The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis
Abstract
:1. Introduction
2. Results
2.1. Detection of Pathogens and Assessment of the Degree of Contamination of Samples
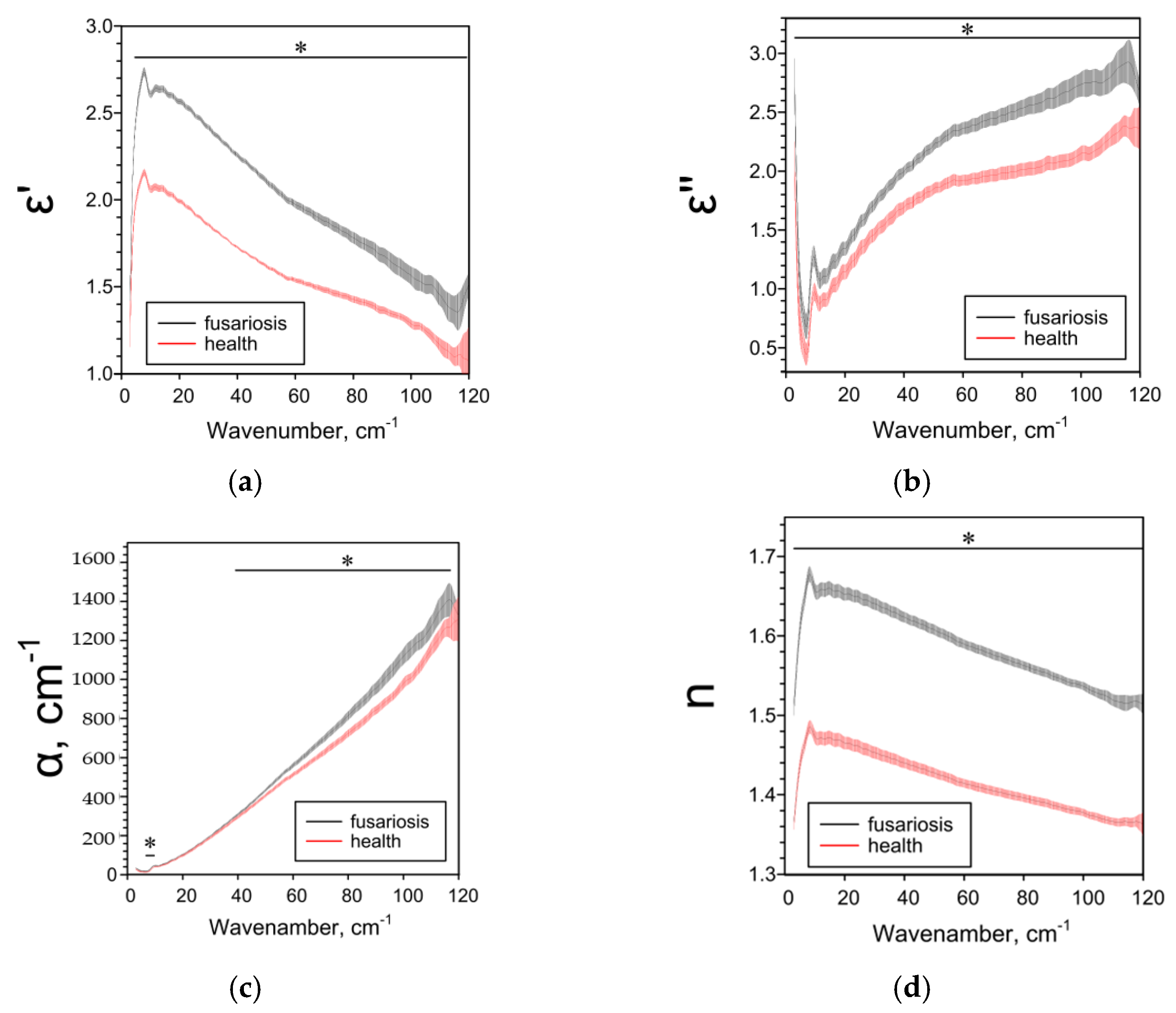
2.2. Effect of Late Blight on Potato Characteristics Determined by the THz-TDS Method
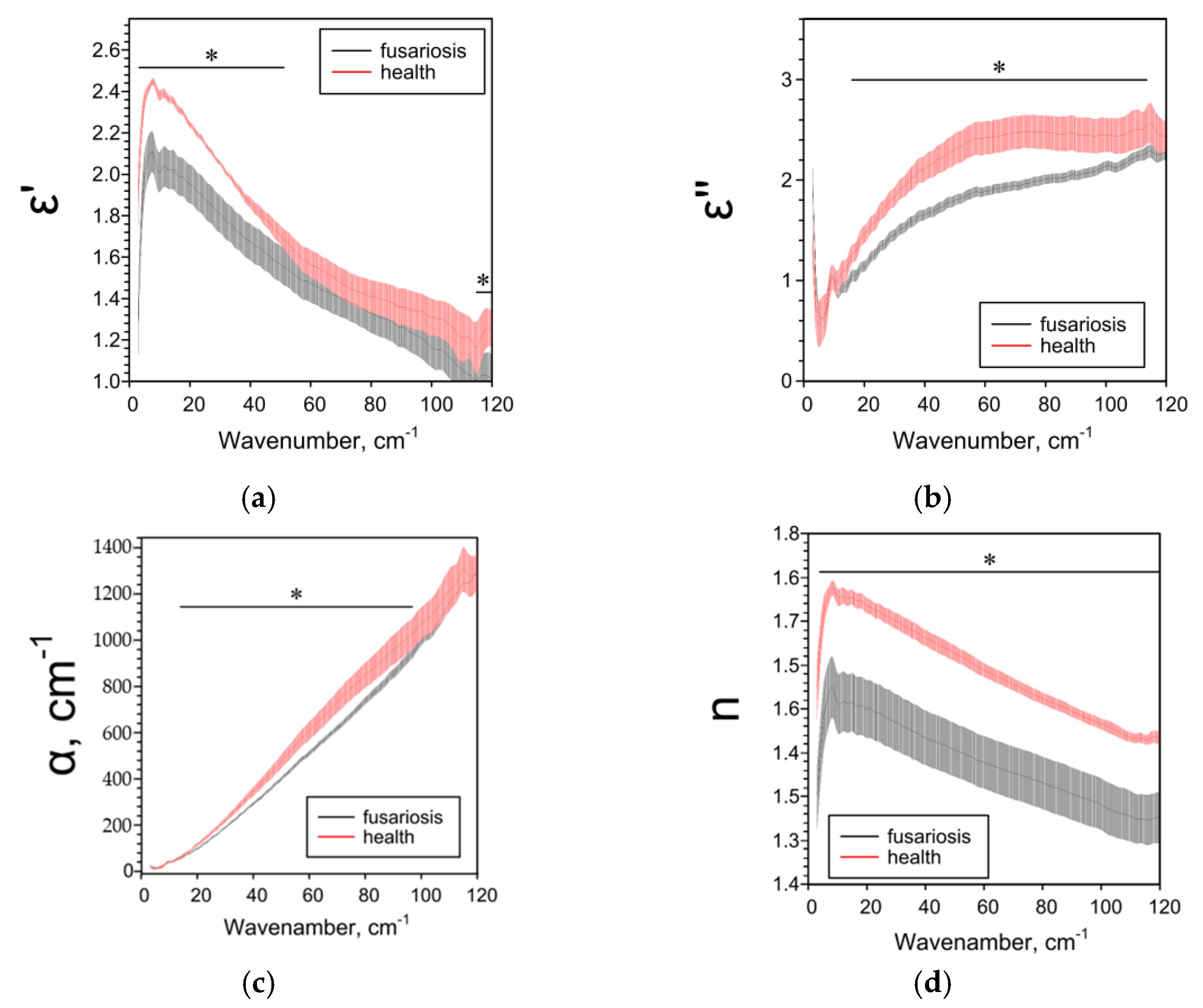
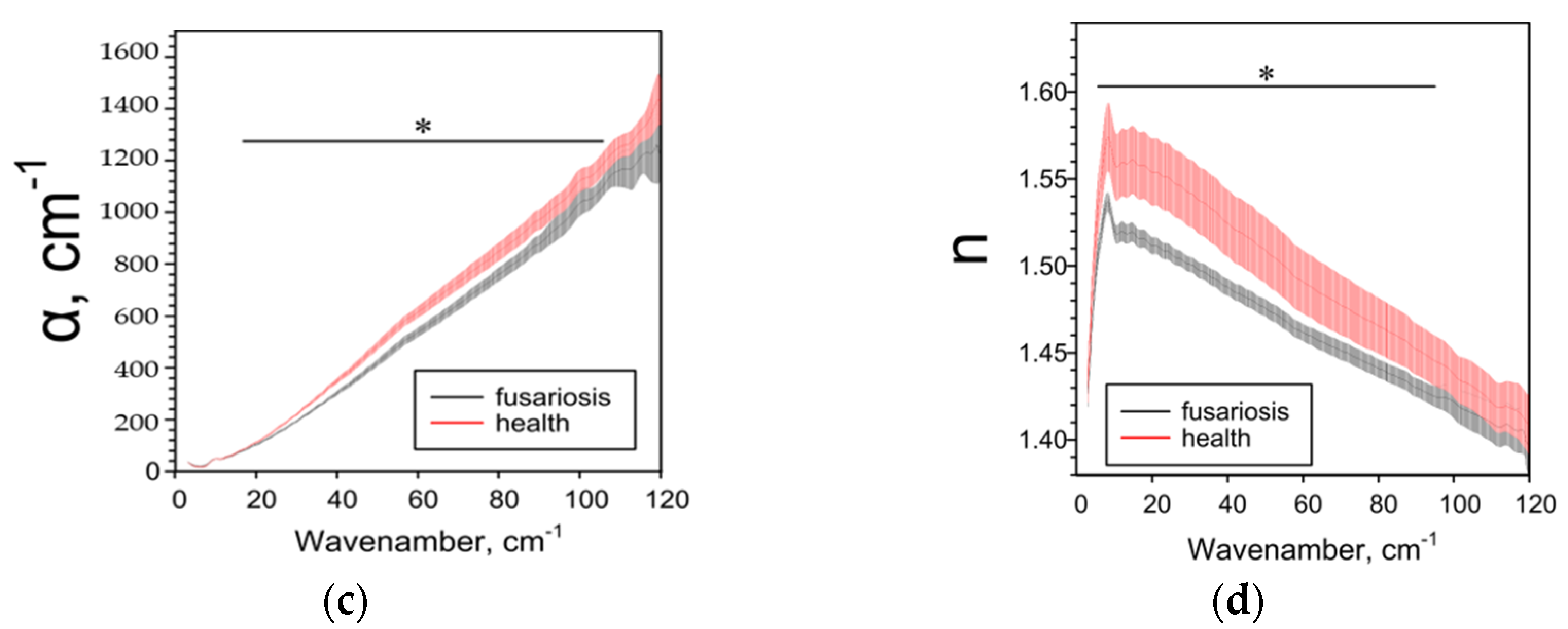
2.3. Effect of Fusarium on the Characteristics of Cereals Determined by the THz-TDS Method
3. Discussion
4. Materials and Methods
4.1. Plants Samples
4.2. DNA Extraction
4.3. Real-Time PCR
4.4. Plants Sample Preparation to THz-TDS
4.5. Terahertz Time-Domain Spectroscopy (THz-TDS)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narouei-Khandan, H.A.; Shakya, S.K.; Garrett, K.A.; Goss, E.M.; Dufault, N.S.; Andrade-Piedra, J.L.; Asseng, S.; Wallach, D.; van Bruggen, A.H.C. BLIGHTSIM: A new potato late blight model simulating the response of phytophthora infestans to diurnal temperature and humidity fluctuations in relation to climate change. Pathogens 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.; Spackman, M.; Freeman, A.; Tre˛bicki, P.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesias, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events—Implications for food production, plant diseases, and pests. Glob. Chang. Human Health 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Cannon, R.J.C. The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob. Chang. Biol. 1998, 4, 785–796. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Akino, S.; Takemoto, D.; Hosaka, K. Phytophthora infestans: A review of past and current studies on potato late blight. J. Gen. Plant Pathol. 2014, 80, 24–37. [Google Scholar] [CrossRef]
