Oral Immunotherapy for Children with Cow’s Milk Allergy
Abstract
:1. Introduction
2. OIT for CMA
3. Indication of patients undergoing OIT for CM
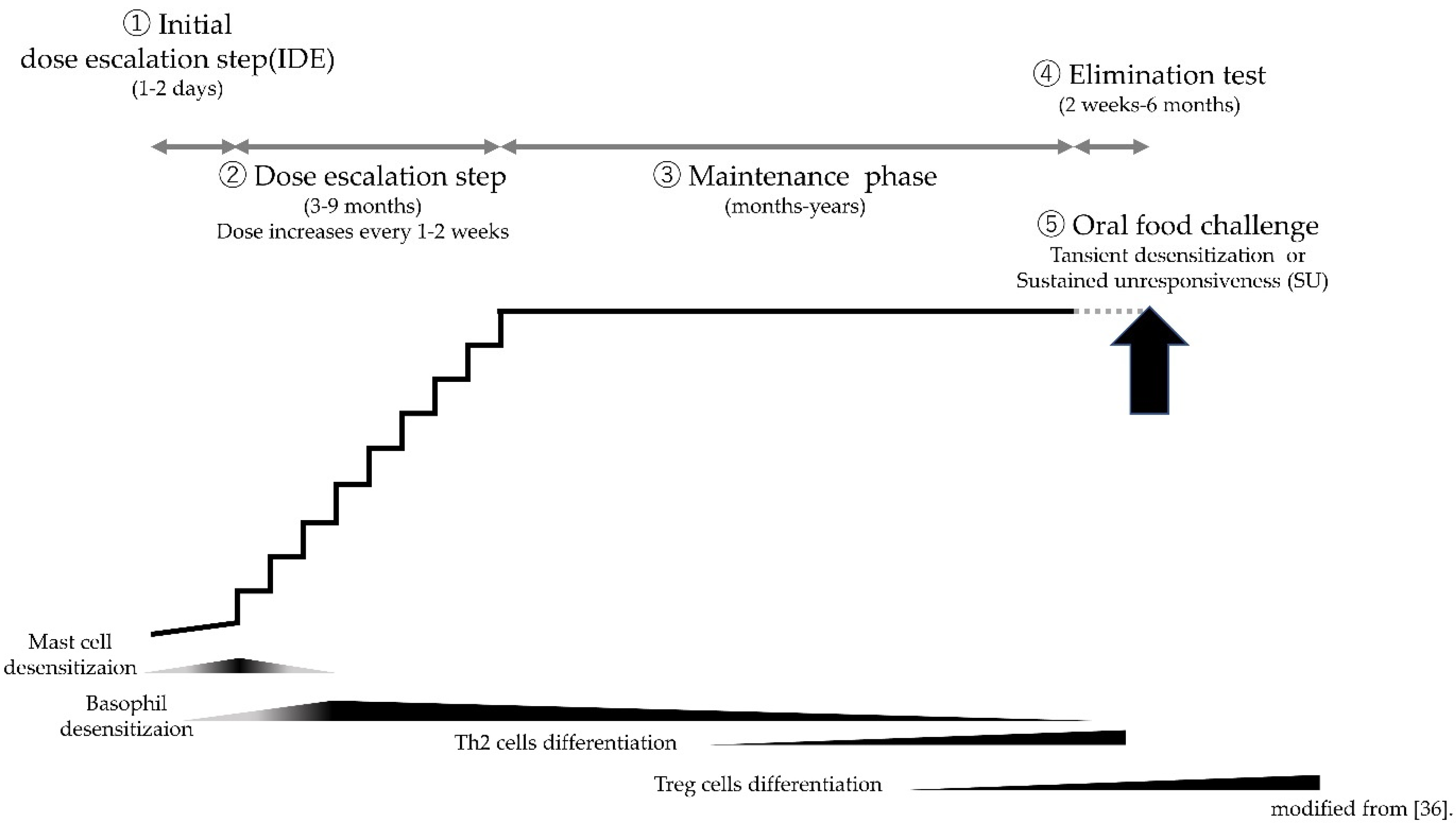
4. Protocols of CM-OIT
5. OIT Issues
5.1. Is It More Effective to Start Treatment at a Young Age?
5.2. Role of An Initial Dose Escalation Phase
5.2.1. Mast Cells
5.2.2. Basophils
5.3. Are Lower Maintenance Doses Safer?
5.4. Is Daily Intake Necessary?
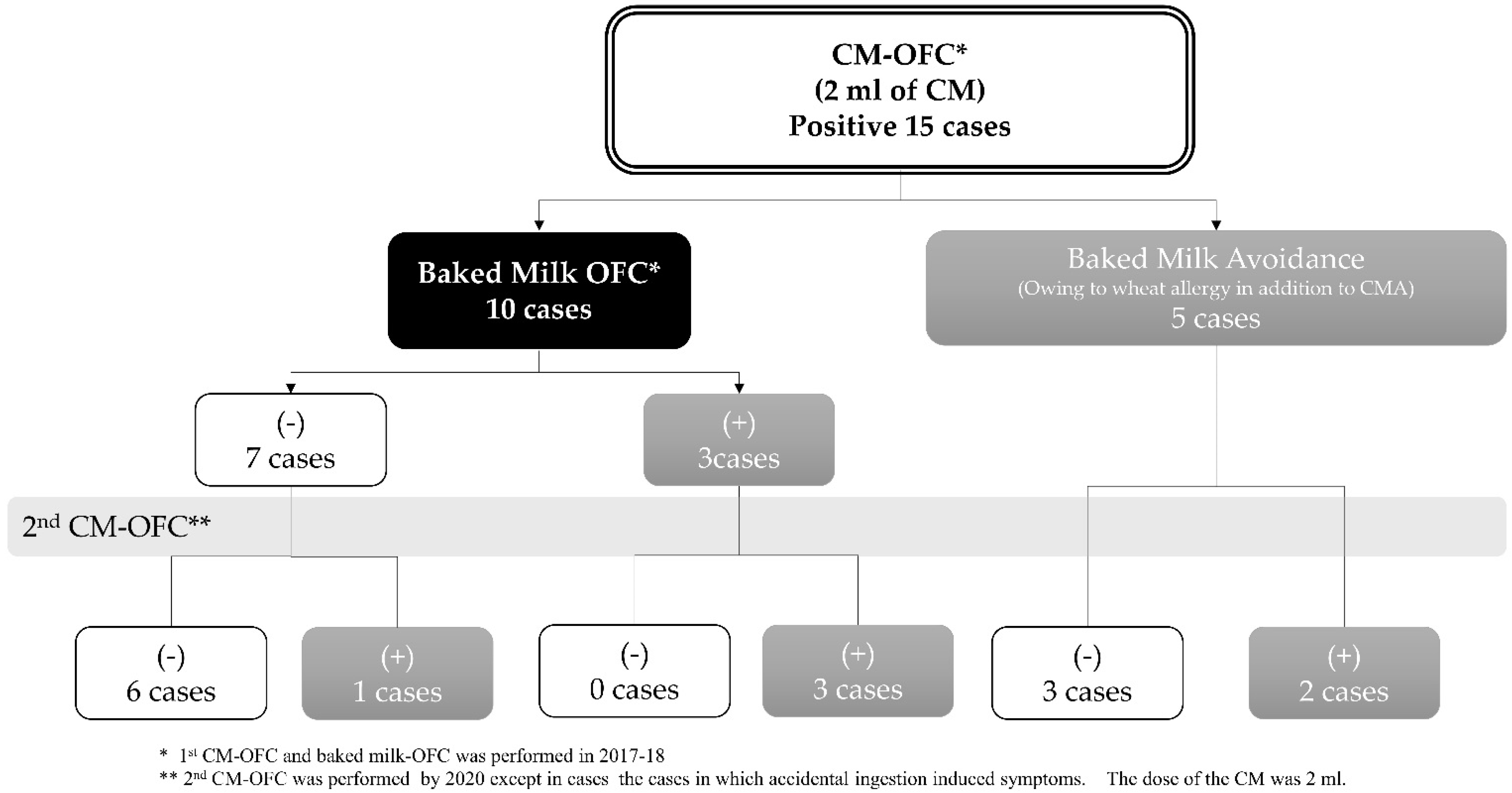
5.5. Is More Prolonged Treatment More Effective?
6. Use of Omalizumab
7. Sublingual Immunotherapy (SLIT) or Epicutaneous Immunotherapy (EPIT)
8. Approaches Other Than Standard OIT
9. Future Prospects in OIT for CMA
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, J.; Sanefuji, M.; Ninomiya, T.; Kawahara, T.; Matsuzaki, H.; Sonoda, Y.; Ogawa, M.; Shimono, M.; Suga, R.; Honjo, S.; et al. Possible association between early formula and reduced risk of cow’s milk allergy: The Japan Environment and Children’s Study. Clin. Exp. Allergy 2021, 51, 99–107. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Pak, K.; Saito-Abe, M.; Yang, L.; Sato, M.; Irahara, M.; Mezawa, H.; Sasaki, H.; Nishizato, M.; Ishitsuka, K.; et al. Allergy and immunology in young children of Japan: The JECS cohort. World Allergy Organ. J. 2020, 13, 100479. [Google Scholar] [CrossRef]
- Singer, A.G.; Kosowan, L.; Soller, L.; Chan, E.S.; Nankissoor, N.N.; Phung, R.R.; Abrams, E.M. Prevalence of Physician-Reported Food Allergy in Canadian Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 193–199. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Allen, K.J.; Lowe, A.J.; Lodge, C.J.; Tang, M.L.K.; Wake, M.; Ponsonby, A.L.; Erbas, B.; Abramson, M.J.; et al. The Prevalence of Food Sensitization Appears Not to Have Changed between 2 Melbourne Cohorts of High-Risk Infants Recruited 15 Years Apart. J. Allergy Clin. Immunol. Pract. 2018, 6, 440–448.e2. [Google Scholar] [CrossRef]
- Hansen, M.M.; Nissen, S.P.; Halken, S.; Høst, A. The natural course of cow’s milk allergy and the development of atopic diseases into adulthood. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2021, 32, 727–733. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiselhart, S.; Podzhilkova, A.; Hoffmann-Sommergruber, K. Cow’s Milk Processing-Friend or Foe in Food Allergy? Foods 2021, 10, 572. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Tsabouri, S.; Douros, K.; Priftis, K.N. Cow’s milk allergenicity. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Dahdah, L.; Albarini, M.; Martelli, A. Cow’s milk allergy in children and adults. Chem. Immunol. Allergy 2015, 101, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Nachshon, L.; Goldberg, M.R.; Schwartz, N.; Sinai, T.; Amitzur-Levy, R.; Elizur, A.; Eisenberg, E.; Katz, Y. Decreased bone mineral density in young adult IgE-mediated cow’s milk-allergic patients. J. Allergy Clin. Immunol. 2014, 134, 1108–1113.e3. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, G.; Perrone, V.; Alos, N.; Dubois, J.; Delvin, E.; Paradis, L.; Des Roches, A. Cow’s Milk Allergy and Bone Mineral Density in Prepubertal Children. Pediatrics 2016, 137, e20151742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinai, T.; Goldberg, M.R.; Nachshon, L.; Amitzur-Levy, R.; Yichie, T.; Katz, Y.; Monsonego-Ornan, E.; Elizur, A. Reduced Final Height and Inadequate Nutritional Intake in Cow’s Milk-Allergic Young Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 509–515. [Google Scholar] [CrossRef]
- Staden, U.; Blumchen, K.; Blankenstein, N.; Dannenberg, N.; Ulbricht, H.; Dobberstein, K.; Ziegert, M.; Niggemann, B.; Wahn, U.; Beyer, K. Rush oral immunotherapy in children with persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 418–419. [Google Scholar] [CrossRef]
