Preparation of a Master Fe–Cu Alloy by Smelting of a Cu-Bearing Direct Reduction Iron Powder
Abstract
:1. Introduction
2. Experimental
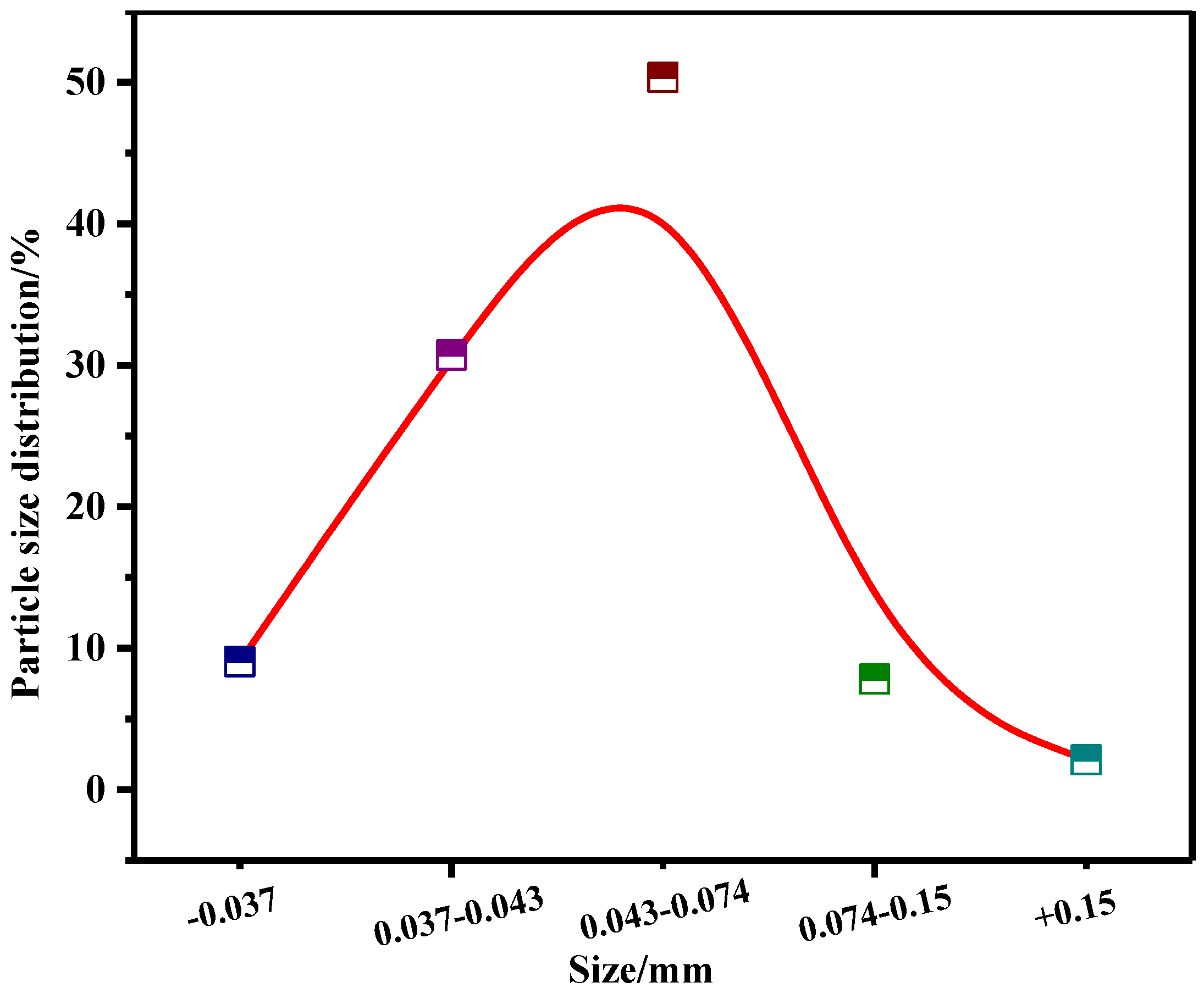
2.1. Raw Materials
2.2. Experimental Methods
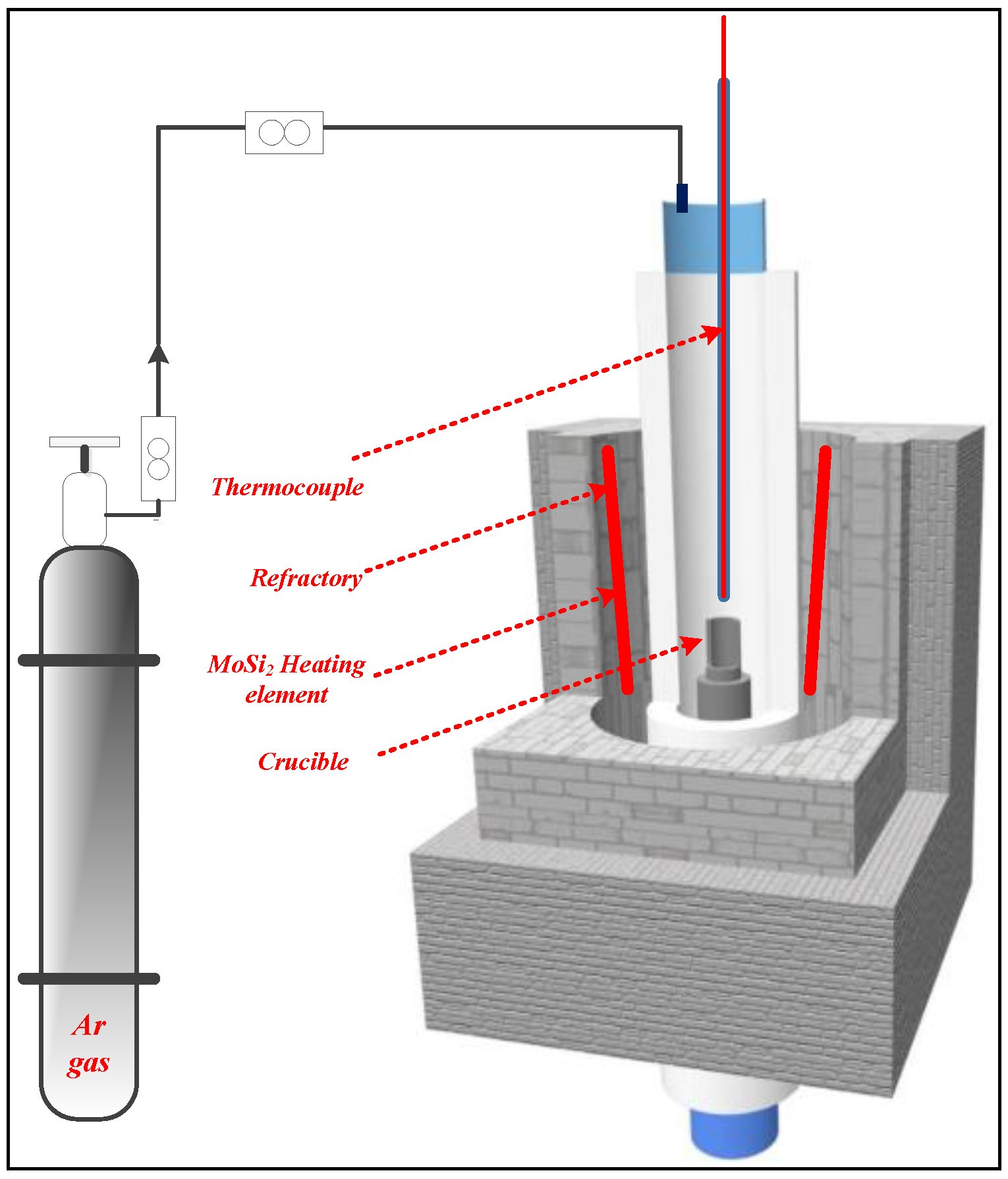
2.2.1. Smelting Process
2.2.2. Analytic Tests
3. Results and Discussion
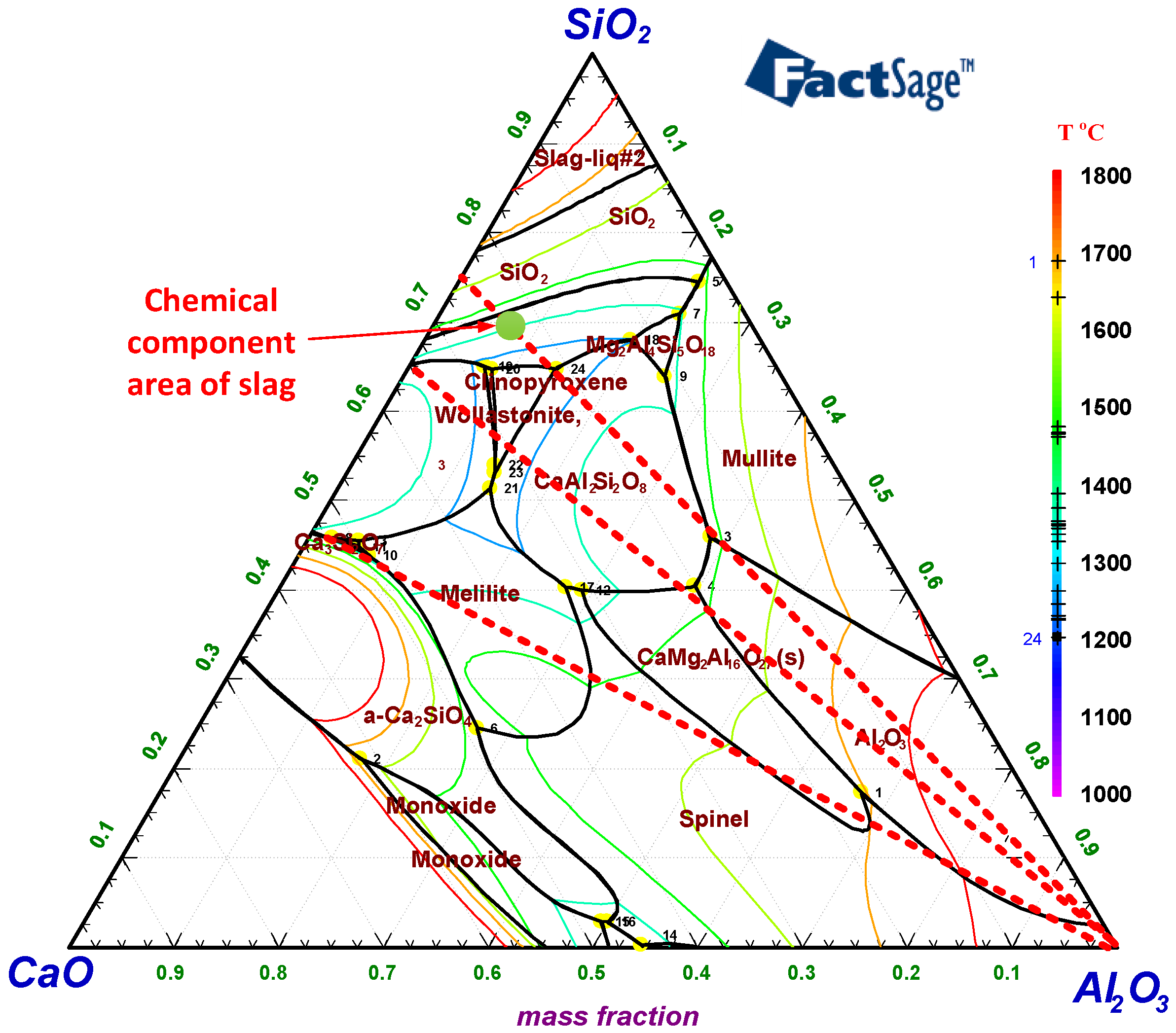
3.1. Thermodynamics Calculations of Smelting Process
3.2. Smelting Process of CBDRI
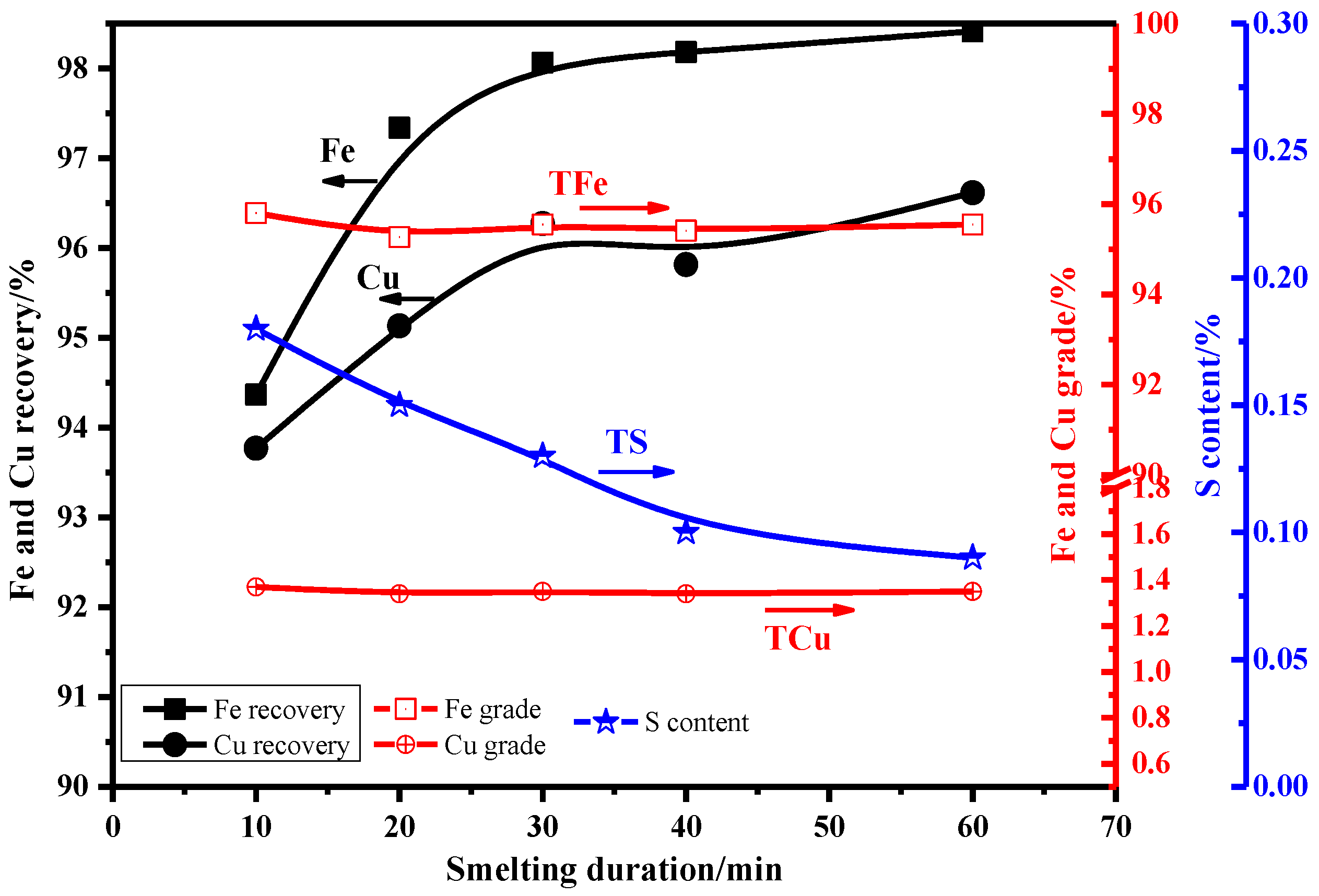
3.2.1. Effect of Smelting Temperature
3.2.2. Effect of Smelting Duration
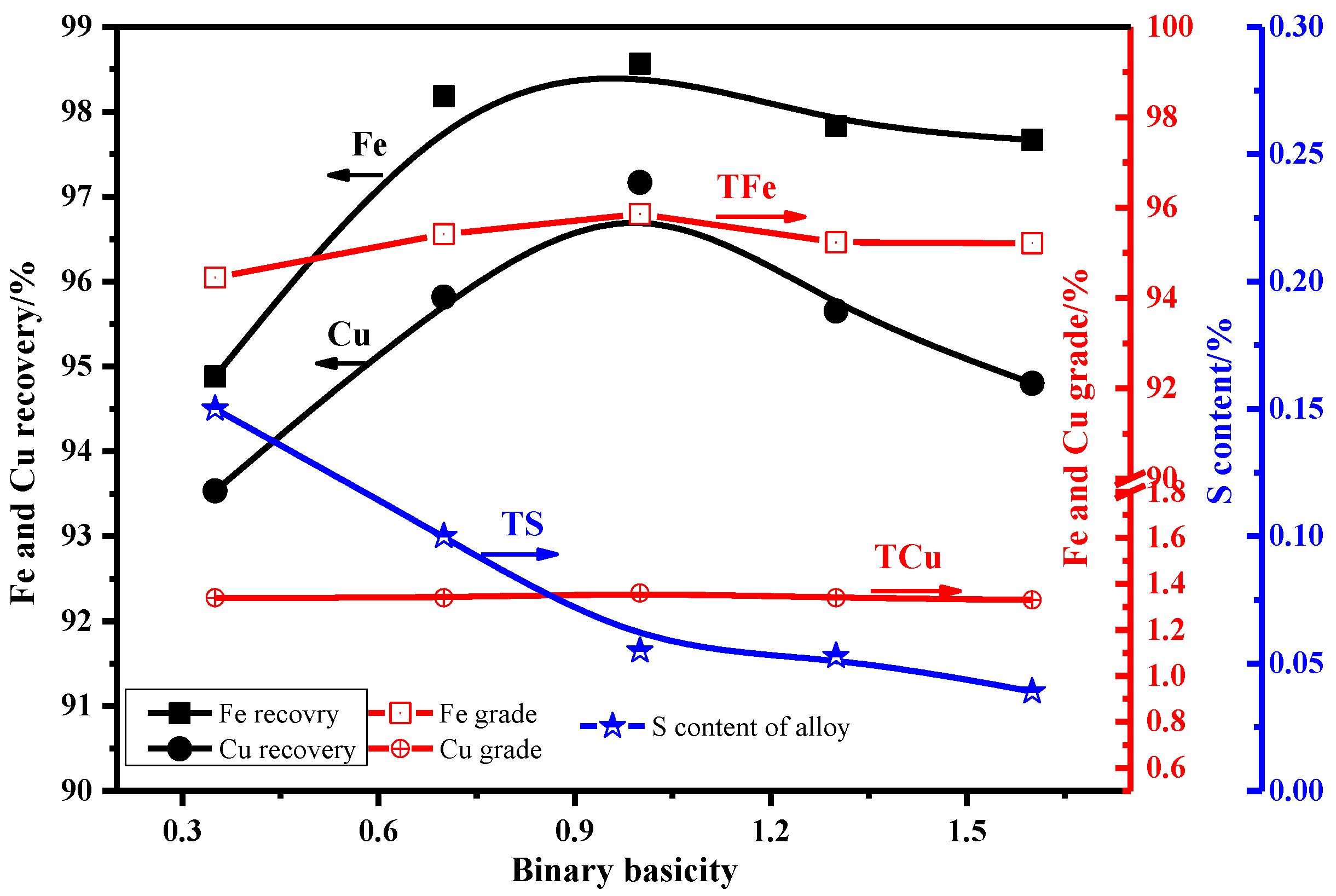
3.2.3. Effect of Binary Basicity
3.3. Mechanism of Desulfurization
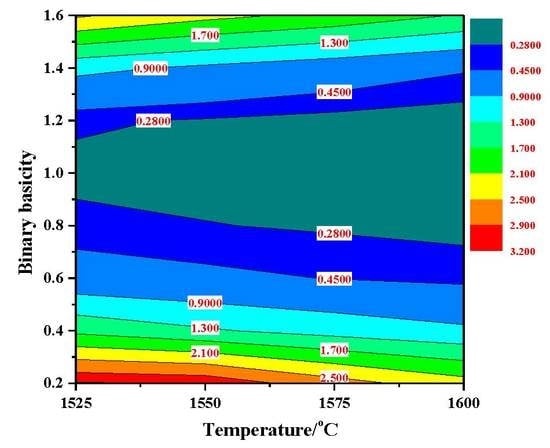
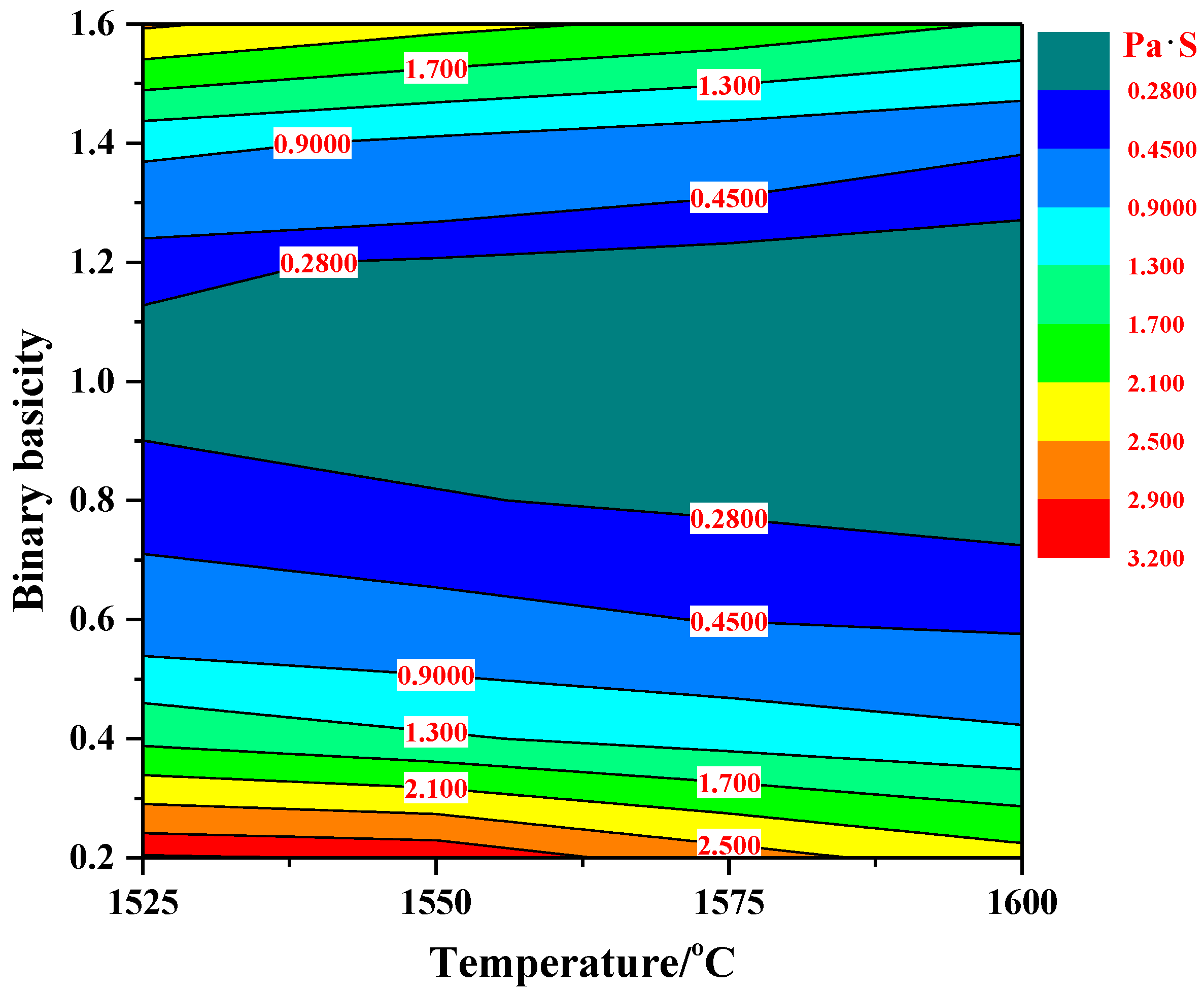
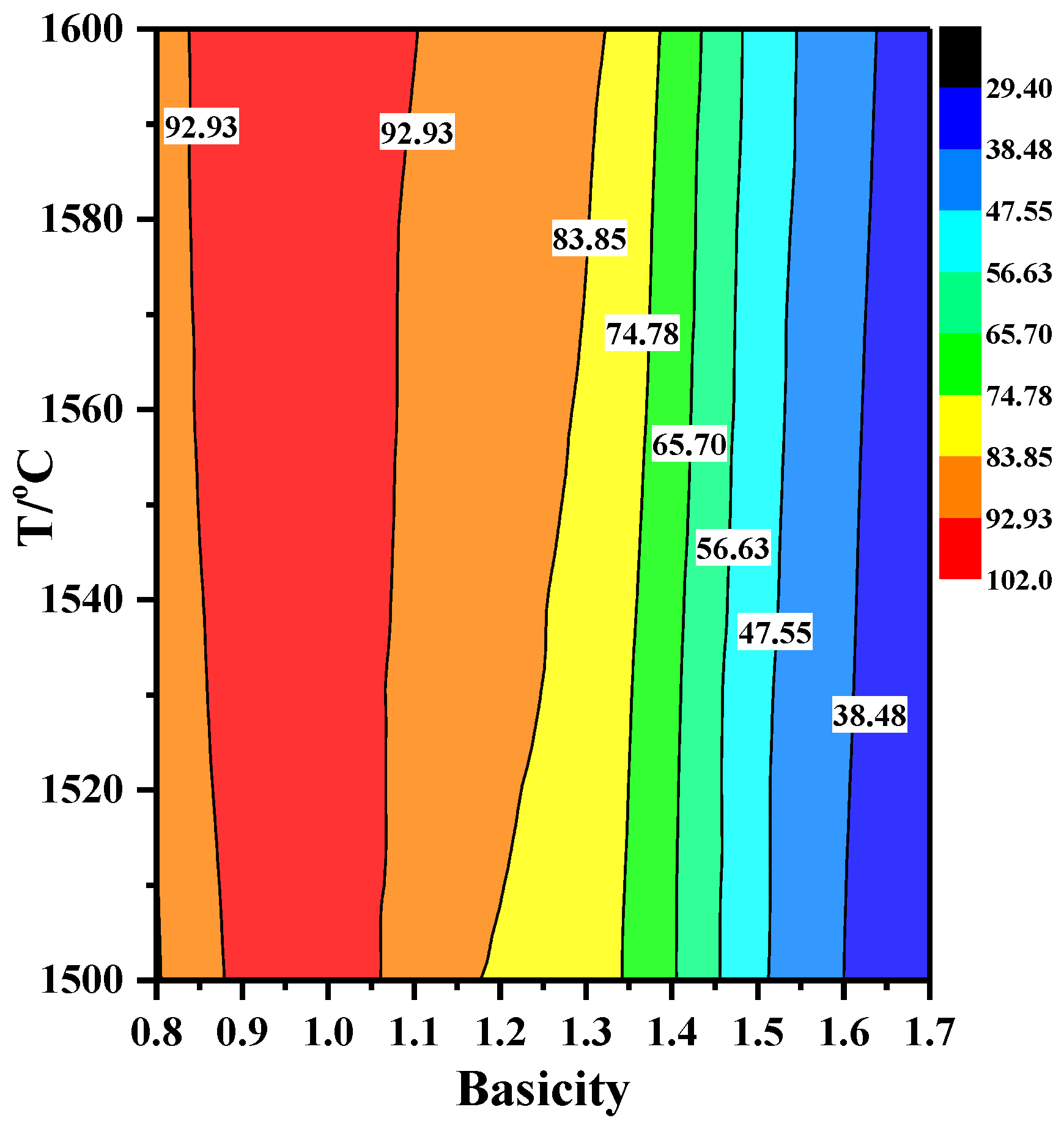
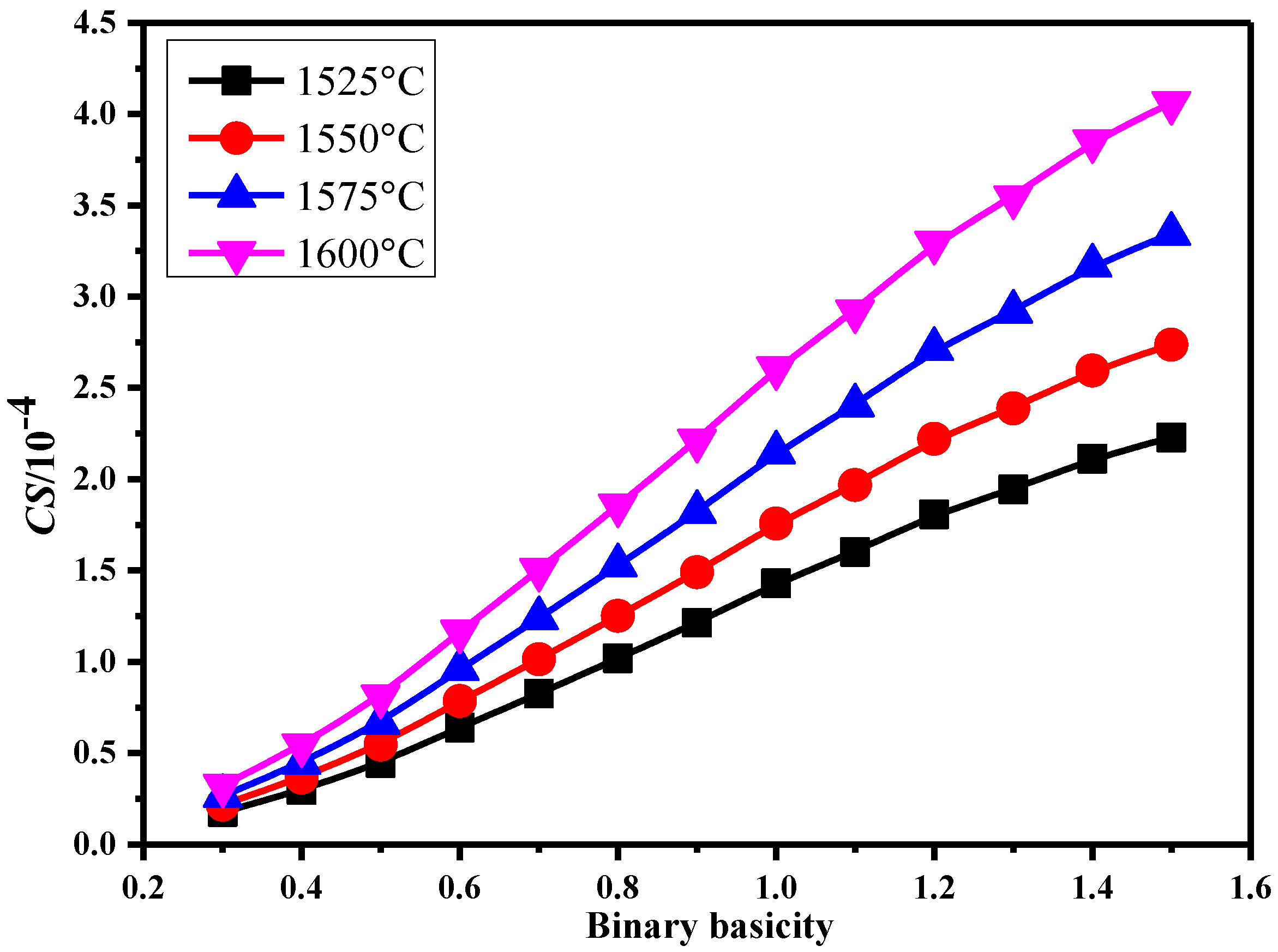
3.3.1. Sulfide Capacity (CS) of Slag
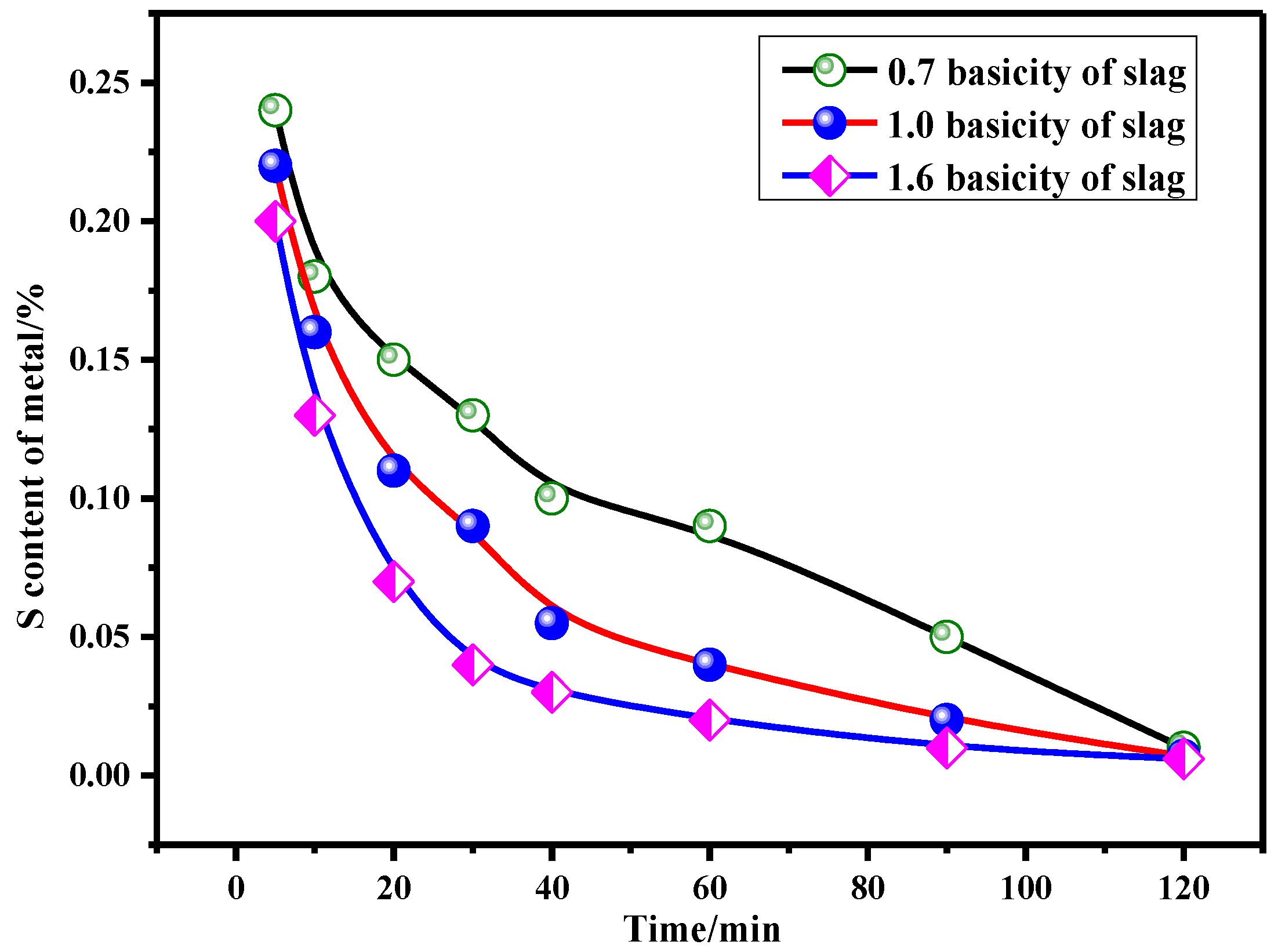
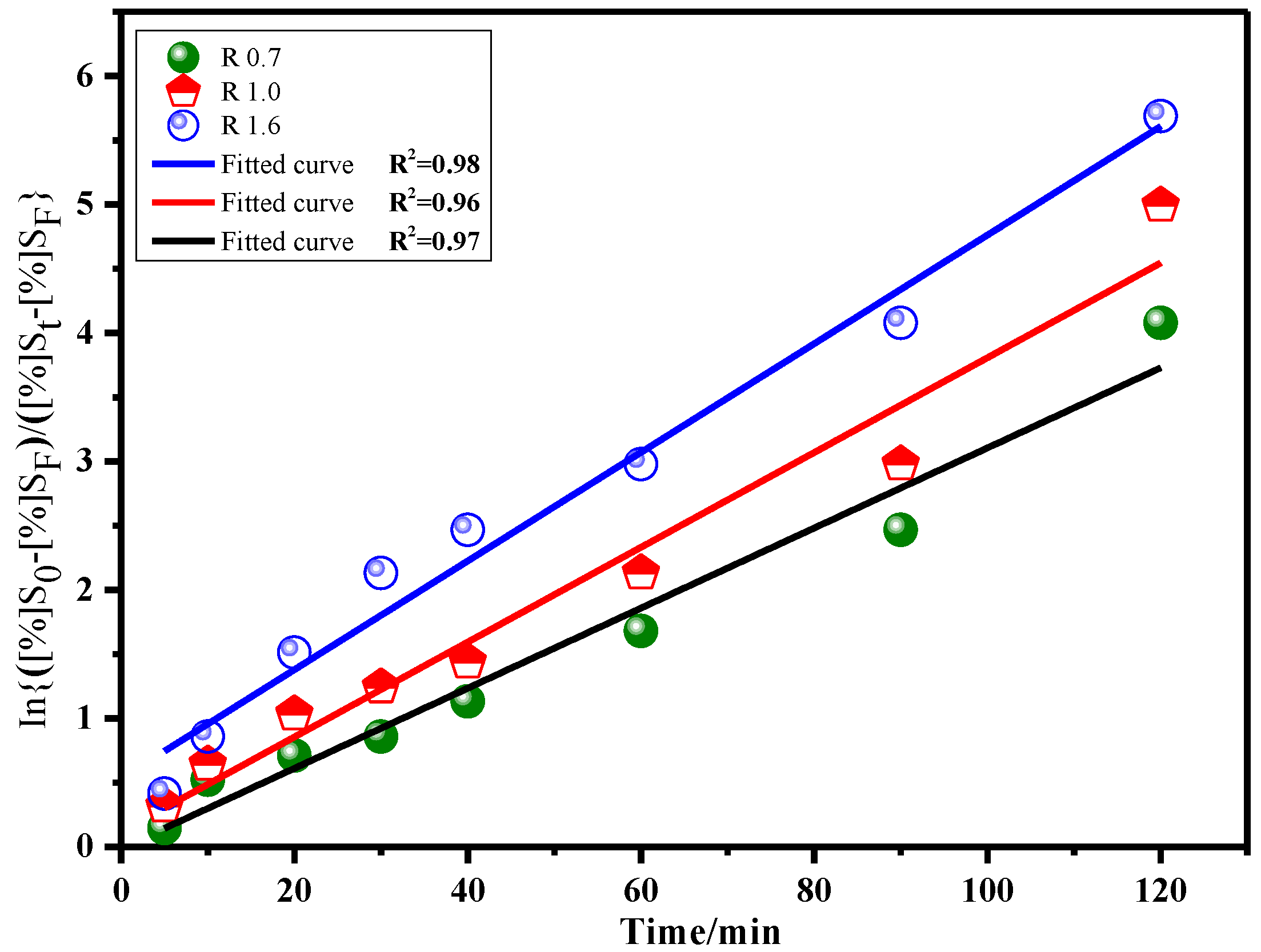
3.3.2. Desulfurization Reaction Kinetics of Slag with Different Basicity