- Hussain, T.; Singh, B.P.; Anwar, F. Development of specific marker for PCR diagnostic of late blight of potato caused by Phytophthora infestans using RAPD based SCAR methodology. J. Saudi Soc. Agric. Sci. 2017, 16, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Govers, F.; Latijnhouwers, M. Late Blight. In Encyclopaedia of Plant and Crop Science, 1st ed.; Goodman, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 1360. [Google Scholar]
- Kelly, B.D. The Great Irish Famine (1845–52) and the Irish asylum system: Remembering, forgetting, and remembering again. Ir. J. Med. Sci. 2019, 188, 953–958. [Google Scholar] [CrossRef]
- Martin, M.D.; Cappellini, E.; Samaniego, J.A.; Zepeda, M.L.; Campos, P.F.; Seguin-Orlando, A.; Wales, N.; Orlando, L.; Ho, S.Y.; Dietrich, F.S.; et al. Reconstructing genome evolution in historic samples of the Irish potato famine pathogen. Nat. Commun. 2013, 4, 2172. [Google Scholar] [CrossRef]
- McKee, M.L.; Zheng, L.; O’Sullivan, E.C.; Kehoe, R.A.; Doyle Prestwich, B.M.; Mackrill, J.J.; McCarthy, F.O. Synthesis and Evaluation of Novel Ellipticines and Derivatives as Inhibitors of Phytophthora infestans. Pathogens 2020, 9, 558. [Google Scholar] [CrossRef]
- De Biazio, G.R.; Leite, G.G.; Tessmann, D.J.; Barbosa-Tessmann, I.P. A new PCR approach for the identification of Fusarium graminearum. Braz. J. Microbiol. 2008, 39, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Li, H.; Naeem, M.; Wu, X.; Yong, T.; Song, C.; Liu, T.; Chen, W.; Yang, W. Diversity of the Seedborne fungi and pathogenicity of fusarium species associated with intercropped soybean. Pathogens 2020, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.-H.; Yang, L.-M.; Kang, J.-G.; Wang, Q.-B.; Wang, X.-W.; Fang, Z.-Y.; Liu, Y.-M.; Zhuang, M.; Zhang, Y.-Y.; Lin, Y.; et al. Development of InDel markers linked to Fusarium wilt resistance in cabbage. Mol. Breed. 2013, 32, 961–967. