tridens, Et. Geoff C.r.Skanc. Soc. Biol. 1923, 88, 866–869. [Google Scholar]
- Marinkelle, C.J.; Duarte, C.A. Trypanosoma pifanoi n. sp. from Colombian bats. J. Protozool. 1968, 15, 621. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Ray, R.; Dasgupta, B. A new species of Trypanosoma from an Indian insectivotous bat, Rhinopoma hardwickei Gray. Acta Protozool. 1982, 21, 189–195. [Google Scholar]
- Liao, G.Y. Biology of a new trypanosome: Trypanosoma (Megatrypanum) scotophila sp.nov. from the bat Scotophilus heathi Horsfield. In Malaria and Other Protozoal Infections; Zhongshan University: Guangzhou, China, 1982. [Google Scholar]
- Keymer, I.F. Blood Protozoa of Insectivores, Bats and Primates in Central Africa. J. Zool. 1971, 163, 421–441. [Google Scholar] [CrossRef]
- Lips, M.; Rodhain, J. Quelques hematozoaires de petits mammiferes du Haut-Katang. Annls. Parasit. Hum. Comp. 1956, 31, 481–488. [Google Scholar] [CrossRef]
- Marinkelle, C.J. Trypanosoma (Herpetosoma) longiflagellum sp.n. from the tomb bat, Taphozous nudiventris, from Iraq. J. Wildl. Dis. 1977, 13, 262–264. [Google Scholar] [CrossRef]
- Austen, J.M.; Ryan, U.M.; Friend, J.A.; Ditcham, W.G.; Reid, S.A. Vector of Trypanosoma copemani identified as Ixodes sp. Parasitology 2011, 138, 866–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latif, A.A.; Bakheit, M.A.; Mohamed, A.E.; Zweygarth, E. High infection rates of the tick Hyalomma anatolicum anatolicum with Trypanosoma theileri. Onderstepoort J. Vet. Res. 2004, 71, 251–256. [Google Scholar] [CrossRef]
- Luu, L.; Bown, K.J.; Palomar, A.M.; Kazimirova, M.; Bell-Sakyi, L. Isolation and partial characterisation of a novel Trypanosoma from the tick Ixodes ricinus. Ticks Tick Borne Dis. 2020, 11, 101501. [Google Scholar] [CrossRef]
- Marotta, R.C.; Dos Santos, P.N.; Cordeiro, M.D.; Matos, P.C.M.; Barros, J.H.D.S.; Madeira, M.F.; Bell Sakyi, L.; Fonseca, A.H. Trypanosoma rhipicephalis sp. nov. (Protozoa: Kinetoplastida) isolated from Rhipicephalus microplus (Acari: Ixodidae) ticks in Rio de Janeiro, Brazil. Parasitol. Open 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Thekisoe, O.M.; Honda, T.; Fujita, H.; Battsetseg, B.; Hatta, T.; Fujisaki, K.; Sugimoto, C.; Inoue, N. A trypanosome species isolated from naturally infected Haemaphysalis hystricis ticks in Kagoshima Prefecture, Japan. Parasitology 2007, 134, 967–974. [Google Scholar] [CrossRef]
- Szentiványi, T.; Christe, P.; Glaizot, O. Bat flies and their microparasites:current knowledge and distribution. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, C.A.; Di Noia, J.M. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas’ disease. Microbes Infect. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Marinkelle, C.J. Developmental stages of Trypanosoma cruzi-like flagellates in Cavernicola pilosa. Rev. Biol. Trop. 1982, 30, 107–111. [Google Scholar]
- D’Alessandro, A.; Saravia, N.G. Trypanosoma rangeli. In Parasitic Protozoa, 2nd ed.; Kreier, J.P., Bake, J.R., Eds.; Academic Press: San Diego, CA, USA, 1992; Volume 2, pp. 1–54. [Google Scholar]
- Stevens, J.; Noyes, H.; Gibson, W. The evolution of trypanosomes infecting humans and primates. Mem. Inst. Oswaldo Cruz 1998, 93, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Castillo-Neyra, R.; Tustin, A.W.; Borrini-Mayori, K.; Naquira, C.; Levy, M.Z. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2015, 92, 331–335. [Google Scholar] [CrossRef]
