Abstract
The horse industry has grown rapidly as a leisure industry in the Republic of Korea (ROK) in parallel with an increased demand for equestrian activities. As a result, there has been an increase in horse breeding and equestrian population and potential exposure to ticks and their associated pathogens. To provide a better understanding of the potential disease risks of veterinary and medical importance, a study was conducted to determine the geographical distribution and diversity of ticks collected from horses and vegetation associated with horse racetracks/ranches throughout the ROK. This included a survey of five associated common pathogens, Anaplasma phagocytophilum, Ehrlichia chaffeensis, Borrelia spp., Babesia caballi, and Theileria equi. A total 9220 ticks were collected from horses and associated pastures. Ticks were identified to species, stage of development, and sex. Two species of ticks, Haemaphysalis longicornis (99.9%) and Ixodes nipponensis (0.1%) were identified. Two of the target pathogens, A. phagocytophilum and Borrelia spp., were detected in 5/1409 tick pools (0.35%) and 4/1409 pools (0.28%) of H. longicornis, respectively, both of which are zoonotic pathogens of medical importance. The results of 16S rRNA phylogenetic analysis of A. phagocytophilum showed a close relationship to strains distributed in China, USA, Germany, Italy, Turkey, and Poland. Borrelia spp. showed a close relationship, based on 16S rRNA gene, to the strains reported from the USA (B. burgdorferi and B. americana) and Japan (B. tanukii and B. garinii). These results provide information about the potential risks of veterinary and medical importance and the development of mitigation strategies for disease prevention.
1. Introduction
The number of horses has grown rapidly following the enactment of the Horse Industry Promotion Act by the Republic of Korea (ROK) government in 2011. The Korean government invested 600 billion won (USD 543,133,883) from 2011–2018 to foster the horse industry [1,2]. By 2019, there were 27,246 horses, 459 horseback riding facilities, and 919,556 riders in the ROK, with increases of 11.4%, 38.7% and 18.1%, respectively, compared to 2013 data [1]. The higher numbers of horses, associated horse facilities, and riders have increased the potential for exposure to ticks and transmission of tick-borne pathogens to both horses and associated equestrian personnel.
A wide range of wild (deer, raccoon dogs, brown bears, red fox, gray wolves) and domestic animals (horses, cattle, dogs, sheep) are hosts of ticks that harbor tick-borne pathogens, e.g., Babesia, Theileria, Anaplasma, and Borrelia species [3,4,5,6,7]. The prevalence of horse ticks varies according to geographical regions, such as Amblyomma cajennense and Anocentor nitens in Brazil [8], Amblyomma americanum, Dermacentor variabilis, Amblyomma maculatum in Oklahoma, US [9], and 13 species belonging to Ixodidae Family in Italy [10]. Ticks and tick-borne diseases (TBD) associated with horses have been reported in different countries (Italy, Spain, Sweden, Guatemala, Taiwan) where selected pathogens have resulted in abortion and decreased animal production [3,11,12,13,14]. Additionally, horse ranch personnel and riders are exposed to biting ticks and associated transmission of tick-borne pathogens [15,16].
In the ROK, Haemaphysalis longicornis harbors various pathogens, e.g., Candidatus Rickettsia longicornii, Ehrlichia canis, and Theileria luwenshuni that have been reported in horses at Jeju Island [17]. However, investigations on the distribution of ticks and associated pathogens that are associated with horses and pasture lands in the ROK have not been conducted. The aims of this study were to identify tick species associated with horses and horse ranches at three metropolitan cities and seven provinces in the ROK, and to detect selected tick-borne pathogens: A. phagocytophilum, E. chaffeensis, Borrelia spp., B. caballi, and T. equi.
2. Results
2.1. Prevalence of Ticks Infesting Horses in ROK
A total 9220 ticks consisting of 2267 larvae; 5433 nymphs; and 1520 adults (412 male and 1108 female) were collected during 2016 and 2017, in which 1686 ticks (18.3%) were collected from horses (1090 adults, 595 nymphs, 1 larva) and 7534 ticks (81.7%) from vegetation associated with racetracks/ranches (430 adults, 4838 nymphs, 2266 larvae) (Table 1). A total of 1532 ticks (16.6%); 2184 ticks (23.7%), and 5504 ticks (59.7%) were collected at racetracks under the Racing Horse Authority (RHA), private horse farms (PHF), and leisure horse-riding ranches (LHR), respectively (Table 2). The largest number of ticks were collected from Jeju Island (6633; 71.9%), followed by Gyeonggi (614; 6.7%) and Jeollanam (380; 4.1%) provinces (Table 1). Nymphs accounted for the highest number of ticks collected (5433; 58.9%), followed by larvae (2267; 24.6%) and adults (1520; 16.5%) (Table 1).