- Robbins, K.A.; Wood, R.A.; Keet, C.A. Milk allergy is associated with decreased growth in US children. J. Allergy Clin. Immunol. 2014, 134, 1466–1468.e6. [Google Scholar] [CrossRef] [Green Version]
- Abrams, E.M.; Kim, H.; Gerdts, J.; Protudjer, J.L.P. Milk allergy most burdensome in multi-food allergic children. Pediatric Allergy Immunol. 2020, 31, 827–834. [Google Scholar] [CrossRef]
- Abdelwadoud, M.; Eftekhari, S.; Jaffee, H.; Carver, M.; Mattingly, T.J. Food allergy treatment value: Child caregiver and patient perspectives. Pediatric Allergy Immunol. 2021, 32, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickery, B.P.; Vereda, A.; Casale, T.B.; Beyer, K.; du Toit, G.; Hourihane, J.O.; Jones, S.M.; Shreffler, W.G.; Marcantonio, A.; Zawadzki, R.; et al. AR101 Oral Immunotherapy for Peanut Allergy. N. Engl. J. Med. 2018, 379, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.B.; Dölle, S.; Moneret-Vautrin, A.; Köhli, A.; Lange, L.; Spindler, T.; Ruëff, F.; Nemat, K.; Maris, I.; Roumpedaki, E.; et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J. Allergy Clin. Immunol. 2016, 137, 1128–1137.e1. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, D.M.; Perry, T.T.; Atkins, D.; Wood, R.A.; Burks, A.W.; Jones, S.M.; Henning, A.K.; Stablein, D.; Sampson, H.A.; Sicherer, S.H. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics 2012, 130, e25–e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen immunotherapy for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy 2017, 72, 1133–1147. [Google Scholar] [CrossRef]
- Keet, C.A.; Frischmeyer-Guerrerio, P.A.; Thyagarajan, A.; Schroeder, J.T.; Hamilton, R.G.; Boden, S.; Steele, P.; Driggers, S.; Burks, A.W.; Wood, R.A. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J. Allergy Clin. Immunol. 2012, 129, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Skripak, J.M.; Nash, S.D.; Rowley, H.; Brereton, N.H.; Oh, S.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Meglio, P.; Giampietro, P.G.; Gianni, S.; Galli, E. Oral desensitization in children with immunoglobulin E-mediated cow’s milk allergy-follow-up at 4 yr and 8 months. Pediatric Allergy Immunol. 2008, 19, 412–419. [Google Scholar] [CrossRef]
- Staden, U.; Rolinck-Werninghaus, C.; Brewe, F.; Wahn, U.; Niggemann, B.; Beyer, K. Specific oral tolerance induction in food allergy in children: Efficacy and clinical patterns of reaction. Allergy 2007, 62, 1261–1269. [Google Scholar] [CrossRef]
- Keet, C.A.; Seopaul, S.; Knorr, S.; Narisety, S.; Skripak, J.; Wood, R.A. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 2013, 132, 737–739.e6. [Google Scholar] [CrossRef] [Green Version]
- Longo, G.; Barbi, E.; Berti, I.; Meneghetti, R.; Pittalis, A.; Ronfani, L.; Ventura, A. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J. Allergy Clin. Immunol. 2008, 121, 343–347. [Google Scholar] [CrossRef]
- De Schryver, S.; Mazer, B.; Clarke, A.E.; St Pierre, Y.; Lejtenyi, D.; Langlois, A.; Torabi, B.; Zhao, W.W.; Chan, E.S.; Baerg, I.; et al. Adverse Events in Oral Immunotherapy for the Desensitization of Cow’s Milk Allergy in Children: A Randomized Controlled Trial. J. Allergy Clin. Immunol. Pract. 2019, 7, 1912–1919. [Google Scholar] [CrossRef]
- Berti, I.; Badina, L.; Cozzi, G.; Giangreco, M.; Bibalo, C.; Ronfani, L.; Barbi, E.; Ventura, A.; Longo, G. Early oral immunotherapy in infants with cow’s milk protein allergy. Pediatric Allergy Immunol. 2019, 30, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Boné Calvo, J.; Clavero Adell, M.; Guallar Abadía, I.; Laliena Aznar, S.; Sancho Rodríguez, M.L.; Claver Monzón, A.; Aliaga Mazas, Y. As soon as possible in IgE-cow’s milk allergy immunotherapy. Eur. J. Pediatrics 2021, 180, 291–294. [Google Scholar] [CrossRef]