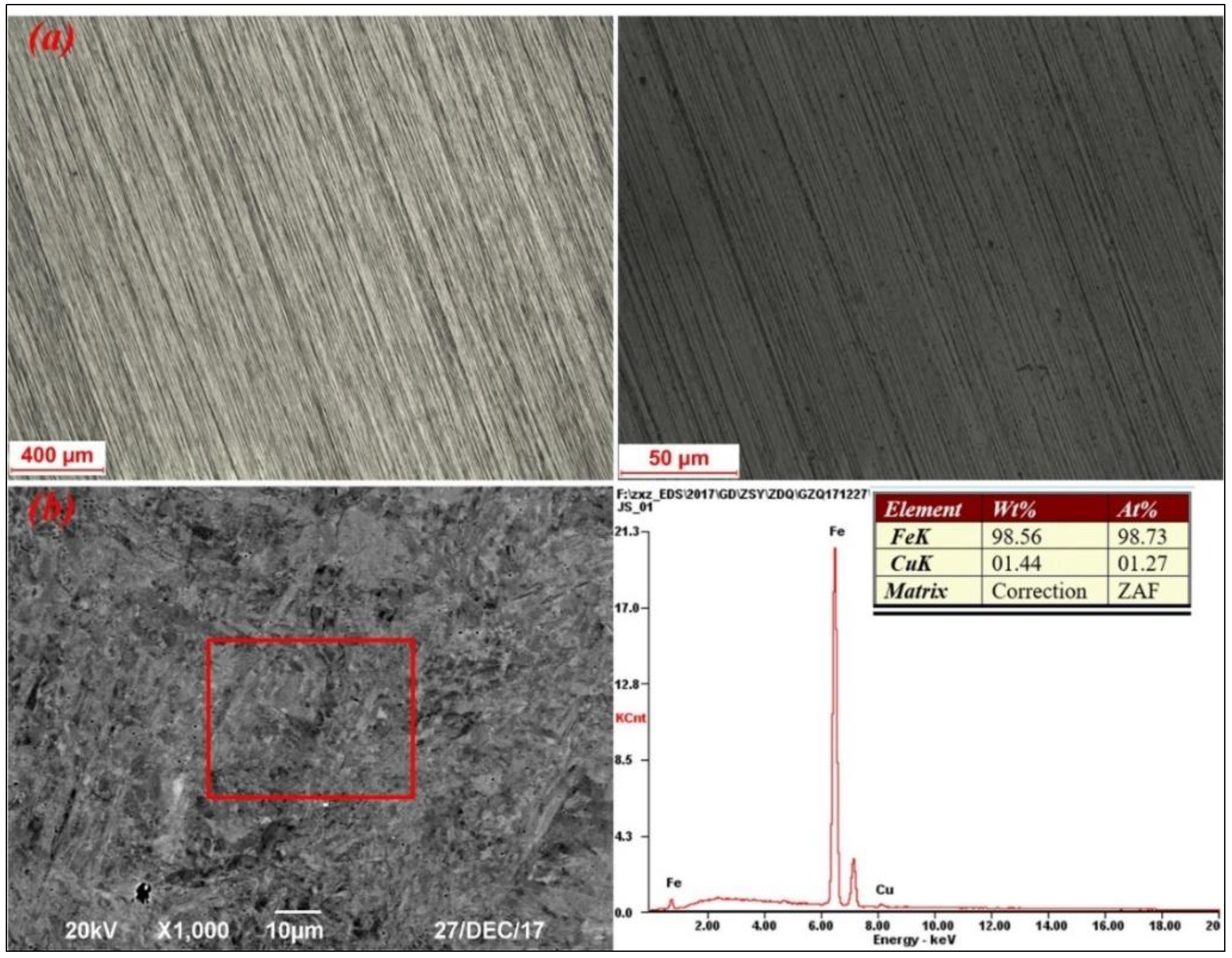
3.4. Characterization of the Fe–Cu Master Alloy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, X.B.; Yan, W.; Xu, D.K.; Yan, M.C.; Yang, C.G.; Shan, Y.Y.; Yang, K. Microbial corrosion resistance of a novel Cu-bearing pipeline steel. J. Mater. Sci. Technol. 2018, 34, 2480–2491. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Zhao, J.; Yang, K. Cu-bearing stainless steel reduces cytotoxicity and crystals adhesion after ureteral epithelial cells exposing to calcium oxalate monohydrate. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Zhao, J.L.; Xi, T.; Shahzad, M.B.; Yang, C.G.; Yang, K. Dissolution and repair of passive film on Cu-bearing 304L stainless steels immersed in H2SO4 solution. J. Mater. Sci. Technol. 2018, 34, 2149–2159. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Chico, B.; Cano, H.; Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef] [Green Version]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Yang, G.Y.; Deng, M.L.; Liu, Y.G. Research Status and Prospect of Copper-bearing Steel. Hot Work. Technol. 2017, 46, 36–39. (In Chinese) [Google Scholar]