Table 1.
Number of ticks collected (pools) at selected metropolitan areas and provinces.

Table 2.
Identification of tick species from horses and vegetative at racetracks and private horse ranches.
Only two species of ticks, Haemaphysalis longicornis (9214; 99.9%) and Ixodes nipponensis (6; 0.1%) were collected directly from horses and associated vegetation at racetracks and horse ranches (Table 1; Figure 1). Haemaphysalis longicornis was collected from all 10 areas, while I. nipponensis was only collected at Gyeonggi and Jeollabuk provinces.
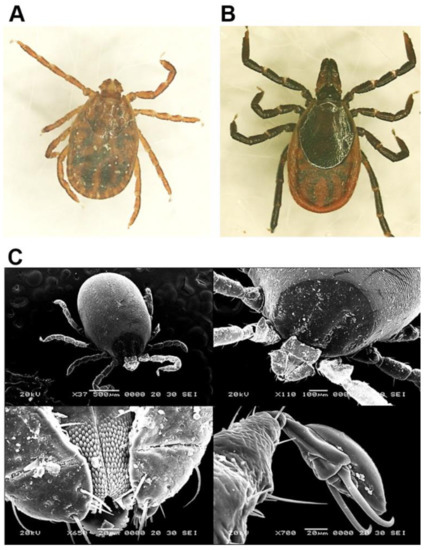
Figure 1.
Morphological characterization of H. longicornis and I. nipponensis using light microscopy and scanning electron microscopy. (A) H. longicornis and (B) I. nipponensis females observed under a light microscope at 40 × magnification. (C) Characterization of H. longicornis was performed using a scanning electron microscope.
2.2. Detection of Tick-Borne Pathogens
Only two, A. phagocytophilum and Borrelia spp., of the five pathogens surveyed were detected (Table 3). Anaplasma phagocytophilum was detected in five pools of H. longicornis ticks with a minimum infection rate (MIR) was 0.54%, and the pools of t A. phagocytophilum-positive ticks were all adults (MIR = 3.30%) (Table 3, Figure 2 and Figure 3). The number of tick pools positive for A. phagocytophilum collected from grasses/herbaceous vegetation associated with horse ranches and directly from horses was 3/5 (60%) and 2/5 (40%), respectively. The distributions of the infected ticks included 1/5 pools (20%) from LHR (Gyeonggi province), while the other four pools (80%) were from LHR (3), RHA (1) at Jeju Island. Anaplasma phagocytophilum-positive ticks from Gyeonggi province and Jeju Island were all adult H. longicornis ticks collected in June 2017.

Table 3.
Tick-borne pathogens detected in ticks collected from horses in the Republic of Korea.
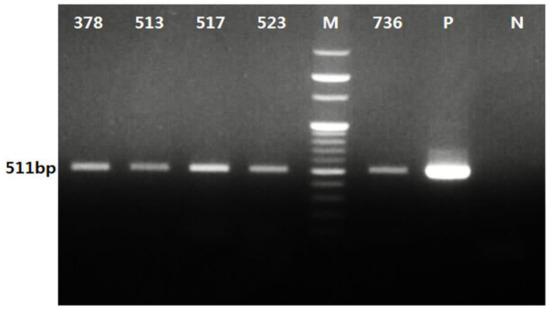
Figure 2.
PCR analysis of A. phagocytophilum from ticks collected from horses and associated vegetation in the Republic of Korea (ROK). Positive detection of A. phagocytophilum in five tick pools (378, 513, 517, 523, and 736 ticks) was confirmed with an expected 511 bp band observed using electrophoresis. “P” and “N” represent a positive control using recombinant A. phagocytophilum DNA and a negative control without a DNA template, respectively. “M” represents a 100 bp DNA marker.
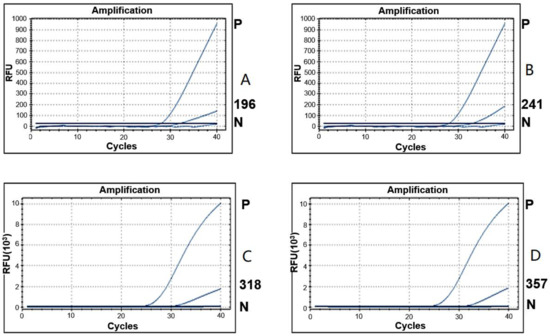
Figure 3.