- Van Den Berghe, L.; Chardome, M.; Peel, E. An African bat trypanosome in Stricticimex brevispinosus Usinger, 1959. J. Protozool. 1963, 10, 135–138. [Google Scholar] [CrossRef]
- Rodhain, J. Mode de transmission de Trypanosoma vespertilionis Battaglia par les arthropodes. C. R. Seances Soc. Biol. Fil. 1939, 131, 814–818. [Google Scholar]
- Mcconnell, E.; Correa, M. Trypanosomes + Other Microorganisms from Panamanian Phlebotomus Sandflies. J. Parasitol. 1964, 50, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.S.; Sciimidt, F.L.; Guaraldo, A.M.A.; Franco, R.M.B.; Dias, V.L.; Passos, L.A.C. Chagas’ Disease as a Foodborne Illness. J. Food Prot. 2009, 72, 441–446. [Google Scholar] [CrossRef]
- Oliveira, M.P.; Cortez, M.; Maeda, F.Y.; Fernandes, M.C.; Haapalainen, E.F.; Yoshida, N.; Mortara, R.A. Unique behavior of Trypanosoma dionisii interacting with mammalian cells: Invasion, intracellular growth, and nuclear localization. Acta Trop. 2009, 110, 65–74. [Google Scholar] [CrossRef]
- Caradonna, K.L.; Burleigh, B.A. Mechanisms of host cell invasion by Trypanosoma cruzi. Adv. Parasitol. 2011, 76, 33–61. [Google Scholar] [CrossRef]
- Machado, F.S.; Dutra, W.O.; Esper, L.; Gollob, K.J.; Teixeira, M.M.; Factor, S.M.; Weiss, L.M.; Nagajyothi, F.; Tanowitz, H.B.; Garg, N.J. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin. Immunopathol. 2012, 34, 753–770. [Google Scholar] [CrossRef]
- Bonney, K.M.; Engman, D.M. Chagas heart disease pathogenesis: One mechanism or many? Curr. Mol. Med. 2008, 8, 510–518. [Google Scholar] [CrossRef]
- Lukes, J.; Butenko, A.; Hashimi, H.; Maslov, D.A.; Votypka, J.; Yurchenko, V. Trypanosomatids Are Much More than Just Trypanosomes: Clues from the Expanded Family Tree. Trends Parasitol. 2018, 34, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.C.; Borelli, P.; Yoshida, N.; Russo, M. Acute Trypanosoma cruzi infection is associated with anemia, thrombocytopenia, leukopenia, and bone marrow hypoplasia: Reversal by nifurtimox treatment. Microbes Infect. 2000, 2, 347–352. [Google Scholar] [CrossRef]
- Murray, M.; Dexter, T.M. Anaemia in bovine African trypanosomiasis. A review. Acta Trop. 1988, 45, 389–432. [Google Scholar]
- Noyes, H.A.; Alimohammadian, M.H.; Agaba, M.; Brass, A.; Fuchs, H.; Gailus-Durner, V.; Hulme, H.; Iraqi, F.; Kemp, S.; Rathkolb, B.; et al. Mechanisms controlling anaemia in Trypanosoma congolense infected mice. PLoS ONE 2009, 4, e5170. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.M.; Pena, S.D. Genetic Variability of Trypanosoma cruzi:Implications for the Pathogenesis of Chagas Disease. Parasitol. Today 1998, 14, 119–124. [Google Scholar] [CrossRef]
- Perez, C.J.; Lymbery, A.J.; Thompson, R.C. Chagas disease: The challenge of polyparasitism? Trends Parasitol. 2014, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wibbelt, G.; Moore, M.S.; Schountz, T.; Voigt, C.C. Emerging diseases in Chiroptera: Why bats? Biol. Lett. 2010, 6, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.T.; Stenner, R.; Gillett, A.K.; Barbosa, A.; Ryan, U.; Irwin, P.J. Trypanosomiasis in an Australian little red flying fox (Pteropus scapulatus). Aust. Vet. J. 2017, 95, 259–261. [Google Scholar] [CrossRef]