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Basallote-Ureba, M.J.; Melero-Vara, J.M.; Abbasi, P.A. Characterization of Fusarium isolates from asparagus fields in southwestern Ontario and influence of soil organic amendments on Fusarium crown and root rot. Phytopathology 2013, 104, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A.O. Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.X.; Dehne, H.-W.; Steiner, U. Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum, and F. verticillioides. Fungal Biol. 2016, 120, 1094–1104. [Google Scholar] [CrossRef]
- Geraldo, M.R.F.; Tessmann, D.J.; Kemmelmeier, C. Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in southern Brazil. Braz. J. Microbiol. 2006, 37, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Faria, C.; Almeida-Ferreira, G.; Gagliardi, K.; Alves, T.; Tessmann, D.; Machinski, M., Jr.; Barbosa-Tessmann, I. Use of the polymerase chain reaction for detection of Fusarium graminearum in bulgur wheat. Food Sci. Technol. 2012, 32, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hwang, S.M.; Lee, J.H.; Oh, M.; Han, J.W.; Choi, G.J. Specific PCR detection of Fusarium oxysporum f. sp. raphani: A causal agent of Fusarium wilt on radish plants. Lett. Appl. Microbiol. 2017, 65, 133–140. [Google Scholar] [CrossRef]
- Khan, M.; Li, B.; Jiang, Y.; Weng, Q.; Chen, Q. Evaluation of different PCR-based assays and LAMP method for rapid detection of Phytophthora infestans by targeting the Ypt1 gene. Front. Microbiol. 2017, 8, 1920. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ara, P.; Ehsani, R.J. A comprehensive review on food applications of terahertz spectroscopy and imaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1563–1621. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Yin, X.; Hu, F.; Hu, C. Discrimination of genetically modified sugar beets based on terahertz spectroscopy. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2016, 153, 586–590. [Google Scholar] [CrossRef]