Detection of Borrelia spp. from ticks collected from horses and associated vegetation by real-time PCR. Amplification curves show positive detection of Borrelia spp. in four tick pools (196(A), 241(B), 318(C), and 357(D) ticks). Tick pool number 196 and 241 were tested together in one PCR performance, and the pool numbers 318 and 357 were done together in another PCR performance. The threshold cycle of Borrelia spp. detection from the four samples was 31.77; 32.81; 30.46; and 31.23, respectively. “P” and “N” represent a positive control using recombinant B. burgdorferi DNA and a negative control without DNA template, respectively. “RFU” indicates relative fluorescence units.
Borrelia spp. were detected by real time PCR in four pools of ticks (MIR = 0.43%) (Table 3, Figure 3). Borrelia spp. was only detected in H. longicornis with an MIR of 0.43‰, in which three (MIR = 0.55%) and one pool (MIR = 0.66‰) were nymphs and adults, respectively. All the Borrelia spp. infected ticks were collected in May and June 2017, in which three pools were collected from grasses/herbaceous vegetation associated with RHA (1; Jeju Island), LHR (2; Jeollanam province (1) and Busan metropolitan city (1)), while one positive pool was collected directly from a horse at Jeju Island (Table 3).
2.3. Sequencing and Phylogenetic Analysis
Sequencing analysis of the 16S rRNA gene of A. phagocytophilum from five tick samples showed 100% amino acid (aa) homology with each other and 99.78 to 100% nucleotide (nt) identity with A. phagocytophilum sequences deposited in the NCBI. Phylogenetic analysis based on the 16S rRNA gene (511 bp) showed that all the detected A. phagocytophilum had the same genotype and shared a close relationship with A. phagocytophilum distributed in China, USA, Canada, and Russia (Figure 4).
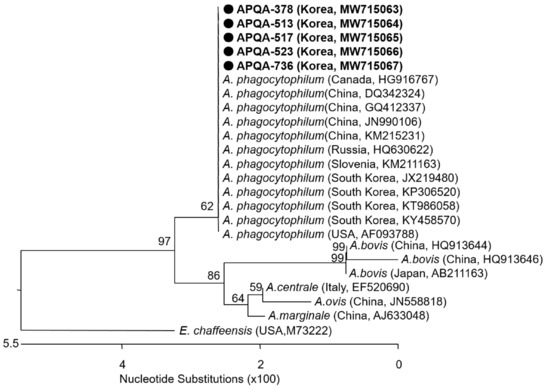
Figure 4.
Phylogenetic relationship of A. phagocytophilum from ticks collected from horses and associated vegetation in the ROK. A neighbor-joining tree was created based on the 16S rRNA gene (511bp) of A. phagocytophilum using MEGA-6 software, with 1000 bootstrap replications. Anaplasma phagocytophilum detected from five tick pools collected in the ROK with isolate names of APQA-378, APQA-513, APQA-517, APQA-523, and APQA-736 and NCBI accession numbers are written in bold. The reference strains detected in different counties and the NCBI accession number are shown.
Phylogenetic analysis of Borrelia spp.-positive samples based on the 16S rRNA gene, showed 99.82 to 100% nt identity with reported sequences of B. burgdorferi listed at NCBI. The phylogenetic analysis showed a close relationship between Borrelia sp. in this study and strains reported from the USA (B. burgdorferi and B. americana) and Japan (B. tanukii and B. garinii) (Figure 5). The derived sequences of pathogens were submitted to the GenBank database under the accession numbers MW715063 - MW715067 (A. phagocytophilum) and MW715293 (Borrelia sp.)
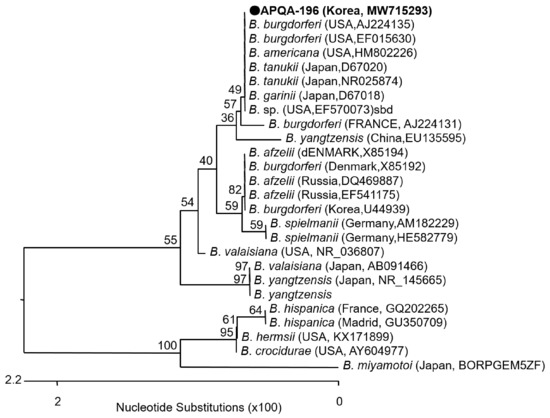
Figure 5.
Phylogenetic relationship of Borrelia sp. from a pool of ticks collected from a horse in the ROK. A neighbor-joining tree was created based on the 16S rRNA gene (622 bp) of Borrelia sp. using MEGA-6 software, with 1,000 bootstrap replications. Borrelia sp. (APQA-196) detected from tick pool number 196 with NCBI accession No.: MW715293 is shown. The reference strains originated from other countries are indicated, with the NCBI accession numbers and country name are shown.