- Martorell, A.; De la Hoz, B.; Ibáñez, M.D.; Bone, J.; Terrados, M.S.; Michavila, A.; Plaza, A.M.; Alonso, E.; Garde, J.; Nevot, S.; et al. Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin. Exp. Allergy 2011, 41, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ortiz, M.; Alvaro-Lozano, M.; Alsina, L.; Garcia-Paba, M.B.; Piquer-Gibert, M.; Giner-Muñoz, M.T.; Lozano, J.; Domínguez-Sánchez, O.; Jiménez, R.; Días, M.; et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: Severity of reaction at oral challenge, specific IgE and prick test. Clin. Exp. Allergy 2013, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Narisety, S.D.; Skripak, J.M.; Steele, P.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2009, 124, 610–612. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, Y.; Yajima, Y.; Ito, Y.M.; Kumon, J.; Muroya, T.; Tsurinaga, Y.; Shigekawa, A.; Takahashi, S.; Iba, N.; Tsuji, T.; et al. Single-Center Noninferiority Randomized Trial on the Efficacy and Safety of Low-and High-Dose Rush Oral Milk Immunotherapy for Severe Milk Allergy. Int. Arch. Allergy Immunol. 2020, 181, 699–705. [Google Scholar] [CrossRef]
- Meglio, P.; Bartone, E.; Plantamura, M.; Arabito, E.; Giampietro, P.G. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004, 59, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, N.; Sato, S.; Asaumi, T.; Okada, Y.; Ogura, K.; Ebisawa, M. A Single-Center, Case-Control Study of Low-Dose-Induction Oral Immunotherapy with Cow’s Milk. Int. Arch. Allergy Immunol. 2015, 168, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.B.; Elizur, A.; Goldberg, M.R.; Nachshon, L.; Katz, Y. Clinical predictors for favorable outcomes in an oral immunotherapy program for IgE-mediated cow’s milk allergy. Ann. Allergy Asthma Immunol. 2014, 112, 58–63.e1. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Kim, J.S.; Lindblad, R.; Nadeau, K.; Henning, A.K.; Dawson, P.; Plaut, M.; Sampson, H.A. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J. Allergy Clin. Immunol. 2016, 137, 1103–1110.e11. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, K.C.; Schneider, L.C.; Hoyte, L.; Borras, I.; Umetsu, D.T. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J. Allergy Clin. Immunol. 2011, 127, 1622–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martorell-Calatayud, C.; Michavila-Gómez, A.; Martorell-Aragonés, A.; Molini-Menchón, N.; Cerdá-Mir, J.C.; Félix-Toledo, R.; De Las Marinas-Álvarez, M.D. Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediatric Allergy Immunol. 2016, 27, 544–546. [Google Scholar] [CrossRef]
- Ibáñez-Sandín, M.D.; Escudero, C.; Candón Morillo, R.; Lasa, E.M.; Marchán-Martín, E.; Sánchez-García, S.; Terrados, S.; González Díaz, C.; Juste, S.; Martorell, A.; et al. Oral immunotherapy in severe cow’s milk allergic patients treated with omalizumab: Real life survey from a Spanish registry. Pediatric Allergy Immunol. 2021, 32, 1287–1295. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347.e2. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatric Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef]
- Gruzelle, V.; Juchet, A.; Martin-Blondel, A.; Michelet, M.; Chabbert-Broue, A.; Didier, A. Benefits of baked milk oral immunotherapy in French children with cow’s milk allergy. Pediatric Allergy Immunol. 2020, 31, 364–370. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Nachshon, L.; Appel, M.Y.; Elizur, A.; Levy, M.B.; Eisenberg, E.; Sampson, H.A.; Katz, Y. Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J. Allergy Clin. Immunol. 2015, 136, 1601–1606. [Google Scholar] [CrossRef]