- Shyi, W.; Powe, K. Effect of alloying elements on the structure and mechanical properties of ultra low carbon bainitic steels. Mater. Sci. 1993, 28, 5169–5175. [Google Scholar]
- Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Zhang, F. Co-reduction of Copper Smelting Slag and Nickel Laterite to Prepare Fe-Ni-Cu Alloy for Weathering Steel. JOM 2018, 70, 150–154. [Google Scholar] [CrossRef]
- Li, S.W.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Xu, J.W.; Chou, J.L. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Yao, W.J.; Xu, W.Q.; Chen, J.A. Effect of Na2CO3 Addition on Carbothermic Reduction of Copper Smelting Slag to Prepare Crude Fe-Cu Alloy. JOM 2017, 69, 1688–1695. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.; Premchand, M. Characteristics and utilization of copper slag-a review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Subrata, R.; Amlan, D.; Sandeep, R. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 34, 43–49. [Google Scholar]
- Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Zhang, F. Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process. J. Clean. Prod. 2018, 199, 891–899. [Google Scholar] [CrossRef]
- Li, Y.; Perederiy, I.; Papangelakis, V.G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, A.; Takasaki, Y.; William, T.; Yamatodani, A.; Higuchi, Y.; Sunagawa, S.; Ono, E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process. J. Hazard. Mater. 2010, 181, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Perederiy, I.; Papangelakis, V.G.; Buarzaiga, M.; Mihaylov, I. Co-treatment of converter slag and pyrrhotite tailings via high pressure oxidative leaching. J. Hazard. Mater. 2011, 194, 399–406. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Dimitrijevic, M.D.; Stevanovic, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 152, 23–34. [Google Scholar] [CrossRef]