- Garcia, L.; Ortiz, S.; Osorio, G.; Torrico, M.C.; Torrico, F.; Solari, A. Phylogenetic analysis of Bolivian bat trypanosomes of the subgenus Schizotrypanum based on cytochrome B sequence and minicircle analyses. PLoS ONE 2012, 7, e36578. [Google Scholar] [CrossRef]
- Lewis, M.D.; Llewellyn, M.S.; Yeo, M.; Acosta, N.; Gaunt, M.W.; Miles, M.A. Recent, independent and anthropogenic origins of Trypanosoma cruzi hybrids. PLoS Negl. Trop. Dis. 2011, 5, e1363. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Mendez, M.L.; Nogueda-Torres, B.; Alejandre-Aguilar, R.; Cortes-Jimenez, M. Experimental Trypanosoma cruzi infection via contaminated water and food. Rev. Lat. Microbiol. 1994, 36, 67–69. [Google Scholar]
- Jansen, A.M.; Moriearty, P.L.; Castro, B.G.; Deane, M.P. Trypanosoma cruzi in the opossum Didelphis marsupialis: An indirect fluorescent antibody test for the diagnosis and follow-up of natural and experimental infections. Trans. R Soc. Trop. Med. Hyg. 1985, 79, 474–477. [Google Scholar] [CrossRef]
- Silva, N.N.; Clausell, D.T.; Nolibos, H.; Mello, A.L.; O’ssanal, J.; Rapone, T.; Snell, T. Surto epidêmico de doença de Chagas com provável contaminação oral. Rev. Inst. Med. Trop Sao Paulo 1968, 10, 265–276. [Google Scholar] [PubMed]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J. Chagas disease: An emerging food-borne entity? J. Infect. Dev. Countries 2008, 2, 149–150. [Google Scholar] [CrossRef]
- Pinto, C.M.; Kalko, E.K.; Cottontail, I.; Wellinghausen, N.; Cottontail, V.M. TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal Zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infect. Genet. Evol. 2012, 12, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Leffer, L. Social Grooming in Bats: Are Vampire Bats Exceptional? PLoS ONE 2015, 10, e0138430. [Google Scholar] [CrossRef]
- Caraballo, A.J. Outbreak of vampire bat biting in a Venezuelan village. Rev. Saude Publica 1996, 30, 483–484. [Google Scholar] [CrossRef]
- Bergner, L.M.; Orton, R.J.; Benavides, J.A.; Becker, D.J.; Tello, C.; Biek, R.; Streicker, D.G. Demographic and environmental drivers of metagenomic viral diversity in vampire bats. Mol. Ecol. 2020, 29, 26–39. [Google Scholar] [CrossRef]
- Johnson, P.D. A case of infection by Trypanosoma lewisi in a child. Trans. R Soc. Trop. Med. Hyg. 1933, 26, 467–468. [Google Scholar] [CrossRef]
- Lun, Z.R.; Reid, S.A.; Lai, D.H.; Li, F.J. Atypical human trypanosomiasis: A neglected disease or just an unlucky accident? Trends Parasitol. 2009, 25, 107–108. [Google Scholar] [CrossRef]
- Sarataphan, N.; Vongpakorn, M.; Nuansrichay, B.; Autarkool, N.; Keowkarnkah, T.; Rodtian, P.; Stich, R.W.; Jittapalapong, S. Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 2007, 56, 1118–1121. [Google Scholar] [CrossRef]
- Joshi, P.P.; Shegokar, V.R.; Powar, R.M.; Herder, S.; Katti, R.; Salkar, H.R.; Dani, V.S.; Bhargava, A.; Jannin, J.; Truc, P. Human trypanosomiasis caused by Trypanosoma evansi in India: The first case report. Am. J. Trop. Med. Hyg. 2005, 73, 491–495. [Google Scholar] [CrossRef]