- Inuo, C.; Tanaka, K.; Suzuki, S.; Nakajima, Y.; Yamawaki, K.; Tsuge, I.; Urisu, A.; Kondo, Y. Oral Immunotherapy Using Partially Hydrolyzed Formula for Cow’s Milk Protein Allergy: A Randomized, Controlled Trial. Int. Arch. Allergy Immunol. 2018, 177, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; Alonso, E.; Echeverría, L.; Escudero, C.; García-Rodríguez, R.; Blasco, C.; Bone, J.; Borja-Segade, J.; Bracamonte, T.; Claver, A.; et al. Oral Immunotherapy for Food Allergy: A Spanish Guideline. Immunotherapy Egg and Milk Spanish Guide (ITEMS Guide). Part I: Cow Milk and Egg Oral Immunotherapy: Introduction, Methodology, Rationale, Current State, Indications, Contraindications, and Oral Immunotherapy Build-up Phase. J. Investig. Allergol. Clin. Immunol. 2017, 27, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; Alonso, E.; Echeverría, L.; Escudero, C.; García-Rodríguez, R.; Blasco, C.; Bone, J.; Borja-Segade, J.; Bracamonte, T.; Claver, A.; et al. Oral Immunotherapy for Food Allergy: A Spanish Guideline. Egg and Milk Immunotherapy Spanish Guide (ITEMS GUIDE). Part II: Maintenance Phase of Cow Milk (CM) and Egg Oral Immunotherapy (OIT), Special Treatment Dosing Schedules. Models of Dosing Schedules of OIT With CM and Egg. J. Investig. Allergol. Clin. Immunol. 2017, 27, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bégin, P.; Chan, E.S.; Kim, H.; Wagner, M.; Cellier, M.S.; Favron-Godbout, C.; Abrams, E.M.; Ben-Shoshan, M.; Cameron, S.B.; Carr, S.; et al. CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2020, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Pepper, A.N.; Assa’ad, A.; Blaiss, M.; Brown, E.; Chinthrajah, S.; Ciaccio, C.; Fasano, M.B.; Gupta, R.; Hong, N.; Lang, D.; et al. Consensus report from the Food Allergy Research & Education (FARE) 2019 Oral Immunotherapy for Food Allergy Summit. J. Allergy Clin. Immunol. 2020, 146, 244–249. [Google Scholar] [CrossRef]
- Kim, E.H.; Burks, A.W. Food allergy immunotherapy: Oral immunotherapy and epicutaneous immunotherapy. Allergy 2020, 75, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Wood, R.A.; French, S.; Fiocchi, A.; Jordana, M.; Waserman, S.; Brożek, J.L.; Schünemann, H.J. Oral immunotherapy for peanut allergy (PACE): A systematic review and meta-analysis of efficacy and safety. Lancet 2019, 393, 2222–2232. [Google Scholar] [CrossRef]
- Petersen, T.H.; Mortz, C.G.; Bindslev-Jensen, C.; Eller, E. Cow’s milk allergic children-Can component-resolved diagnostics predict duration and severity? Pediatric Allergy Immunol. 2018, 29, 194–199. [Google Scholar] [CrossRef]
- Sampson, H.A.; Berin, M.C.; Plaut, M.; Sicherer, S.H.; Jones, S.; Burks, A.W.; Lindblad, R.; Leung, D.Y.M.; Wood, R.A. The Consortium for Food Allergy Research (CoFAR): The first generation. J. Allergy Clin. Immunol. 2019, 143, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Koike, Y.; Sato, S.; Yanagida, N.; Asaumi, T.; Ogura, K.; Ohtani, K.; Imai, T.; Ebisawa, M. Predictors of Persistent Milk Allergy in Children: A Retrospective Cohort Study. Int. Arch. Allergy Immunol. 2018, 175, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.K.; Paassilta, M.; Kukkonen, A.K.; Kuitunen, M.; Pelkonen, A.S.; Makela, M.J. Outcome of oral immunotherapy for persistent cow’s milk allergy from 11 years of experience in Finland. Pediatric Allergy Immunol. 2019, 30, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.D.; Patil, S.U.; Wambre, E.; Vickery, B.P. Immune mechanisms of oral immunotherapy. J. Allergy Clin. Immunol. 2018, 141, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barshow, S.M.; Kulis, M.D.; Burks, A.W.; Kim, E.H. Mechanisms of oral immunotherapy. Clin. Exp. Allergy 2021, 51, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Rios, E.J.; Tsai, M.; Kalesnikoff, J.; Galli, S.J. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J. Allergy Clin. Immunol. 2013, 132, 922–932.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, W.X.; Church, A.M.; Kulis, M.; Choi, H.W.; Burks, A.W.; Abraham, S.N. Mast cell desensitization inhibits calcium flux and aberrantly remodels actin. J. Clin. Investig. 2016, 126, 4103–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Tsai, M.; Mukai, K.; Chinthrajah, R.S.; Nadeau, K.C.; Galli, S.J. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J. Allergy Clin. Immunol. 2020, 145, 885–896.e6. [Google Scholar] [CrossRef] [Green Version]