- Zuo, Z.L.; Yu, Q.B.; Xie, H.Q.; Wang, K.; Liu, S.H.; Yang, F.; Qin, Q.; Qi, Z.F. Mechanical and reduction characteristics of cold-pressed copper slag pellets composited within biomass and lignite. Renew. Energy 2018, 125, 206–224. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Zhang, F. Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Chun, T.; Mu, G.; Di, Z.; Long, H.; Ning, C.; Li, D. Recovery of iron from copper slag by carbothermic thermic reduction and magnetic separation in the presence of CaO. Arch. Metall. Mater. 2018, 63, 299–305. [Google Scholar]
- Geng, C.; Wang, H.J.; Hu, W.T.; Li, L.; Shi, C.S. Recovery of iron and copper from copper tailings by coal-based direct reduction and magnetic separation. J. Iron Steel Res. Int. 2017, 24, 991–997. [Google Scholar] [CrossRef]
- Jiao, R.M.; Xing, P.; Wang, C.Y.; Ma, B.Z.; Chen, Y.Q. Recovery of iron from copper tailings via low-temperature direct reduction and magnetic separation: Process optimization and mineralogical study. Int. J. Min. Met. Mater. 2017, 24, 974–982. [Google Scholar] [CrossRef]
- Wang, X.H. Ferrous Metallurgy (Steelmaking Part), 1st ed.; Metallurgical Industry Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Liu, Y.; Lv, X.; Xu, J.; Zhang, S.; Bai, C. Preparation of stainless steel master alloy by direct smelting reduction of Fe–Ni–Cr Sinter at 1600 °C. Ironmak. Steelmak. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; Pang, Z.; He, W.; Liang, D.; Bai, C. Transition of Blast Furnace Slag from Silicate Based to Aluminate Based: Sulfide Capacity. Metall. Mater. Trans. B 2017, 48, 2607–2613. [Google Scholar] [CrossRef]
- Guo, H. Metallurgical Physical Chemistry, 2nd ed.; China Metallurgical Industry Press: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Young, R.W.; Duffy, J.A.; Hassall, G.J. Use of optical basicity concept of determining phoshphorus and sulfur slag-metal partitions. Ironmak. Steelmak. 1992, 19, 201–209. [Google Scholar]
- Wang, L.; Wu, S.; Kou, M.; Du, B.; Lu, Y.; Gu, K. Improving the Desulphurization in COREX Process by Adjusting the Hot Metal Chemical Composition. Metall. Mater. Trans. B 2018, 49, 89–96. [Google Scholar] [CrossRef]