3. Discussion
Haemaphysalis longicornis was the predominant tick species collected from horses in the ROK, which is consistent with previous reports that horses in the ROK are primarily infested with H. longicornis ticks [18,19,20]. The prevalence of ticks in the ROK was identified with a predominance of H. longicornis, followed by H. flava, and other less abundant species, such as I. nipponensis, I. persulcatus, H. japonica, Amblyomma testudinarium, and I. granulatus [21]. However, only two tick species, H. longicornis and I. nipponensis were detected in horses in this study. This implies that the habitat of tick species might have been affected by land use and the presence of animal reservoirs [22,23,24,25,26].
Detection of E. chaffeensis, T. equi, and B. caballi from cattle grazing in ROK during 2010 and 2011 showed that 19.4%, 7.2%, and 0.35%, respectively, of the tick pools were positive for the three pathogens [27], and T. equi infections in horses have been serologically confirmed [28]. However, the three pathogens were not detected in ticks collected from horses and associated vegetation in the ROK during 2016 and 2017 in this study. The collection of ticks and detection of associated pathogens provide information for disease risks of veterinary and medical importance and are critical for assessing disease risks and development of tick-borne disease mitigation strategies [29]. However, more rapid detection methods should be developed, such as point-of-care diagnostics from ticks collected from horses and associated vegetation, for early detection and instituting early control measure of tick-borne diseases in the future [30].
The two tick-borne pathogens, Anaplasma phagocytophilum and Borrelia spp., detected from ticks collected from horses and associated vegetation are causative agents of anaplasmosis and Lyme borreliosis in humans [31,32]. Even though the positive rate of A. phagocytophilum-positive ticks was very low (MIR = 0.54‰) from horse ticks in this study compared to 9.9% of ticks collected from different domestic and wild animals reported in the ROK in 2003 [33], the potential risk of transmission of this zoonotic pathogen to humans was identified [34]. Analyzing the relationship of the outbreak of human granulocytic anaplasmosis (HGA) and the collection areas of A. phagocytophilum-positive ticks has not been conducted due to lack of data of HGA outbreaks at areas, such as Hwasong in Gyeonggi province, and Jeju and Seoguipo cities at Jeju Island.
Borrelia spp. were detected in ticks associated with horses in the ROK for the first time. While the seroprevalence of B. burgdorferi in horses in the ROK was 5.2% during 2009 through 2013 [35], its prevalence in ticks collected from horses and associated vegetation during 2016-2017 was very low (MIR = 0.43%). Borrelia burgdorferi, the causative agent of Lyme disease, is the most prevalent zoonotic TBD worldwide. Domestic animals that are susceptible to B. burgdorferi infections include various species, e.g., dogs, cats, horses, and ruminants [36]. In this study, Borrelia spp.-positive ticks were collected from vegetation associated with leisure horseback riding ranches and horse racing parks, in addition to directly from horses. However, Borrelia spp.-infections in horses has not been determined in this study, while human cases of Lyme disease are reported annually in Korea [26]. Therefore, there is a need to investigate the horse infectious status of Borrelia spp. for regions of Borrelia spp.-infected ticks to control and prevent zoonotic TBD in the future.
The high homology of A. phagocytophilum from different areas (100%) demonstrates the low variation of A. phagocytophilum distributed throughout the ROK. In addition, the A. phagocytophilum sequences detected in ticks during this study demonstrated 100% similarity to those previously detected in infected horses [37]. Various gene fragments have been used for identification of A. phagocytophilum [38]. However, other genes, e.g., groEL and msp2, were shown not to be helpful for the detection of A. phagocytophilum in the ROK [37].
Borrelia spp. were detected by specific probe-based real-time PCR and then confirmed based on sequencing analysis of the 16S rRNA gene. However, sequence results of 16S rRNA gene was not useful for phylogenetic identification of B. burgdorferi sensu lato because the sequence also shared 100% identity to B. tanukii. Therefore, further analysis using various primer sets of alternate gene fragments [39,40], and specific primers for each species detection are necessary. Unfortunately, the nucleic acids extracted from the positive samples were exhausted. Therefore, we could not conduct further analysis for the detected Borrelia spp. in this study.
A nationwide surveillance of tick prevalence and tick-borne pathogens harbored by horse ticks was conducted in this study for the first time. The result revealed that horses in the ROK are infested by two tick species, H. longicornis and I. nipponensis, with majority of H. longicornis (99%). These ticks are vectors of two important tick-borne pathogens, A. phagocytophilum and Borrelia spp., among the selected five targets for detection (A. phagocytophilum, E. chaffeensis, Borrelia spp., B. caballi, and T. equi). The survey of tick-borne pathogens harbored by horse ticks should be further extended for other important pathogens, such as Rickettsia, by which a strategy for diagnosis and prevention of the related diseases could be established.