- Austen, J.M.; Reid, S.A.; Robinson, D.R.; Friend, J.A.; Ditcham, W.G.; Irwin, P.J.; Ryan, U. Investigation of the morphological diversity of the potentially zoonotic Trypanosoma copemani in quokkas and Gilbert’s potoroos. Parasitology 2015, 142, 1443–1452. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Austen, J.; Portas, T.J.; Friend, J.A.; Ahlstrom, L.A.; Oskam, C.L.; Ryan, U.M.; Irwin, P.J. Sequence analyses at mitochondrial and nuclear loci reveal a novel Theileria sp. and aid in the phylogenetic resolution of piroplasms from Australian marsupials and ticks. PLoS ONE 2019, 14, e0225822. [Google Scholar] [CrossRef]
- Austen, J.M.; Paparini, A.; Reid, S.A.; Friend, J.A.; Ditcham, W.G.; Ryan, U. Molecular characterization of native Australian trypanosomes in quokka (Setonix brachyurus) populations from Western Australia. Parasitol. Int. 2016, 65, 205–208. [Google Scholar] [CrossRef][Green Version]
- Ledezma, A.P.; Blandon, R.; Schijman, A.G.; Benatar, A.; Saldana, A.; Osuna, A. Mixed infections by different Trypanosoma cruzi discrete typing units among Chagas disease patients in an endemic community in Panama. PLoS ONE 2020, 15, e0241921. [Google Scholar] [CrossRef]
- Burgos, J.M.; Altcheh, J.; Bisio, M.; Duffy, T.; Valadares, H.M.; Seidenstein, M.E.; Piccinali, R.; Freitas, J.M.; Levin, M.J.; Macchi, L.; et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int. J. Parasitol. 2007, 37, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, O.; Santos, S.S.; Cupolillo, E.; Mendonca, B.; Derre, R.; Junqueira, A.C.; Santos, L.C.; Sturm, N.R.; Naiff, R.D.; Barret, T.V.; et al. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans. R Soc. Trop. Med. Hyg. 2001, 95, 97–99. [Google Scholar] [CrossRef]
- Fernandes, O.; Sturm, N.R.; Derre, R.; Campbell, D.A. The mini-exon gene: A genetic marker for zymodeme III of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1998, 95, 129–133. [Google Scholar] [CrossRef]
- Jaramillo, N.; Moreno, J.; Triana, O.; Arcos-Burgos, M.; Munoz, S.; Solari, A. Genetic structure and phylogenetic relationships of Colombian Trypanosoma cruzi populations as determined by schizodeme and isoenzyme markers. Am. J. Trop. Med. Hyg. 1999, 61, 986–993. [Google Scholar] [CrossRef][Green Version]
- Triana, O.; Ortiz, S.; Dujardin, J.C.; Solari, A. Trypanosoma cruzi: Variability of stocks from Colombia determined by molecular karyotype and minicircle Southern blot analysis. Exp. Parasitol. 2006, 113, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Lima, L.; Xavier, S.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Int. J. Parasitol. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.; Florez, O.; Rincon, G.; Gonzalez, C.I. Comparison of seven diagnostic tests to detect Trypanosoma cruzi infection in patients in chronic phase of Chagas disease. Colomb. Med. (Cali) 2014, 45, 61–66. [Google Scholar] [CrossRef]
- Lewis, M.D.; Ma, J.; Yeo, M.; Carrasco, H.J.; Llewellyn, M.S.; Miles, M.A. Genotyping of Trypanosoma cruzi: Systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am. J. Trop. Med. Hyg. 2009, 81, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.A.; Cabello, P.H.; Jansen, A.M. Growth behaviour of two Trypanosoma cruzi strains in single and mixed infections: In vitro and in the intestinal tract of the blood-sucking bug, Triatoma brasiliensis. Acta Trop. 2007, 101, 225–231. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Gofton, A.W.; Paparini, A.; Codello, A.; Greay, T.; Gillett, A.; Warren, K.; Irwin, P.; Ryan, U. Increased genetic diversity and prevalence of co-infection with Trypanosoma spp. in koalas (Phascolarctos cinereus) and their ticks identified using next-generation sequencing (NGS). PLoS ONE 2017, 12, e0181279. [Google Scholar] [CrossRef]