- Fawole, O.; Tabib-Azar, M. Terahertz quantification of ethanol and sugar concentrations in water and its application for noninvasive real-time monitoring of fermentation. In Proceedings of the 2016 IEEE MTT-S International Microwave Symposium (IMS), San Francisco, CA, USA, 22–27 May 2016; pp. 1–4. [Google Scholar]
- Ge, H.; Jiang, Y.; Xu, Z.; Lian, F.; Zhang, Y.; Xia, S. Identification of wheat quality using THz spectrum. Opt. Express 2014, 22, 12533–12544. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.W.; Pu, H.; Pan, T. Recent developments in methods and techniques for rapid monitoring of sugar metabolism in fruits. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1067–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jepsen, P.U.; Møller, U.; Merbold, H. Investigation of aqueous alcohol and sugar solutions with reflection terahertz time-domain spectroscopy. Opt. Express 2007, 15, 14717–14737. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Kołodziejczyk, K.; Markowski, J.; Mieszczakowska, M.; Ginies, C.; Renard, C.M.G.C. Co-products of black-currant and apple juice production: Hydration properties and polysaccharide composition. LWT Food Sci. Technol. 2010, 43, 173–180. [Google Scholar] [CrossRef]
- Nakajima, S.; Shiraga, K.; Suzuki, T.; Kondo, N.; Ogawa, Y. Quantification of starch content in germinating mung bean seedlings by terahertz spectroscopy. Food Chem. 2019, 294, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, D.-W.; Pu, H. Emerging non-destructive terahertz spectroscopic imaging technique: Principle and applications in the agri-food industry. Trends Food Sci. Technol. 2017, 67, 93–105. [Google Scholar] [CrossRef]