4. Materials and Methods
4.1. Tick Collection and Identification
Ticks were collected directly from horses and associated vegetation at 72 sites, including horse racetracks (2) and stud farms (3) operated by the Racing Horse Authority, PHF (11), and LHR (56) in the ROK. Twenty four of the ranches were located in Gyeonggi and Gangwon provinces in Northern ROK, while the other 48 ranches were located in central (2) and southern (46) provinces and metropolitan cities (Figure 6). Ticks on horses were removed by securing the mouthparts with fine forceps as close to the skin as possible and gently pulling the tick away to avoid breaking off the mouthparts, while ticks were collected from the vegetation by the dragging/flagging method. Ticks were placed in 15 mL or 50 mL plastic vials with screw tops. At the end of each collection, the ticks were placed in a cooler where they were transported to the Parasitic and Honeybee Disease Laboratory, the Animal and Plant Quarantine Agency and stored at −80 °C until further identified.
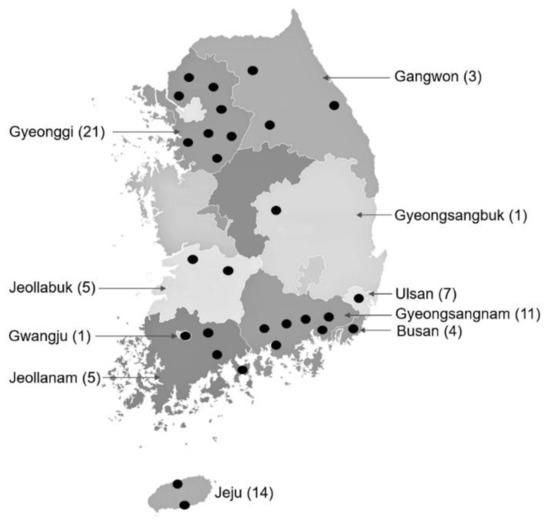
Figure 6.
Collection sites of ticks from horses and associated vegetation at horse ranches and racetracks in the ROK. Collection sites in each province or metropolitan city are indicated by black circles. Number of ranches where samples were collected in each province or metropolitan city are shown in parentheses.
Sex determination, identification of species and developmental stages were carried out using morphological keys [41,42,43] under a light for all tick individuals, and a subset of ticks was identified using electron microscopy. After identification, the ticks were transferred to 1.5 mL cryovials according to species, stage of development, and sex (adults) and returned to the −80 °C freezer until they were processed for the detection of selected tick-borne agents.
4.2. Extraction of Nucleic Acids
Ticks were pooled based on the collection date, location, species, developmental stage, and sex. Each pool consisted of adults (1–5), nymphs (1–30), or larvae (1–50). Ticks from each pool and 300 µL of PBS solution were added in a tissue-homogenizing tube with steel beads (SNC, Hanam, Korea), the sample was homogenized using a Precellys 24 Tissue Homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). Maxwell RSC Viral Total Nucleic Acid Purification Kit (Promega, Madison, WI, USA) was used for total nucleic acids extraction. The homogenate, 300 µL of lysis buffer, and 30 µL of proteinase K solution were added in a new 1.5 mL microcentrifuge tube. After incubating at 56 ℃ for 10 min purification of nucleic acids was done by using an automated Maxwell RSC Instrument (Promega, Madison, WI, USA). Isolated material was stored at −80 °C until further molecular analysis.
4.3. Polymerase Chain Reaction (PCR) and Real-Time PCR
The detection of Babesia spp., B. caballi, T. equi, A. phagocytophilum, and E. chaffeensis was performed using conventional PCR, and the AccuPower ProFi Taq PCR PreMix (Bioneer, Daejeon, Korea). Each 20 µL reaction mix included 5 µL DNA template, 1 µL (10 pmol) of each primer, 13 µL of double-distilled water (ddH2O). The PCR conditions used to amplify each target are shown in Table 4. Detection of Borrelia spp. was performed using real-time PCR (CFX96 Touch Real-time PCR Detection System; Bio-Rad Laboratories, Inc., Hercules, CA). Each 20 µL reaction mixture consisted of 1 µL (10 pmol) of each primer, 1 µL (5 pmol) of probe, 10 µL of PCR premix (IQ supermix, Bio-Rad Laboratories, Inc., Hercules, CA, USA), 5 µL of DNA template, and 2 µL of ddH2O (Table 4). The volume of DNA template (5 µL) used for each PCR was examined without PCR inhibition (Supplementary Figure S1).

Table 4.
PCR primer sets and conditions used for detecting tick-borne pathogens in ticks collected from horses and associated vegetation.