- Northover, A.S.; Godfrey, S.S.; Keatley, S.; Lymbery, A.J.; Wayne, A.F.; Cooper, C.; Pallant, L.; Morris, K.; Thompson, R.C.A. Increased Trypanosoma spp. richness and prevalence of haemoparasite co-infection following translocation. Parasit. Vectors 2019, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Petney, T.N.; Andrews, R.H. Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 1998, 28, 377–393. [Google Scholar] [CrossRef]
- Pullan, R.; Brooker, S. The health impact of polyparasitism in humans: Are we under-estimating the burden of parasitic diseases? Parasitology 2008, 135, 783–794. [Google Scholar] [CrossRef]
- Grybchuk-Ieremenko, A.; Losev, A.; Kostygov, A.Y.; Lukes, J.; Yurchenko, V. High prevalence of trypanosome co-infections in freshwater fishes. Folia Parasitol. 2014, 61, 495–504. [Google Scholar] [CrossRef]
- Spodareva, V.V.; Grybchuk-Ieremenko, A.; Losev, A.; Votypka, J.; Lukes, J.; Yurchenko, V.; Kostygov, A.Y. Diversity and evolution of anuran trypanosomes: Insights from the study o European species. Parasite. Vector 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.Y.; Lukes, J.; Jirku, M.; Maslov, D.A. Selective recovery of the cultivation-prone components from mixed trypanosomatid infections: A case of several novel species isolated from Neotropical Heteroptera. Int. J. Syst. Evol. Microbiol. 2009, 59, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Keatley, S.; Northover, A.; Gofton, A.W.; Brigg, F.; Lymbery, A.J.; Pallant, L.; Clode, P.L.; Thompson, R.C.A. Next generation sequencing reveals widespread trypanosome diversity and polyparasitism in marsupials from Western Australia. Int. J. Parasitol. Parasites Wildl. 2018, 7, 58–67. [Google Scholar] [CrossRef]
- Dario, M.A.; Moratelli, R.; Schwabl, P.; Jansen, A.M.; Llewellyn, M.S. Small subunit ribosomal metabarcoding reveals extraordinary trypanosomatid diversity in Brazilian bats. PLoS Negl. Trop. Dis. 2017, 11, e0005790. [Google Scholar] [CrossRef]
- Anez, N.; Crisante, G.; Soriano, P.J. Trypanosoma cruzi congenital transmission in wild bats. Acta Trop. 2009, 109, 78–80. [Google Scholar] [CrossRef]
- Noireau, F.; Diosque, P.; Jansen, A.M. Trypanosoma cruzi: Adaptation to its vectors and its hosts. Vet. Res. 2009, 40, 26. [Google Scholar] [CrossRef]
- Balouz, V.; Melli, L.J.; Volcovich, R.; Moscatelli, G.; Moroni, S.; Gonzalez, N.; Ballering, G.; Bisio, M.; Ciocchini, A.E.; Buscaglia, C.A.; et al. The Trypomastigote Small Surface Antigen from Trypanosoma cruzi Improves Treatment Evaluation and Diagnosis in Pediatric Chagas Disease. J. Clin. Microbiol. 2017, 55, 3444–3453. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Disease: Poverty and pathogens. Nature 2016, 531, S61–S63. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- Lisboa, C.V.; Pinho, A.P.; Herrera, H.M.; Gerhardt, M.; Cupolillo, E.; Jansen, A.M. Trypanosoma cruzi (Kinetoplastida, Trypanosomatidae) genotypes in neotropical bats in Brazil. Vet. Parasitol. 