- Osawa, H.; Suzuki, N.; Akino, S.; Araki, H.; Asano, K.; Akai, K.; Kondo, N. Quantification of Phytophthora infestans population densities and their changes in potato field soil using real-time PCR. Sci. Rep. 2021, 11, 6266. [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Gavdush, A.A.; Chernomyrdin, N.V.; Yurchenko, S.O. Highly accurate in vivo terahertz spectroscopy of healthy skin: Variation of refractive index and absorption coefficient along the human body. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 817–827. [Google Scholar] [CrossRef]
- Gavdush, A.A.; Chernomyrdin, N.V.; Komandin, G.A.; Dolganova, I.N.; Nikitin, P.V.; Musina, G.R.; Katyba, G.M.; Kucheryavenko, A.S.; Reshetov, I.V.; Potapov, A.A.; et al. Terahertz dielectric spectroscopy of human brain gliomas and intact tissues ex vivo: Double-debye and double-overdamped-oscillator models of dielectric response. Biomed. Opt. Express 2021, 12, 69–83. [Google Scholar] [CrossRef]
- Yakovlev, E.V.; Zaytsev, K.I.; Chernomyrdin, N.V.; Gavdush, A.A.; Zotov, A.K.; Nikonovich, M.Y.; Yurchenko, S.O. Non-destructive testing of composite materials using terahertz time-domain spectroscopy. Opt. Sens. Detect. IV 2016, 9899, 98990. [Google Scholar]
- Nazarov, M.M.; Shkurinov, A.P.; Kuleshov, E.A.; Tuchin, V.V. Terahertz time-domain spectroscopy of biological tissues. Quantum Electron. 2008, 38, 647. [Google Scholar] [CrossRef]
- Fischer, B.M.; Helm, H.; Jepsen, P.U. Chemical recognition with broadband THz spectroscopy. Proc. IEEE 2007, 95, 1592–1604. [Google Scholar] [CrossRef] [Green Version]
- Terao, W.; Mori, T.; Fujii, Y.; Koreeda, A.; Kabeya, M.; Kojima, S. Boson peak dynamics of natural polymer starch investigated by terahertz time-domain spectroscopy and low-frequency Raman scattering. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2018, 192, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Kabeya, M.; Mori, T.; Fujii, Y.; Koreeda, A.; Lee, B.W.; Ko, J.-H.; Kojima, S. Boson peak dynamics of glassy glucose studied by integrated terahertz-band spectroscopy. Phys. Rev. B 2016, 94, 224204. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, H.; Shiba, H.; Ikeda, A. Continuum limit of the vibrational properties of amorphous solids. Proc. Natl. Acad. Sci. USA 2017, 114, E9767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Girolamo, F.V.; Pagano, M.; Tredicucci, A.; Bitossi, M.; Paoletti, R.; Barzanti, G.P.; Benvenuti, C.; Roversi, P.F.; Toncelli, A. Detection of fungal infections in chestnuts: A terahertz imaging-based approach. Food Control 2021, 123, 107700. [Google Scholar] [CrossRef]
- Fry, W. Phytophthora infestans: The plant (and R gene) destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Yuen, J. Pathogens which threaten food security: Phytophthora infestans, the potato late blight pathogen. Food Secur. 2021, 13, 247–253. [Google Scholar] [CrossRef]
- Simakov, E.A.; Anisimov, B.V.; Zhevora, S.V.; Mityushkin, A.V.; Meleshin, A.A.; Apshev, H.H.; Zhuravlev, A.A.; Mityushkin, A.V.; Zharova, V.A.; Salyukov, S.S.; et al. Potato Varieties of Russian Selection; Russian Potato Research Center: Moscow, Russia, 2018; p. 120. (In Russian) [Google Scholar]
- Yang, L.-N.; Liu, H.; Wang, Y.-P.; Seematti, J.; Grenville-Briggs, L.J.; Wang, Z.; Zhan, J. Pathogen-mediated stomatal opening: A previously overlooked pathogenicity strategy in the oomycete pathogen Phytophthora infestans. Front. Plant Sci. 2021, 12, 668797. [Google Scholar] [CrossRef]
- Beakes, G.W.; Honda, D.; Thines, M. 3 systematics of the straminipila: Labyrinthulomycota, Hyphochytriomycota, and Oomycota. In Systematics and Evolution: Part A; McLaughlin, D.J., Spatafora, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–97. [Google Scholar]
- Wang, H.; Tsuchikawa, S.; Inagaki, T. Terahertz time-domain spectroscopy as a novel tool for crystallographic analysis in cellulose: The potentiality of being a new standard for evaluating crystallinity. Cellulose 2021, 28, 5293–5304. [Google Scholar] [CrossRef]