Results of positive detections were expressed as a minimum infection rate (MIR) that assumed that every positive pool contains only one infected tick. The MIR was calculated using the formula: MIR = number of positive pools/total number of tested ticks × 1000 [44,45].
4.4. Phylogenetic Analysis
Positive samples of Borrelia spp. detected by real-time PCR were analyzed phylogenetically using the 16S rRNA gene and PCR products (622 bp; Table 4) amplified and sequenced. Phylogenetic analysis of A. phagocytophilum was performed using the 16S rRNA gene and PCR products of positive samples purified using a QIA Quick Purification Kit (Qiagen, Hilden, Germany) and Macrogen (Seoul, Korea) sequenced the PCR products. The homologies of the generated sequences were analysed using the BLASTn tool of the National Center for Biotechnology Information (NCBI) GenBank database. The sequences were aligned using the Clustal W with MegAlign software version 7.1 (DNA-STAR, Madison, WI, USA) and phylogenetic trees generated using the neighbor-joining algorithm in MEGA-6 software [46] with 1000 bootstrap replications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10091069/s1, Figure S1: Checking PCR inhibition of tick nucleic acids solution.
Author Contributions
Conceptualization, Y.S.C. and H.-J.S.; methodology, H.-J.S. and K.-H.K.; software, J.-Y.L.; validation, Y.S.C., H.-J.S. and A.-T.T.; formal analysis, Y.S.C., A.-T.T., H.-C.K., and T.A.K.; investigation, Y.S.C., K.-H.K., J.-Y.L., and S.M.; resources, J.-Y.L. and S.M.; data curation, M.-S.Y.; writing—original draft preparation, H.-J.S. and A.-T.T.; writing—review and editing, Y.S.C.; visualization, A.-T.T., H.-J.S. and Y.S.C.; supervision, Y.S.C.; project administration, S.-S.Y.; funding acquisition, Y.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency in Korea, grant number P-1543084-2019-21-0101. Partial funding was provided by the Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance (GEIS) Section, ProMIS ID P0025_17_ME.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Joon-Seok Chae at Seoul National University for his cooperation and critical advice for this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Horse Industry Status Report (HISR), Ministry of Agriculture, Food and Rural Affairs. 2019. Available online: www.horsepia.com/industry/stat/horseAllStat.do (accessed on 10 February 2021).
- Kim, J.-Y. The Present Condition and Prospect of Korean Horse Industry. Int. J. Multimed. Ubiquitous Eng. 2015, 10, 119–124. [Google Scholar] [CrossRef]
- Laus, F.; Veronesi, F.; Passamonti, F.; Paggi, E.; Cerquetella, M.; Hyatt, D.; Tesei, B.; Fioretti, D.P. Prevalence of Tick Borne Pathogens in Horses from Italy. J. Vet. Med. Sci. 2013, 75, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.; Knowles, D.P. Persistently Infected Horses Are Reservoirs for Intrastadial Tick-Borne Transmission of the Apicomplexan Parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Liu, J.-Q.; Xu, B.-L.; Lv, S.; Xia, S.; Zhou, X.-N. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in central China. Parasites Vectors 2014, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-J.; Park, J.; Lee, Y.-S.; Chae, J.-S.; Yu, D.-H.; Park, B.-K.; Kim, H.-C.; Choi, K.-S. Molecular identification of selected tick-borne pathogens in wild deer and raccoon dogs from the Republic of Korea. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö.; Emir, H. Identification of tick-borne pathogens in ticks collected from wild animals in Turkey. Parasitol. Res. 2020, 119, 3083–3091. [Google Scholar] [CrossRef]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.; Gennari, S. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef]
- Duell, J.R.; Carmichael, R.; Herrin, B.H.; Holbrook, T.C.; Talley, J.; Little, S.E. Prevalence and species of ticks on horses in central Oklahoma. J. Med. EÈntomol. 2013, 50, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.; Manilla, G.; Maroli, M. Le zecche parassite del cavallo in Italia. Osservazioni sulla distribuzione e sul ruolo patogeno [Parasitic horse ticks in Italy. Observations on their distribution and pathogenic role]. Parassitologia 1994, 36, 273–279. [Google Scholar]