2008, 156, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Santos de Pinho, A.P.; Lisboa, C.V.; Cupolillo, E.; Mangia, R.H.; Fernandes, O. The sylvatic cycle of Trypanosoma cruzi: A still unsolved puzzle. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. 1), 203–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samudio, F.; Ortega-Barria, E.; Saldana, A.; Calzada, J. Predominance of Trypanosoma cruzi I among Panamanian sylvatic isolates. Acta Trop. 2007, 101, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Roellig, D.M.; Brown, E.L.; Barnabe, C.; Tibayrenc, M.; Steurer, F.J.; Yabsley, M.J. Molecular typing of Trypanosoma cruzi isolates, United States. Emerg. Infect. Dis. 2008, 14, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Bosseno, M.F.; Barnabe, C.; Magallon Gastelum, E.; Lozano Kasten, F.; Ramsey, J.; Espinoza, B.; Breniere, S.F. Predominance of Trypanosoma cruzi lineage I in Mexico. J. Clin. Microbiol. 2002, 40, 627–632. [Google Scholar] [CrossRef]
- Anez, N.; Crisante, G.; da Silva, F.M.; Rojas, A.; Carrasco, H.; Umezawa, E.S.; Stolf, A.M.; Ramirez, J.L.; Teixeira, M.M. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas’ disease. Trop. Med. Int. Health 2004, 9, 1319–1326. [Google Scholar] [CrossRef]
- Cuervo, P.; Cupolillo, E.; Segura, I.; Saravia, N.; Fernandes, O. Genetic diversity of Colombian sylvatic Trypanosoma cruzi isolates revealed by the ribosomal DNA. Mem. Inst. Oswaldo Cruz 2002, 97, 877–880. [Google Scholar] [CrossRef][Green Version]
- Herrera, C.; Bargues, M.D.; Fajardo, A.; Montilla, M.; Triana, O.; Vallejo, G.A.; Guhl, F. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect. Genet. Evol. 2007, 7, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Cedillos, R.A.; Povoa, M.M.; de Souza, A.A.; Prata, A.; Macedo, V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas’ disease? Lancet 1981, 1, 1338–1340. [Google Scholar] [CrossRef]
- Marcili, A.; Lima, L.; Cavazzana, M.; Junqueira, A.C.; Veludo, H.H.; Maia Da Silva, F.; Campaner, M.; Paiva, F.; Nunes, V.L.; Teixeira, M.M. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology 2009, 136, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Acosta, N.; Llewellyn, M.; Sanchez, H.; Adamson, S.; Miles, G.A.; Lopez, E.; Gonzalez, N.; Patterson, J.S.; Gaunt, M.W.; et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int. J. Parasitol. 2005, 35, 225–233. [Google Scholar] [CrossRef]
- Guhl, F.; Auderheide, A.; Ramirez, J.D. From ancient to contemporary molecular eco-epidemiology of Chagas disease in the Americas. Int. J. Parasitol. 2014, 44, 605–612. [Google Scholar] [CrossRef]
- Ramirez, J.D.; Hernandez, C.; Montilla, M.; Zambrano, P.; Florez, A.C.; Parra, E.; Cucunuba, Z.M. First report of human Trypanosoma cruzi infection attributed to TcBat genotype. Zoonoses Public Health 2014, 61, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Junqueira, A.C. Ecological diversity of Trypanosoma cruzi transmission in the Amazon basin. The main scenaries in the Brazilian Amazon. Acta Trop. 2015, 151, 51–57. [Google Scholar] [CrossRef]
- Lent, H.; Wygodzinsky, W. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. AMNH 1979, 163, 123–520. [Google Scholar]