- Poi, N.P.V.; Joye, I.J. dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar]
- De Santis, M.A.; Kosik, O.; Passmore, D.; Flagella, Z.; Shewry, P.R.; Lovegrove, A. Comparison of the dietary fibre composition of old and modern durum wheat (Triticum turgidum spp. durum) genotypes. Food Chem. 2018, 244, 304–310. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Naim, M.S.; Sharoubeem, H.H. Carbon and nitrogen requirements of Fusarium oxysporum causing cotton wilt. Mycopathol. Mycol. Appl. 1964, 22, 59–64. [Google Scholar] [CrossRef]
- Šterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical composition of covered and naked spring barley varieties and their potential for food production. Pol. J. Food Nutr. Sci. 2017, 67, 151–158. [Google Scholar] [CrossRef]
- Ge, H.; Jiang, Y.; Zhang, Y. THz spectroscopic investigation of wheat-quality by using multi-source data fusion. Sensors 2018, 18, 3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboul-Maaty, N.A.-F.; Oraby, H.A.-S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S. Principles of Teacher Science and Technology, 1st ed.; Springer: Boston, MA, USA, 2009; p. 340. [Google Scholar]

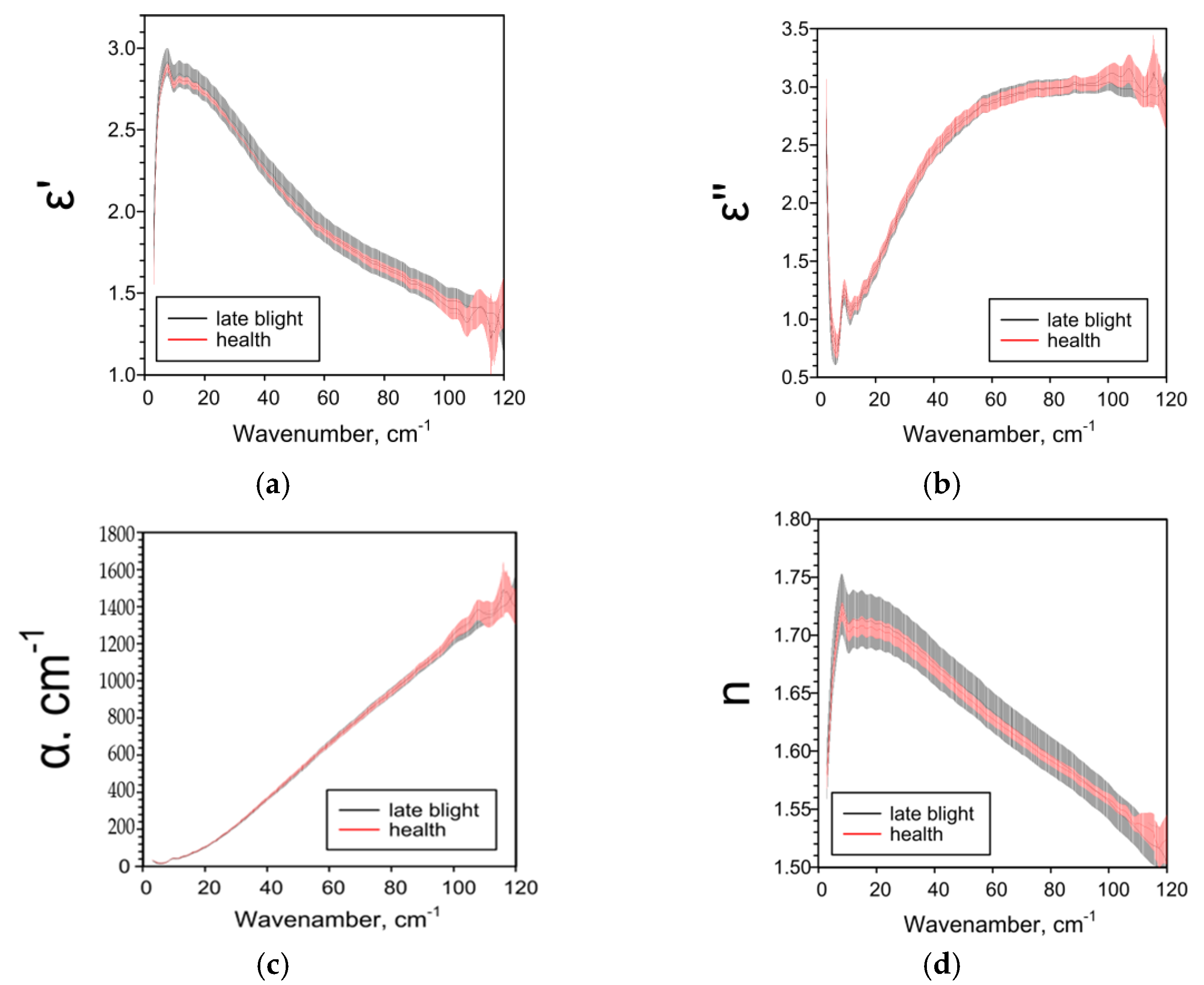
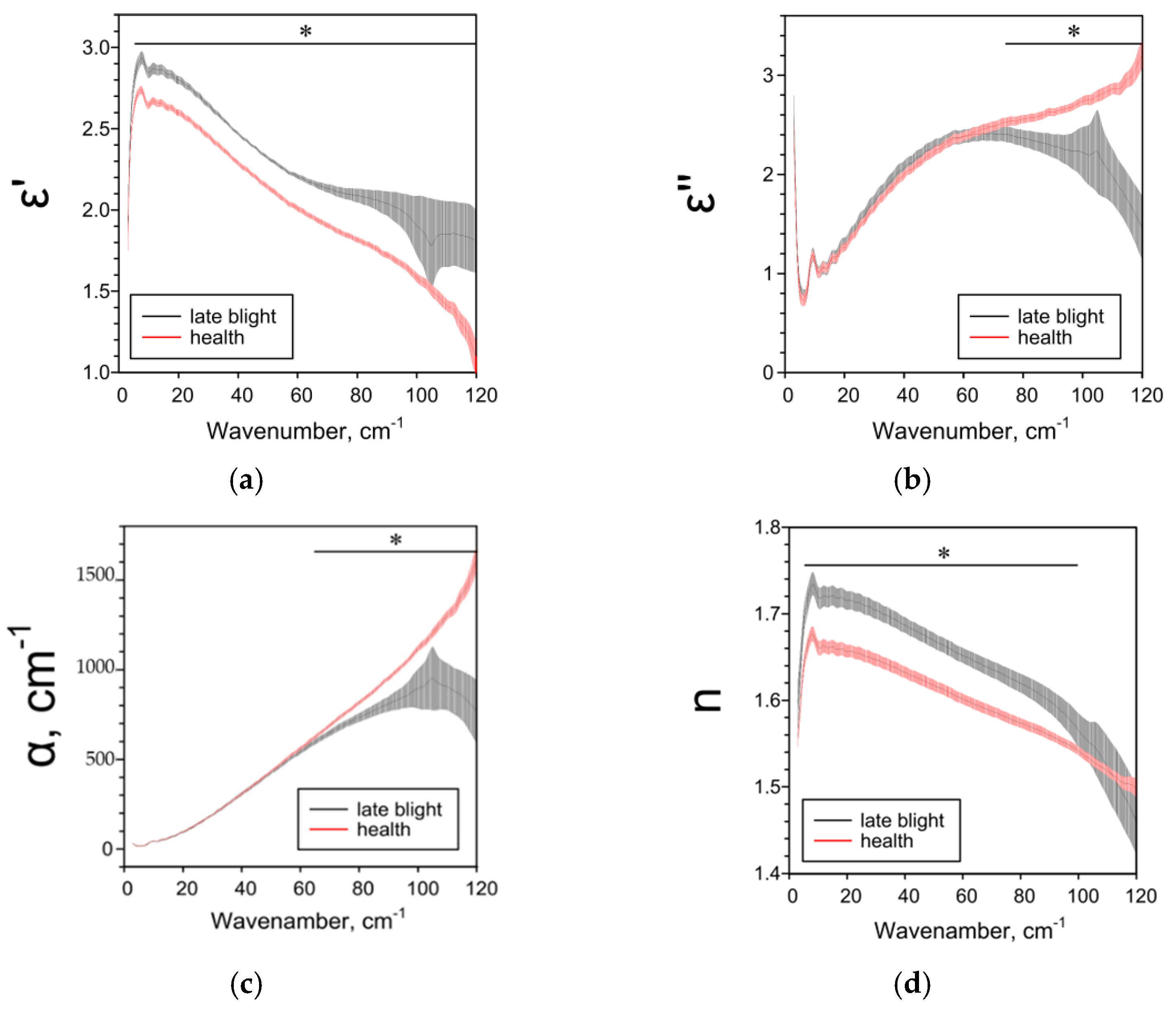
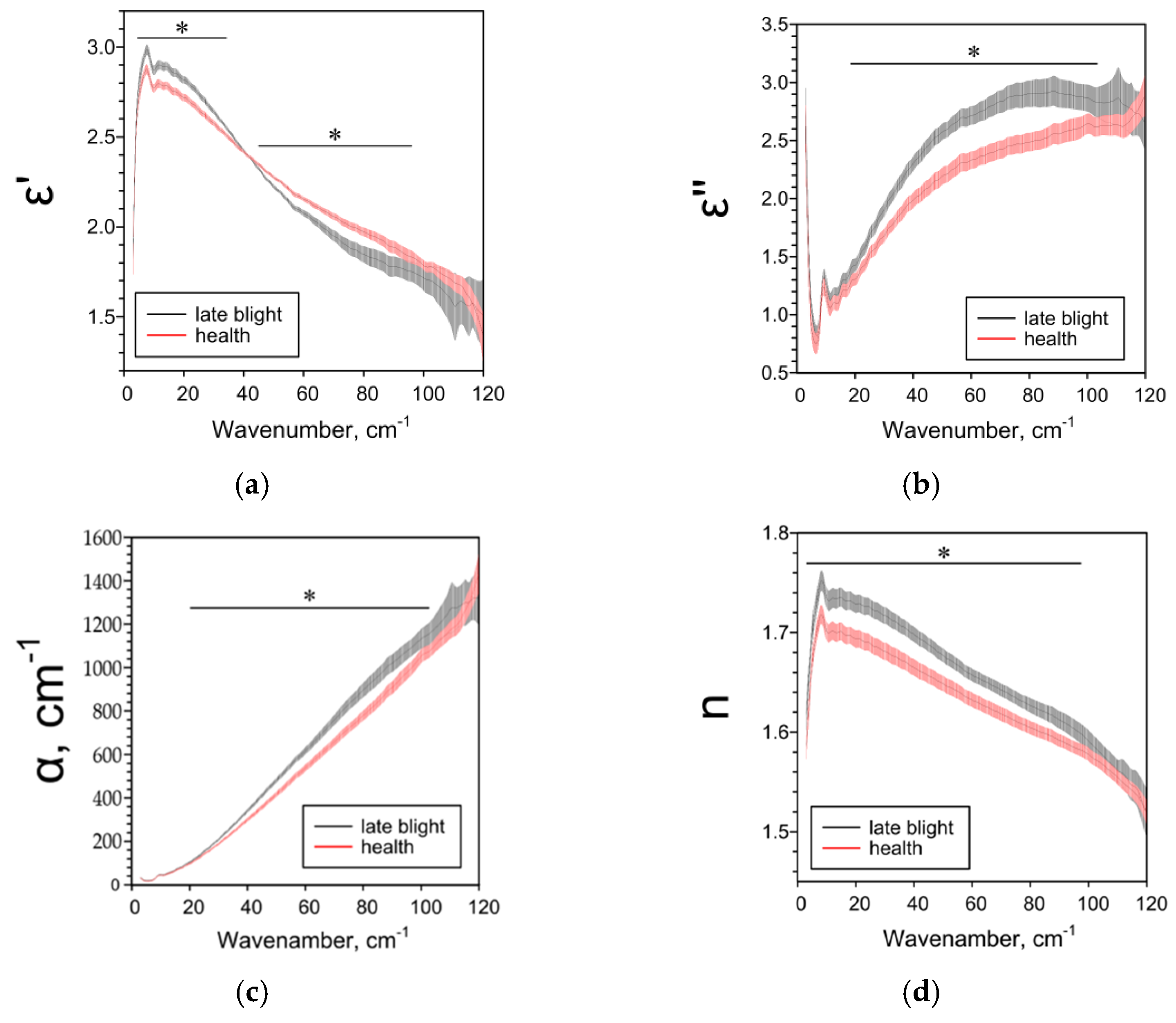
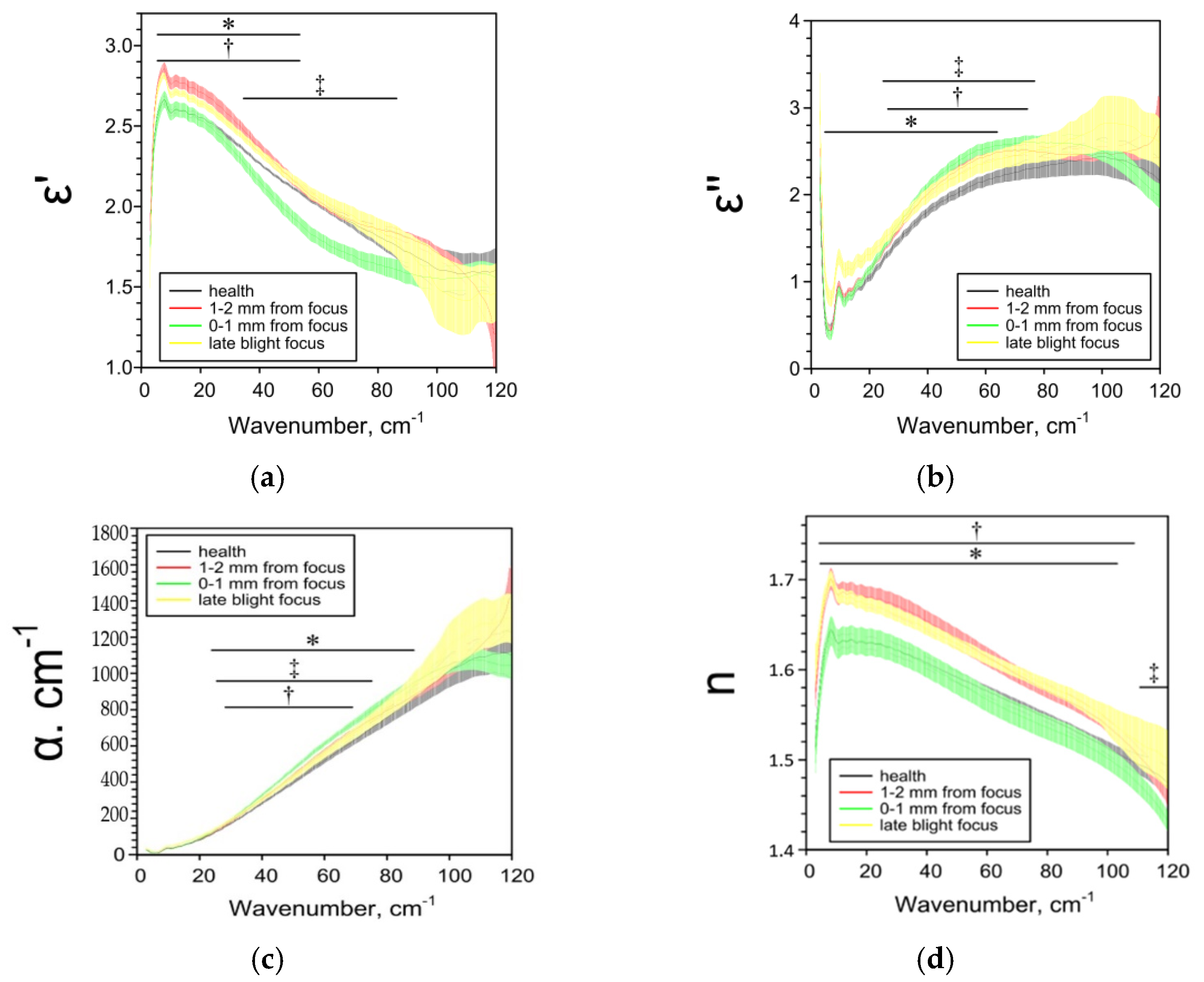

| Parameter | The Extent of the Late Blight | |||
|---|---|---|---|---|
| Ultra Low | Low | Intermediate | Severe | |
| “Meteor” without peel | “Meteor” with peel | “Nadezhda” without peel | “Nadezhda” with peel | |
| ε’ | ― | ↓1 25–85 cm−1 | ↓ 5–38 cm−1 ↑ 44–95 cm−1 | ↑ 4–120 cm−1 |
| ε’’ | ― | ↑ 6–8, 11–93, 116–120 cm−1 | ↑ 18–104 cm−1 | ↓ 73–120 cm−1 |
| α, cm−1 | ― | ↑ 11–95, 110–114 cm−1 | ↑ 20–104 cm−1 | ↓ 62–120 cm−1 |