- Camacho, A.T.; Guitian, J.; Pallas, E.; Gestal, J.J.; Olmeda, A.S.; Habela, M.A.; Iii, S.R.T.; Spielman, A. Theileria (Babesia) equi and Babesia caballi Infections in Horses in Galicia, Spain. Trop. Anim. Health Prod. 2005, 37, 293–302. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wang, C.H.; Wu, Y.L. Serological Survey of Equine Piroplasmosis, Equine Granulocytic Anaplasmosis, and Equine Lyme Disease in Taiwan. Taiwan Vet. J. 2010, 36, 261–267. [Google Scholar]
- Engvall, E.O.; Pettersson, B.; Persson, M.; Artursson, K.; Johansson, K.E. A 16S rRNA-based PCR assay for detection and identification of granulocytic Ehrlichia species in dogs, horses, and cattle. J. Clin. Microbiol. 1996, 34, 2170–2174. [Google Scholar] [CrossRef]
- Teglas, M.; Matern, E.; Lein, S.; Foley, P.; Mahan, S.M.; Foley, J. Ticks and tick-borne disease in Guatemalan cattle and horses. Vet. Parasitol. 2005, 131, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Aguero-Rosenfeld, M.E. Diagnosis of Human Granulocytic Ehrlichiosis: State of the Art. Vector-Borne Zoonotic Dis. 2002, 2, 233–239. [Google Scholar] [CrossRef]
- Strle, F. Human granulocytic ehrlichiosis in Europe. Int. J. Med. Microbiol. 2004, 293S, 27–35. [Google Scholar] [CrossRef]
- Seo, M.; Kwon, O.; Kwak, D. Diversity and genotypic analysis of tick-borne pathogens carried by ticks infesting horses in Korea. Med. Vet. EÈntomol. 2021, 35, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeong, Y.E.; Yun, S.-M.; Lee, I.Y.; Han, M.G.; Ju, Y.R. Molecular evidence for tick-borne encephalitis virus in ticks in South Korea. Med. Vet. EÈntomol. 2009, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Na, D.-K.; Kim, C.-M.; Li, Y.-H.; Cho, Y.-H.; Park, J.-H.; Lee, J.-H.; Eo, S.-K.; A Klein, T.; Chae, J.-S. Identification and prevalence of Ehrlichia chaffeensis infection in Haemaphysalis longicornis ticks from Korea by PCR, sequencing and phylogenetic analysis based on 16S rRNA gene. J. Vet. Sci. 2005, 6, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Kwon, O.-D.; Kwak, D. Molecular Identification of Borrelia afzelii from Ticks Parasitizing Domestic and Wild Animals in South Korea. Microorganisms 2020, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Baek, J.; Durey, A.; Kwon, H.Y.; Chung, M.-H.; Lee, J.-S. Current Status of Tick-Borne Diseases in South Korea. Vector-Borne Zoonotic Dis. 2019, 19, 225–233. [Google Scholar] [CrossRef]
- Lado, P.; Smith, M.L.; Carstens, B.C.; Klompen, H. Population genetic structure and demographic history of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae): New evidence supporting old records. Mol. Ecol. 2020, 29, 2810–2823. [Google Scholar] [CrossRef]
- Macdonald, A.J. Abiotic and habitat drivers of tick vector abundance, diversity, phenology and human encounter risk in southern California. PLoS ONE 2018, 13, e0201665. [Google Scholar] [CrossRef]
- Fryxell, R.T.T.; Moore, J.E.; Collins, M.D.; Kwon, Y.; Jean-Philippe, S.R.; Schaeffer, S.M.; Odoi, A.; Kennedy, M.; Houston, A.E. Habitat and Vegetation Variables Are Not Enough When Predicting Tick Populations in the Southeastern United States. PLoS ONE 2015, 10, e0144092. [Google Scholar] [CrossRef]
- Uspensky, I. Tick pests and vectors (Acari: Ixodoidea) in European towns: Introduction, persistence and management. Ticks Tick-Borne Dis. 2014, 5, 41–47. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Gonzalez, M.; Sánchez, M.; Tercero, J.M.; Elhachimi, L.; Carbonell, J.D.; Olmeda, A.S. Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Doan, H.T.T.; Choe, S.E.; Noh, J.H.; Yoo, M.S.; Reddy, K.E.; Kim, Y.H.; Kweon, C.H.; Jung, S.C.; Chang, K.Y. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol. Int. 2013, 62, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-J.; Kim, K.-H.; Lee, S.K.; Min, S.; Lim, J.-Y.; Yang, S.-J.; Yoo, M.-S.; Jung, S.; Yoon, S.-S.; Cho, Y.S. Molecular and serological surveillance of equine piroplasmosis in the Republic of Korea between 2016 and 2017. Korean J. Vet. Res. 2021, 61, 4. [Google Scholar] [CrossRef]
- Sanders, D.M.; Parker, J.E.; Walker, W.W.; Buchholz, M.W.; Blount, K.; Kiel, J.L. Field collection and genetic classification of tick-borne Rickettsiae and Rickettsiae-like pathogens from South Texas: Coxiella burnetii isolated from field-collected Amblyomma cajennense. Ann. N. Y. Acad. Sci. 2008, 1149, 208–211. [Google Scholar] [CrossRef]