- Burks, A.W.; Jones, S.M.; Wood, R.A.; Fleischer, D.M.; Sicherer, S.H.; Lindblad, R.W.; Stablein, D.; Henning, A.K.; Vickery, B.P.; Liu, A.H.; et al. Oral immunotherapy for treatment of egg allergy in children. N. Engl. J. Med. 2012, 367, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.U.; Steinbrecher, J.; Calatroni, A.; Smith, N.; Ma, A.; Ruiter, B.; Virkud, Y.; Schneider, M.; Shreffler, W.G. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J. Allergy Clin. Immunol. 2019, 144, 1310–1319.e4. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, M.; Narisety, S.D.; Guerrerio, A.L.; Chichester, K.L.; Keet, C.A.; Bieneman, A.P.; Hamilton, R.G.; Wood, R.A.; Schroeder, J.T.; Frischmeyer-Guerrerio, P.A. Suppression of the immunologic response to peanut during immunotherapy is often transient. J. Allergy Clin. Immunol. 2015, 135, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Pajno, G.B.; Caminiti, L.; Chiera, F.; Crisafulli, G.; Salzano, G.; Arasi, S.; Passalacqua, G. Safety profile of oral immunotherapy with cow’s milk and hen egg: A 10-year experience in controlled trials. Allergy Asthma Proc. 2016, 37, 400–403. [Google Scholar] [CrossRef]
- Kido, J.; Hirata, M.; Ueno, H.; Nishi, N.; Mochinaga, M.; Ueno, Y.; Yanai, M.; Johno, M.; Matsumoto, T. Evaluation of the skin-prick test for predicting the outgrowth of cow’s milk allergy. Allergy Rhinol. 2016, 7, 139–143. [Google Scholar] [CrossRef]
- Schoos, A.M.; Chawes, B.L.; Følsgaard, N.V.; Samandari, N.; Bønnelykke, K.; Bisgaard, H. Disagreement between skin prick test and specific IgE in young children. Allergy 2015, 70, 41–48. [Google Scholar] [CrossRef]
- Samsoe-Jensen, T.; Hauge-Kristensen, K. In-vitro fixation of skin-sensitizing antibodies to skin cells and mesenchymal tissue. Acta Allergol. 1960, 15, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Hosking, C.S.; Reyes-Benito, L.V. Reducing the need for food allergen challenges in young children: A comparison of in vitro with in vivo tests. Clin. Exp. Allergy 2001, 31, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Macaubas, C.; Sly, P.D.; Burton, P.; Tiller, K.; Yabuhara, A.; Holt, B.J.; Smallacombe, T.B.; Kendall, G.; Jenmalm, M.C.; Holt, P.G. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin. Exp. Allergy 1999, 29, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Yue, X.; Guo, R.; Zhang, H.; Orgel, K.; Ye, P.; Li, Q.; Liu, Y.; Kim, E.; Burks, A.W.; et al. High- and low-dose oral immunotherapy similarly suppress pro-allergic cytokines and basophil activation in young children. Clin. Exp. Allergy 2019, 49, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, S.A.; Laubach, S.; Wang, J. Integrating oral immunotherapy into clinical practice. J. Allergy Clin. Immunol. 2021, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lomas, J.M.; Bingemann, T.A. Clinical Predictors for Favorable Outcomes in an Oral Immunotherapy Program for IgE-Mediated Cow’s Milk Allergy. Pediatrics 2014, 134, S156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, W.M.; Vlieg-Boerstra, B.J.; Kruizinga, A.G.; van der Heide, S.; Houben, G.F.; Dubois, A.E. Threshold dose distributions for 5 major allergenic foods in children. J. Allergy Clin. Immunol. 2013, 131, 172–179. [Google Scholar] [CrossRef]
- Houben, G.F.; Baumert, J.L.; Blom, W.M.; Kruizinga, A.G.; Meima, M.Y.; Remington, B.C.; Wheeler, M.W.; Westerhout, J.; Taylor, S.L. Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization. Food Chem. Toxicol. 2020, 146, 111831. [Google Scholar] [CrossRef]
- Klemans, R.J.; Blom, W.M.; van Erp, F.C.; Masthoff, L.J.; Rubingh, C.M.; van der Ent, C.K.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; Pasmans, S.G.; Meijer, Y.; et al. Objective eliciting doses of peanut-allergic adults and children can be combined for risk assessment purposes. Clin. Exp. Allergy 2015, 45, 1237–1244. [Google Scholar] [CrossRef]