- Gaunt, M.; Miles, M. The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem. Inst. Oswaldo Cruz 2000, 95, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Llewellyn, M.S.; Lewis, M.D.; Yeo, M.; Baleela, R.; Fitzpatrick, S.; Gaunt, M.W.; Mauricio, I.L. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: Looking back and to the future. Parasitology 2009, 136, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, R.E.; Cardinal, M.V. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015, 151, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Jaramillo, A.M.; Agudelo-Uribe, L.A.; Dib, J.C.; Ortiz, S.; Solari, A.; Triana-Chavez, O. Genotyping of Trypanosoma cruzi in a hyper-endemic area of Colombia reveals an overlap among domestic and sylvatic cycles of Chagas disease. Parasit. Vectors 2014, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Guerenstein, P.G.; Lazzari, C.R. Host-seeking: How triatomines acquire and make use of information to find blood. Acta Trop. 2009, 110, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Arias-Giraldo, L.M.; Munoz, M.; Hernandez, C.; Herrera, G.; Velasquez-Ortiz, N.; Cantillo-Barraza, O.; Urbano, P.; Cuervo, A.; Ramirez, J.D. Identification of blood-feeding sources in Panstrongylus, Psammolestes, Rhodnius and Triatoma using amplicon-based next-generation sequencing. Parasit. Vectors 2020, 13, 434. [Google Scholar] [CrossRef]
- Thomas, M.E.; Rasweiler Iv, J.J.; D’Alessandro, A. Experimental transmission of the parasitic flagellates Trypanosoma cruzi and Trypanosoma rangeli between triatomine bugs or mice and captive neotropical bats. Mem. Inst. Oswaldo Cruz 2007, 102, 559–565. [Google Scholar] [CrossRef]
- Thompson, C.K.; Thompson, R.C.A. Trypanosomes of Australian Mammals: Knowledge Gaps Regarding Transmission and Biosecurity. Trends Parasitol. 2015, 31, 553–562. [Google Scholar] [CrossRef]
- Thompson, R.C.A. Exotic Parasite Threats to Australia’s Biosecurity-Trade, Health, and Conservation. Trop. Med. Infect. Dis. 2018, 3, 76. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.A.; Stevens, J.R.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef]
- Backhouse, T.C.; Bolliger, A. Transmission of Chagas’ disease to the Australian marsupial Trichosurus Vulpecula. Trans. R Soc. Trop. Med. Hyg. 1951, 44, 521–533. [Google Scholar] [CrossRef]


| Trypanosoma (Schizotrypanum) sp. | Geographic Distribution | Bats (Family) | Other Host Species | Transmission Routes | Clinical Significance | Ref. |
|---|---|---|---|---|---|---|
| Bat-restricted trypanosomes | ||||||
| T. cruzi marinkellei | Central/South America | Phyllostomidae Vespertilionidae Mormoopidae | Unknown | Biological | Unknown | [30,48] |
| T. erneyi | Africa | Molossidae | Unknown | Unknown | Unknown | [49] |
| T. livingstonei | Africa | Rhinolophidae Hipposideridae | Unknown | Unknown | Unknown | [51] |
| T. cf. livingstonei | Africa | Nycteridae Miniopteridae | Unknown | Unknown | Unknown | [60] |
| T. madeirae | Central/South America | Phyllostomidae | Unknown | Unknown | Unknown | [69] |
| T. teixeirae | Australia | Pterpodidae | Unknown | Unknown | Pathogenic | [47] |
| T. vespertillionis | Europe | Vespertilionidae | Unknown | Biological | Unknown | [48] |
| T. vespertilionis-like G1 | Africa | Vespertilionidae | Unknown | Unknown | Unknown | [26] |
| T. vespertilionis-like G2 | Africa | Vespertilionidae | Unknown | Unknown | Unknown | [26] |