| n | ― | ↓84–105 cm−1 | ↑ 3–97 cm−1 | ↑ 3–100 cm 1 |
| Parameter | Species and IDF/SDF Ratios 1 | ||
|---|---|---|---|
| Barley H. vulgare ~4.5 2 | Wheat T. aestivum ~5.3 | Oat A. sativa ~6.3 | |
| ε’ | ↑3 3–120 cm−1 | ↓5–29 cm−1 ↑50–86 cm−1 | ↓3–51, 116–120 cm−1 |
| ε’’ | ↑5–120 cm−1 | ↓16–96 cm−1 | ↓14–116 cm−1 |
| α, cm−1 | ↑7–10, 39–117 cm−1 | ↓17–106 cm−1 | ↓15–96 cm−1 |
| n | ↑3–120 cm−1 | ↓5–96 cm−1 | ↓3–120 cm−1 |
| Species and Target | Primers (F and R) | Ref. |
|---|---|---|
| F. graminearu Intergenic Spacer of rDNA (IGS region) | 5′-GTTGATGGGTAAAAGTGTG-3′ 5′-CTCTCATATACCCTCCG-3′ | [5] |
| F. avenaceum gene translation elongation factor 1-alpha (TEF1) | 5′-ATGGGTAAGGARGACAAGAC-3′ 5′-GGARGTACCAGTSATCATG-3′ | [5,6] |
| P. infestans sites ITS1 and ITS2 | 5′-AACCCAATAGTTGGGGGTCTTAC-3′ 5′-TCGTCCCCACAGTATAATCAGTATTAA-3′ | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penkov, N.V.; Goltyaev, M.V.; Astashev, M.E.; Serov, D.A.; Moskovskiy, M.N.; Khort, D.O.; Gudkov, S.V. The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis. Pathogens 2021, 10, 1336. https://doi.org/10.3390/pathogens10101336
Penkov NV, Goltyaev MV, Astashev ME, Serov DA, Moskovskiy MN, Khort DO, Gudkov SV. The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis. Pathogens. 2021; 10(10):1336. https://doi.org/10.3390/pathogens10101336
Chicago/Turabian StylePenkov, Nikita V., Mikhail V. Goltyaev, Maxim E. Astashev, Dmitry A. Serov, Maxim N. Moskovskiy, Dmitriy O. Khort, and Sergey V. Gudkov. 2021. "The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis" Pathogens 10, no. 10: 1336. https://doi.org/10.3390/pathogens10101336
APA StylePenkov, N. V., Goltyaev, M. V., Astashev, M. E., Serov, D. A., Moskovskiy, M. N., Khort, D. O., & Gudkov, S. V. (2021). The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis. Pathogens, 10(10), 1336. https://doi.org/10.3390/pathogens10101336