- Nachshon, L.; Schwartz, N.; Tsviban, L.; Levy, M.B.; Goldberg, M.R.; Epstein-Rigby, N.; Katz, Y.; Elizur, A. Patient Characteristics and Risk Factors for Home Epinephrine-Treated Reactions During Oral Immunotherapy for Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 185–192.e3. [Google Scholar] [CrossRef]
- Pajno, G.B.; Caminiti, L.; Salzano, G.; Crisafulli, G.; Aversa, T.; Messina, M.F.; Wasniewska, M.; Passalacqua, G. Comparison between two maintenance feeding regimens after successful cow’s milk oral desensitization. Pediatric Allergy Immunol. 2013, 24, 376–381. [Google Scholar] [CrossRef]
- Epstein-Rigbi, N.; Goldberg, M.R.; Levy, M.B.; Nachshon, L.; Elizur, A. Quality of Life of Food-Allergic Patients Before, During, and After Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2019, 7, 429–436.e2. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Suprun, M.; Chang, H.L.; Gimenez, G.; Grishina, G.; Getts, R.; Nadeau, K.; Wood, R.A.; Sampson, H.A. Predicting development of sustained unresponsiveness to milk oral immunotherapy using epitope-specific antibody binding profiles. J. Allergy Clin. Immunol. 2019, 143, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- Savilahti, E.M.; Kuitunen, M.; Valori, M.; Rantanen, V.; Bardina, L.; Gimenez, G.; Mäkelä, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2014, 25, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.M.; Burks, A.W.; Keet, C.; Vickery, B.P.; Scurlock, A.M.; Wood, R.A.; Liu, A.H.; Sicherer, S.H.; Henning, A.K.; Lindblad, R.W.; et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J. Allergy Clin. Immunol. 2016, 137, 1117–1127.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickery, B.P.; Scurlock, A.M.; Kulis, M.; Steele, P.H.; Kamilaris, J.; Berglund, J.P.; Burk, C.; Hiegel, A.; Carlisle, S.; Christie, L.; et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy Clin. Immunol. 2014, 133, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, J.H.; Courneya, J.P.; Sterba, P.M.; Macglashan, D.W.; Saini, S.S.; Wood, R.A. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J. Allergy Clin. Immunol. 2012, 130, 1123–1129.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranks, A.J.; Minnicozzi, S.C.; Miller, S.J.; Burton, O.T.; Logsdon, S.L.; Spergel, J.M.; Nadeau, K.C.; Pongracic, J.A.; Umetsu, D.T.; Rachid, R.; et al. Immunoglobulin E blockade during food allergen ingestion enhances the induction of inhibitory immunoglobulin G antibodies. Ann Allergy Asthma Immunol. 2019, 122, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Bedoret, D.; Singh, A.K.; Shaw, V.; Hoyte, E.G.; Hamilton, R.; DeKruyff, R.H.; Schneider, L.C.; Nadeau, K.C.; Umetsu, D.T. Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol. 2012, 5, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gadir, A.; Schneider, L.; Casini, A.; Charbonnier, L.M.; Little, S.V.; Harrington, T.; Umetsu, D.T.; Rachid, R.; Chatila, T.A. Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin. Exp. Allergy 2018, 48, 825–836. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Charbonnier, L.M.; Georgiev, P.; Oettgen, H.C.; Rachid, R.; Chatila, T.A. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015, 42, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, J.A.; Wood, R.A. Omalizumab as an adjuvant in food allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 278–285. [Google Scholar] [CrossRef]
- Andrew, J.; Long, P. Dupilumab and Milk OIT for the Treatment of Cow’s Milk Allergy. Available online: https://ClinicalTrials.gov/show/NCT04148352 (accessed on 29 August 2021).
- Study in Pediatric Subjects with Peanut Allergy to Evaluate Efficacy and Safety of Dupilumab as Adjunct to AR101 (Peanut Oral Immunotherapy). Available online: https://ClinicalTrials.gov/show/NCT03682770 (accessed on 29 August 2021).