| T. wauwau | Central/South America | Mormoopidae | Unknown | Unknown | Unknown | [70] |
| Trypanosoma sp. HochG3 | Africa | Vespertilionidae | Unknown | Unknown | Unknown | [26] |
| T. sp. bat | Africa | Pteropodidae | Unknown | Unknown | Unknown | [54] |
| Trypanosoma sp. NeoBat | Central/South America | Phyllostomidae | Unknown | Unknown | Unknown | [54] |
| T. sp. 1 | Africa Europe | Miniopteridae | Unknown | Unknown | Unknown | [60] |
| T. sp. 2 | Africa | Miniopteridae | Unknown | Unknown | Unknown | [60] |
| Zoonotic bat trypanosomes | ||||||
| T. cruzi cruzi DTU (TcI–TcIV) | North/Central/ South America | Phyllostomidae Vespertilionidae Noctionidae Mormoopidae Thyropteridae | Generalist | Biological Mechanical Vertical | Pathogenic | [26,48] |
| T. rangeli | Central/South America | Phyllostomidae | Generalist | Biological Mechanical | Non-pathogenic | [48] |
| T. dionisii | Cosmopolitan | Molossidae Phyllostomidae Vespertilionidae | Human Opossum | Biological | Potentially Pathogenic | [16,18] |
| T. sp. TcBat | Central/South America | Pteropodidae | Human | Unknown | Non-pathogenic | [70] |
| Trypanosoma sp. | Host Species | Geographic Location | Ref. |
|---|---|---|---|
| Megatrypanum | |||
| T. heybergi | Nycteris hispida Nycteris capensis Pipistrellus kuhlii | Congo Kenya Egypt | [24,81] |
| T. incertum | Pipistrellus pipistrellu | United Kingdom | [32] |
| T. leleupi | Hipposideros caffer | Congo | [5,82] |
| T. leonidasdeanei | Saccopterix bilineata | Costa Rica | [5,83] |
| T. lizae | Hipposideros cyclops | Gabon | [32,84] |
| T. magadermae | Lavia frons P. kuhlii | Sudan Egypt | [24,85] |
| T. megachiropterum | Pteropus tonganus | Tonga | [32,86] |
| T. morinorum | Asellia tridens | Senegal | [5,87] |
| T. mpapuense | Nycteris aethiopica | Tanganyika | [5] |
| T. pessoai | D. rotundus | Brazil | [5,19] |
| T. pifanoi | Artibeus lituratus Phillostomus hastatus | Colombia | [5,88] |
| T. possoai | Desmodus rotundus P. kuhlii | Brazil | [19] |
| T. rhinopoma | Rhinopoma hardwickei | India | [32,89] |
| T. scotophila | Scotophilus heathi horsfield | China | [32,90] |
| T. thomasi | Nycteris macrotis | Congo | [91,92] |
| Herpetosoma | |||
| T. lineatum | Vampyrops lineatum | Venezuela | [93] |
| T. longiflagellum | Taphozous nudiventris | Iraq | [93] |
| T. anauwa | Miniopterus tristris | Papua New Guinea | [27] |
| Trypanozoon | |||
| T. evansi | D. rotundus | Latin America | [5] |
| Uncharacterised subgenus | |||
| T. vegrandis | Chalinolobus gouldii Nyctophilus geoffroyi Pteropus Alecto Pteropus scapulatus | Australia | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austen, J.M.; Barbosa, A.D. Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens 2021, 10, 1148. https://doi.org/10.3390/pathogens10091148
Austen JM, Barbosa AD. Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens. 2021; 10(9):1148. https://doi.org/10.3390/pathogens10091148
Chicago/Turabian StyleAusten, Jill M., and Amanda D. Barbosa. 2021. "Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective" Pathogens 10, no. 9: 1148. https://doi.org/10.3390/pathogens10091148
APA StyleAusten, J. M., & Barbosa, A. D. (2021). Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens, 10(9), 1148. https://doi.org/10.3390/pathogens10091148