- Wang, X.L. Ferrous Metallurgy (Ironmaking Part), 3rd ed.; China Metallurgical Industry Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Kang, J.G.; Shin, J.H.; Chung, Y.; Park, J.H. Effect of Slag Chemistry on the Desulfurization Kinetics in Secondary Refining Processes. Metall. Mater. Trans. B 2017, 48, 2123–2135. [Google Scholar] [CrossRef]
- Peter, J.; Peaslee, K.D.; Robertson, D.G.C.; Thomas, B.G. Proceedings of the AISTech 2005; AIST: Warrendale, PA, USA, 2005; pp. 959–967. [Google Scholar]
- Robertson, D.G.C.; Staples, B.B. Process Engineering of Pyrometallurgy; IMM: London, UK, 1974; pp. 51–59. [Google Scholar]


| Fe | Cu | S | MFe | SiO2 | Al2O3 | CaO | MgO | Na2O | C | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 90.33 | 1.29 | 0.30 | 87.54 | 3.62 | 0.56 | 1.09 | 0.29 | 0.07 | 0.78 | 0.012 |
| CaO | Al2O3 | SiO2 | MgO | P | S | MnO | LOI |
|---|---|---|---|---|---|---|---|
| 55.73 | 1.19 | 4.13 | 2.05 | 0.001 | 0.001 | 0.02 | 41.52 |
| Items | Basicity R | ||
|---|---|---|---|
| 0.7 | 1.0 | 1.6 | |
| Diffusion coefficient | 0.78 × 10−3 | 0.92 × 10−3 | 1.05 × 10−3 |