- Frischmeyer-Guerrerio, P.A.; Masilamani, M.; Gu, W.; Brittain, E.; Wood, R.; Kim, J.; Nadeau, K.; Jarvinen, K.M.; Grishin, A.; Lindblad, R.; et al. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J. Allergy Clin. Immunol. 2017, 140, 1043–1053.e8. [Google Scholar] [CrossRef] [Green Version]
- Burks, A.W.; Sampson, H.A.; Plaut, M.; Lack, G.; Akdis, C.A. Treatment for food allergy. J. Allergy Clin. Immunol. 2018, 141, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Yang, L.; Ye, P.; Guo, R.; Li, Q.; Kulis, M.D.; Burks, A.W. Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization. J. Allergy Clin. Immunol. 2019, 144, 1320–1326.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burks, A.W.; Wood, R.A.; Jones, S.M.; Sicherer, S.H.; Fleischer, D.M.; Scurlock, A.M.; Vickery, B.P.; Liu, A.H.; Henning, A.K.; Lindblad, R.; et al. Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J. Allergy Clin. Immunol. 2015, 135, 1240–1248.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scurlock, A.M.; Burks, A.W.; Sicherer, S.H.; Leung, D.Y.M.; Kim, E.H.; Henning, A.K.; Dawson, P.; Lindblad, R.W.; Berin, M.C.; Cho, C.B.; et al. Epicutaneous immunotherapy for treatment of peanut allergy: Follow-up from the Consortium for Food Allergy Research. J. Allergy Clin. Immunol. 2021, 147, 992–1003.e5. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Muir, A.B.; Liacouras, C.A.; Burke, D.; Lewis, M.O.; Brown-Whitehorn, T.; Cianferoni, A. Sustained milk consumption after 2 years post-milk epicutaneous immunotherapy for eosinophilic esophagitis. Allergy 2021, 76, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hirobe, S.; Kuwabara, Y.; Nagao, M.; Saito, M.; Quan, Y.S.; Kamiyama, F.; Fujisawa, T.; Okada, N. Immunogenicity of Milk Protein-Containing Hydrophilic Gel Patch for Epicutaneous Immunotherapy for Milk Allergy. Pharm. Res. 2020, 37, 35. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Kalach, N.; Soulaines, P.; Legoué-Morillon, S.; Piloquet, H.; Benhamou, P.H. Cow’s milk epicutaneous immunotherapy in children: A pilot trial of safety, acceptability, and impact on allergic reactivity. J. Allergy Clin. Immunol. 2010, 125, 1165–1167. [Google Scholar] [CrossRef]
- Uncuoglu, A.; Yologlu, N.; Simsek, I.E.; Uyan, Z.S.; Aydogan, M. Tolerance to baked and fermented cow’s milk in children with IgE-mediated and non-IgE-mediated cow’s milk allergy in patients under two years of age. Allergol. Immunopathol. 2017, 45, 560–566. [Google Scholar] [CrossRef]
- Kim, J.S.; Nowak-Węgrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J. Allergy Clin. Immunol. 2011, 128, 125–131.e2. [Google Scholar] [CrossRef] [Green Version]
- Wanich, N.; Nowak-Wegrzyn, A.; Sampson, H.A.; Shreffler, W.G. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J. Allergy Clin. Immunol. 2009, 123, 789–794.e20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lin, J.; Bardina, L.; Goldis, M.; Nowak-Wegrzyn, A.; Shreffler, W.G.; Sampson, H.A. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J. Allergy Clin. Immunol. 2010, 125, 695–702.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreffler, W.G.; Wanich, N.; Moloney, M.; Nowak-Wegrzyn, A.; Sampson, H.A. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J. Allergy Clin. Immunol. 2009, 123, 43–52.e7. [Google Scholar] [CrossRef]
- Dang, T.D.; Peters, R.L.; Allen, K.J. Debates in allergy medicine: Baked egg and milk do not accelerate tolerance to egg and milk. World Allergy Organ. J. 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kido, J.; Nishi, N.; Sakaguchi, M.; Matsumoto, T. Most cases of cow’s milk allergy are able to ingest a partially hydrolyzed formula. Ann Allergy Asthma Immunol. 2015, 115, 330–331.e2. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Matsubara, T.; Nakazato, Y.; Namba, K.; Takeda, Y. Evaluation of the antigenicity of hydrolyzed cow’s milk protein formulas using the mouse basophil activation test. Toxicol. Lett. 2016, 242, 53–59. [Google Scholar] [CrossRef]
- Inuo, C.; Tanaka, K.; Nakajima, Y.; Yamawaki, K.; Matsubara, T.; Iwamoto, H.; Tsuge, I.; Urisu, A.; Kondo, Y. Tolerability of partially and extensively hydrolysed milk formulas in children with cow’s milk allergy. Asia Pac. J. Clin. Nutr. 2019, 28, 49–56. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, D.M.; Sicherer, S.; Greenhawt, M.; Campbell, D.; Chan, E.; Muraro, A.; Halken, S.; Katz, Y.; Ebisawa, M.; Eichenfield, L.; et al. Consensus Communication on Early Peanut Introduction and the Prevention of Peanut Allergy in High-risk Infants. Pediatrics 2015, 136, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef] [Green Version]
| Protein Name | Allergen Name | Molecular Mass (kDa) | Allergenicity | Others |
|---|---|---|---|---|
| Curd—Casein family: 80% | ||||