- Noden, B.H.; Martin, J.; Carrillo, Y.; Talley, J.L.; Ochoa-Corona, F. Development of a loop-mediated isothermal amplification (LAMP) assay for rapid screening of ticks and fleas for spotted fever group rickettsia. PLoS ONE 2018, 13, e0192331. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Médecine Maladies Infectieuses 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Saleem, S.; Ijaz, M.; Farooqi, S.H.; Ghaffar, A.; Ali, A.; Iqbal, K.; Mehmood, K.; Zhang, H. Equine Granulocytic Anaplasmosis 28 years later. Microb. Pathog. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Kim, C.M.; Kim, M.S.; Park, M.S.; Park, J.H.; Chae, J.S. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector-Borne Zoonotic Dis. 2003, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yun, S.-H.; Choi, E.; Park, Y.-S.; Lee, S.-E.; Cho, G.-J.; Kwon, O.-D.; Kwak, D. Serological Detection of Borrelia burgdorferi among Horses in Korea. Korean J. Parasitol. 2016, 54, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B. Lyme disease. Rev. Sci. Tech. 2015, 34, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Ouh, I.-O.; Choi, E.; Kwon, O.-D.; Kwak, D. Molecular Detection and Phylogenetic Analysis of Anaplasma phagocytophilum in Horses in Korea. Korean J. Parasitol. 2018, 56, 559–565. [Google Scholar] [CrossRef]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Notter, I.; Fingerle, V. Species Identification and Phylogenetic Analysis of Borrelia burgdorferi Sensu Lato Using Molecular Biological Methods. Adv. Struct. Saf. Stud. 2017, 1690, 13–33. [Google Scholar] [CrossRef]
- Urwin, R.; Maiden, M.C. Multi-locus sequence typing: A tool for global epidemiology. Trends Microbiol. 2003, 11, 479–487. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Roberts, F.H.; Kohls, G.M.; Tipton, V.J. Review of Haemaphysalis (Kaiserinana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Norteastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 1968, 54, 1197–1213. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Wassef, H.Y. The Haemaphysalis Ticks (Ixodoidea: Ixodidae) of Birds. 3. H. (Ornithophysalis) Subgen. N.: Definition, Species, Hosts, and Distribution in the Oriental, Palearctic, Malagasy, and Ethiopian Faunal Regions. J. Parasitol. 1973, 59, 1099. [Google Scholar] [CrossRef]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu islands. Brigh. Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1–226. [Google Scholar]
- Kramer, V.L.; Gutierrez, A.G.; Hui, L.T.; E Irwin, W.; Randolph, M.P.; Vugia, D.J. Detection of the agents of human ehrlichioses in ixodid ticks from California. Am. J. Trop. Med. Hyg. 1999, 60, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.; Ulrich, K.; Ginsbach, P.; Öhme, R.; Bock-Hensley, O.; Falk, U.; Teinert, M.; Lenhard, T. Tick-borne encephalitis virus (TBEV) prevalence in field-collected ticks (Ixodes ricinus) and phylogenetic, structural and virulence analysis in a TBE high-risk endemic area in southwestern Germany. Parasites Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.; Allsopp, M.; Mank, R.; Rijpkema, S.; Figueroa, J.; Jongejan, F. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): A review. Exp. Appl. Acarol. 1999, 23, 929–960. [Google Scholar] [CrossRef]
- Hancock, S.I.; Breitschwerdt, E.B.; Pitulle, C. Differentiation of Ehrlichia platys and E. equi Infections in Dogs by Using 16S Ribosomal DNA-Based PCR. J. Clin. Microbiol. 2001, 39, 4577–4578. [Google Scholar] [CrossRef][Green Version]
- Anderson, B.E.; Sumner, J.W.; Dawson, J.E.; Tzianabos, T.; Greene, C.R.; Olson, J.G.; Fishbein, D.B.; Olsen-Rasmussen, M.; Holloway, B.P.; George, E.H. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 775–780. [Google Scholar] [CrossRef]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; Logiudice, K.; Schmidt, K.A.; Keesing, F. Co-Infection of Blacklegged Ticks with Babesia microti and Borrelia burgdorferi is Higher than Expected and Acquired from Small Mammal Hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Zhai, B.; Niu, Q.; Yang, J.; Liu, Z.; Liu, J.; Yin, H.; Zeng, Q. Identification and molecular survey of Borrelia burgdorferi sensu lato in Sika deer (Cervus nippon) from Jilin Province, north-eastern China. Acta Trop. 2017, 166, 54–57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).