| Fe | Cu | S | SiO2 | Al2O3 | CaO | MgO | Na2O | P | Zn | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| 95.86 | 1.36 | 0.055 | 0.10 | 0.32 | 0.053 | 0.027 | 0.005 | 0.01 | 0.01 | 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Zhu, D.; Guo, Z.; Pan, J. Preparation of a Master Fe–Cu Alloy by Smelting of a Cu-Bearing Direct Reduction Iron Powder. Metals 2019, 9, 701. https://doi.org/10.3390/met9060701
Pan L, Zhu D, Guo Z, Pan J. Preparation of a Master Fe–Cu Alloy by Smelting of a Cu-Bearing Direct Reduction Iron Powder. Metals. 2019; 9(6):701. https://doi.org/10.3390/met9060701
Chicago/Turabian StylePan, Liaoting, Deqing Zhu, Zhengqi Guo, and Jian Pan. 2019. "Preparation of a Master Fe–Cu Alloy by Smelting of a Cu-Bearing Direct Reduction Iron Powder" Metals 9, no. 6: 701. https://doi.org/10.3390/met9060701
APA StylePan, L., Zhu, D., Guo, Z., & Pan, J. (2019). Preparation of a Master Fe–Cu Alloy by Smelting of a Cu-Bearing Direct Reduction Iron Powder. Metals, 9(6), 701. https://doi.org/10.3390/met9060701