| Caseins | Bos d 8 | 20–30 | Major | Main protein fraction of cow’s milk consists of αs1-, αs2-, β-, and κ-caseins |
| αs1-casein | Bos d 9 | 23.6 | Major | Main fraction of casein |
| αs2-casein | Bos d 10 | 25.2 | Major | |
| β-casein | Bos d 11 | 24 | Major | |
| κ-casein | Bos d 12 | 19 | Major | |
| Whey (lactoserum): 20% | Loses IgE binding following 15–20 min of boiling at >90 °C | |||
| α-lactalbumin | Bos d 4 | 14.2 | Major | Present in the milk of almost all mammals |
| β-lactoglobulin; protein family: Lipocalins | Bos d 5 | 18.3 | Major | −65% of all whey proteins absent in human milk |
| Bovine serum albumin; family: Serum albumins | Bos d 6 | 67 | Minor | Clinical cross-reactivity to raw beef |
| Immunoglobulins; family: Immunoglobulins | Bos d 7 | 160 | Minor | Mostly IgG |
| Lactoferrin; family: Transferrins | 800 | Minor | Loses IgE binding following 15–20 min of boiling at >90 °C |
| Author (Year) | n | Age (Years) | Maintenance Dose of CM (mL) | Anaphylaxis during OIT | Target Dose of CM (mL) | Complete Desensitization | Others |
|---|---|---|---|---|---|---|---|
| Keet (2012) [27] | 30 | 6–17 | 0.2 mL (SLIT) and 30 or 60 mL (OIT) | Adrenalin was used for two SLIT doses and four OIT doses | 240 | 10% (SLIT) and 70% (OIT) | SLIT |
| Skripak (2008) [28] | 13 | 6–17 | 15 | Four subjects used adrenalin | 100 | 30.8% | |
| Staden (2007) [30] | 25 | 0.6–12.9 | 100 | 8% of subjects experienced wheezing | 150 | 48% | |
| Longo (2008) [32] | 30 | 5–17 | 150 | Four subjects used adrenalin injector in the hospital, and one used it at home | 150 | 36% | |
| De Schryver (2019) [33] | 41 | 6–18 | 200 | 15.8% (two cases experienced severe anaphylaxis) | 200 | 73.2% | |
| Berti (2019) [34] | 73 | 0.25–0.9 | 150 | No infants needed an adrenalin injection | 150 | 97% | OIT for infants |
| Boné Calvo (2021) [35] | 335 | <1 | 150–200 (infant formulae) | 1.3% | 150–200 (infant formulae) | 98% | OIT for infants |
| Martorell (2011) [36] | 30 | 2–3 | 200 | One subject used an adrenalin injection | 200 | 90% | OIT for infants |
| Vázquez-Ortiz (2013) [37] | 81 | 5–18 | 200 | Nine children were administered an adrenalin injection | 200 | 71.6% | |
| Narisety (2009) [38] | 15 | 6–16 | 3–480 | Four subjects used an adrenalin injection | 480 | 33% | |
| Takaoka (2020) [39] | 33 | 9 (median) | 20 (low-dose group) or 100 (high-dose group) | 0.1% (low-dose group) and 0.5% (high-dose group) | 20 or 100 | 90% (20 mL of CM) 34% (100 mL of CM) | Low-dose OIT |
| Meglio (2004) [40] | 21 | 6–10 | 200 | Two subjects experienced moderate asthma | 200 | 71.4% | |
| Yanagida (2015) [41] | 12 | >5 | 3 | A severe reaction occurred in one of 3795 doses | 3 | 75.0% | Low-dose OIT |
| Levy (2014) [42] | 280 | 3–27 | 240 | 45.7% (induction phase) and 15.7% (home dosing) | 240 | 62% | |
| Wood (2016) [43] | 28 | 11.7 | 300 | One participant used an adrenalin injection | 180 | 88.9% | Omalizumab |
| Nadeau (2011) [44] | 11 | 7–17 | 60 | Three subjects used an adrenalin injection | 60 | 81.8% | Omalizumab |
| Martorell (2016) [45] | 5 | 3–11 | 200 | Three subjects experienced anaphylaxis after discontinuing omalizumab | 200 | 100.0% | Omalizumab |
| Ibáñez-Sandín (2021) [46] | 58 | 6.3–13.2 | 180 | 36.4% of subjects who discontinued omalizumab experienced anaphylaxis | 180 | 83.0% | Omalizumab |
| Nowak-Wegrzyn (2008) [47] | 100 | 2.1–17.3 | 1.3 g of baked CM protein | None | 240 | 9% | Baked milk |
| Esmaeilzadeh (2018) [48] | 42 | 0.5–3 | First, 1.3 g of baked CM protein, and then 4.6 g of it | No data | 240 | 88.1% | Baked milk |
| Gruzelle (2020) [49] | 64 | 2–16 | 168.6 mg of baked CM protein | Six subjects experienced asthma (one subject used two injections of adrenalin) | 254 | 42.2% | Baked milk |
| Goldberg (2015) [50] | 15 | 6–12 | 1.3 g of baked CM protein | Three subjects used an adrenalin injection | 1.3 g of baked milk protein | 21% | Baked milk |
| Inuo (2018) [51] | 25 | 1–9 | 20 mL of pHF | None | 20 mL of pHF | (The threshold dose of pHF increased) | pHF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, M.; Kido, J.; Nakamura, K. Oral Immunotherapy for Children with Cow’s Milk Allergy. Pathogens 2021, 10, 1328. https://doi.org/10.3390/pathogens10101328
Ogata M, Kido J, Nakamura K. Oral Immunotherapy for Children with Cow’s Milk Allergy. Pathogens. 2021; 10(10):1328. https://doi.org/10.3390/pathogens10101328
Chicago/Turabian StyleOgata, Mika, Jun Kido, and Kimitoshi Nakamura. 2021. "Oral Immunotherapy for Children with Cow’s Milk Allergy" Pathogens 10, no. 10: 1328. https://doi.org/10.3390/pathogens10101328
APA StyleOgata, M., Kido, J., & Nakamura, K. (2021). Oral Immunotherapy for Children with Cow’s Milk Allergy. Pathogens, 10(10), 1328. https://doi.org/10.3390/pathogens10101328






