New Ferritic Stainless Steel for Service Temperatures up to 1050 °C Utilizing Intermetallic Phase Transformation
Abstract
:1. Introduction
2. Methodology
2.1. Thermo-Calc and TC-Prisma
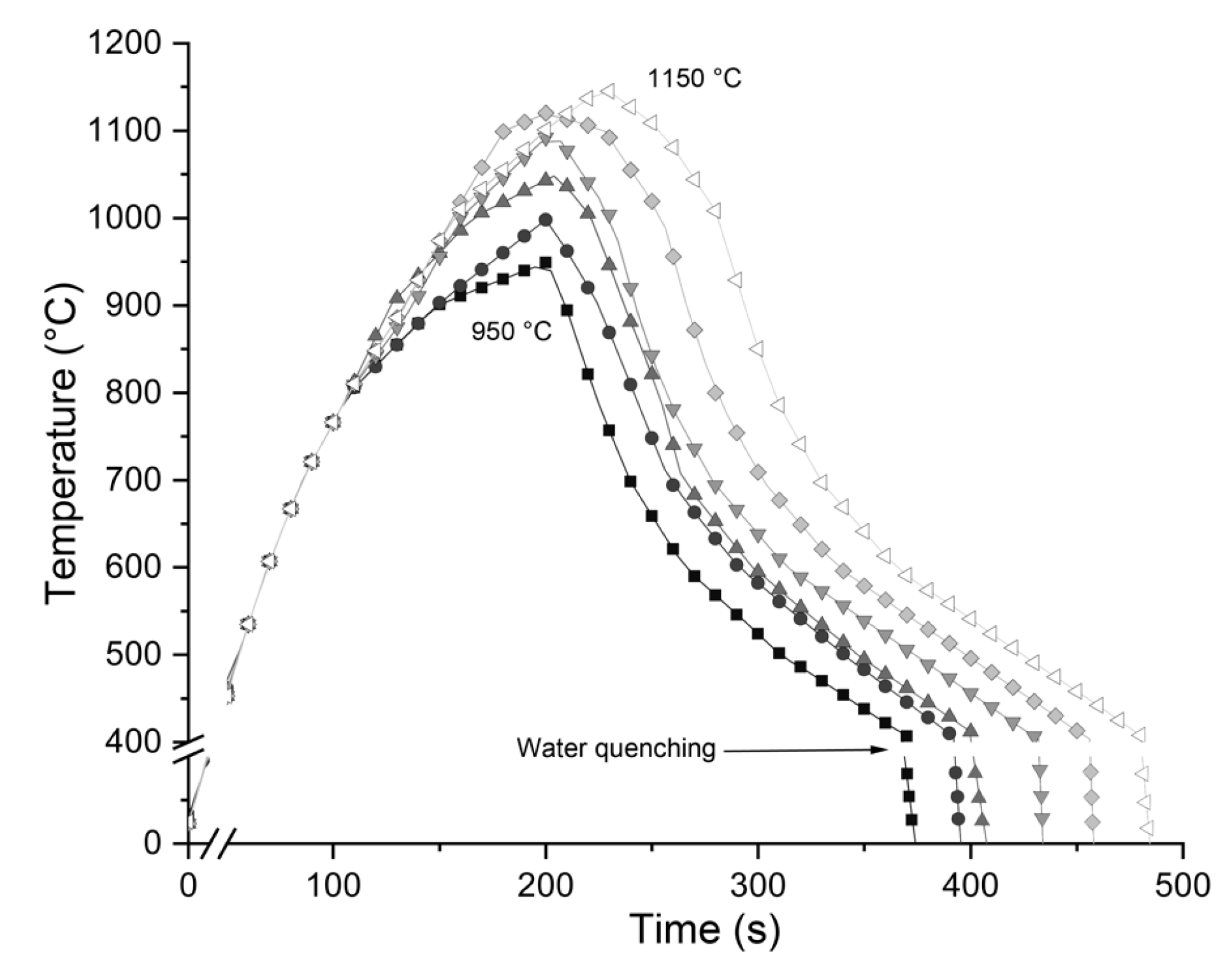
2.2. Sag Tests
3. Results
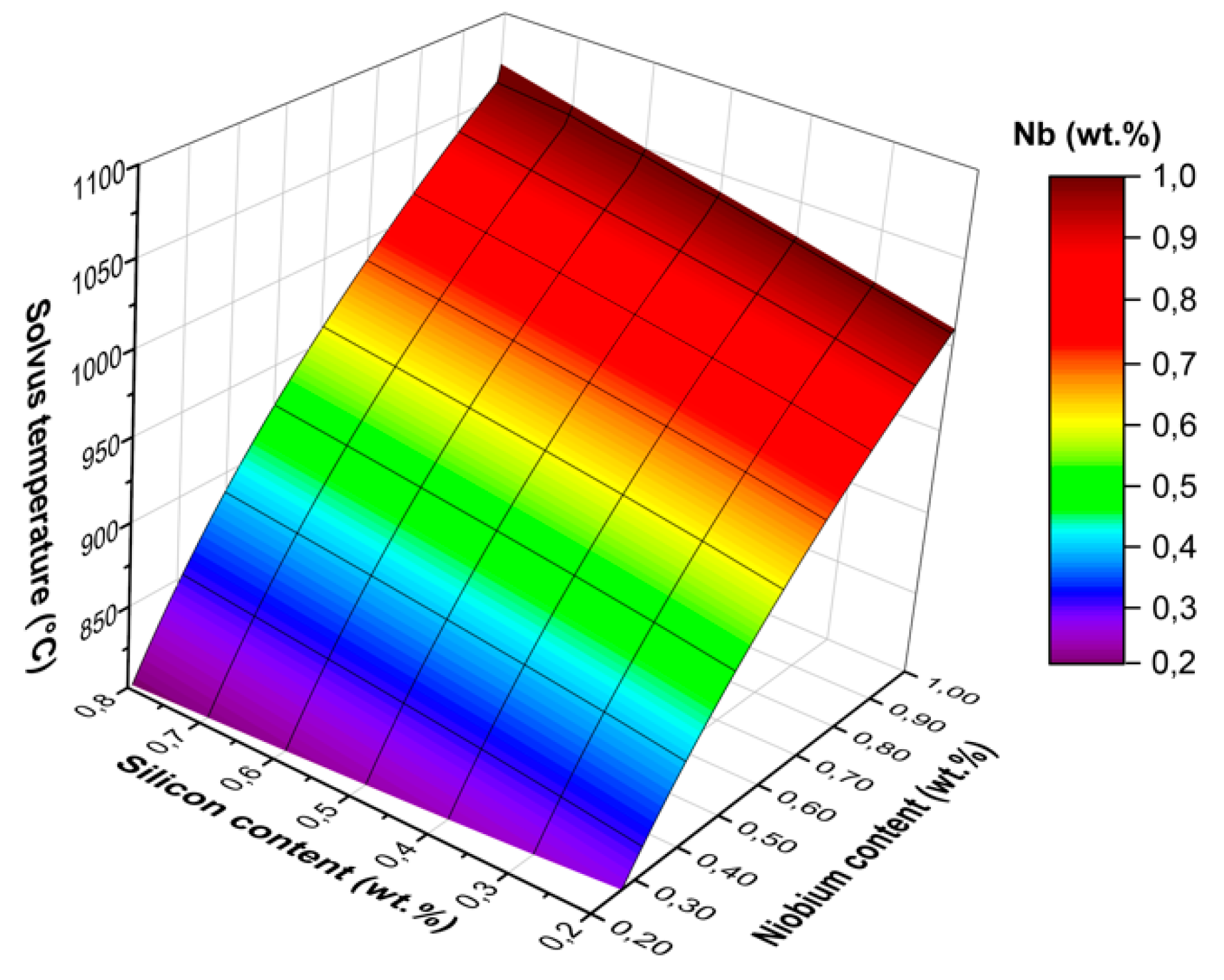
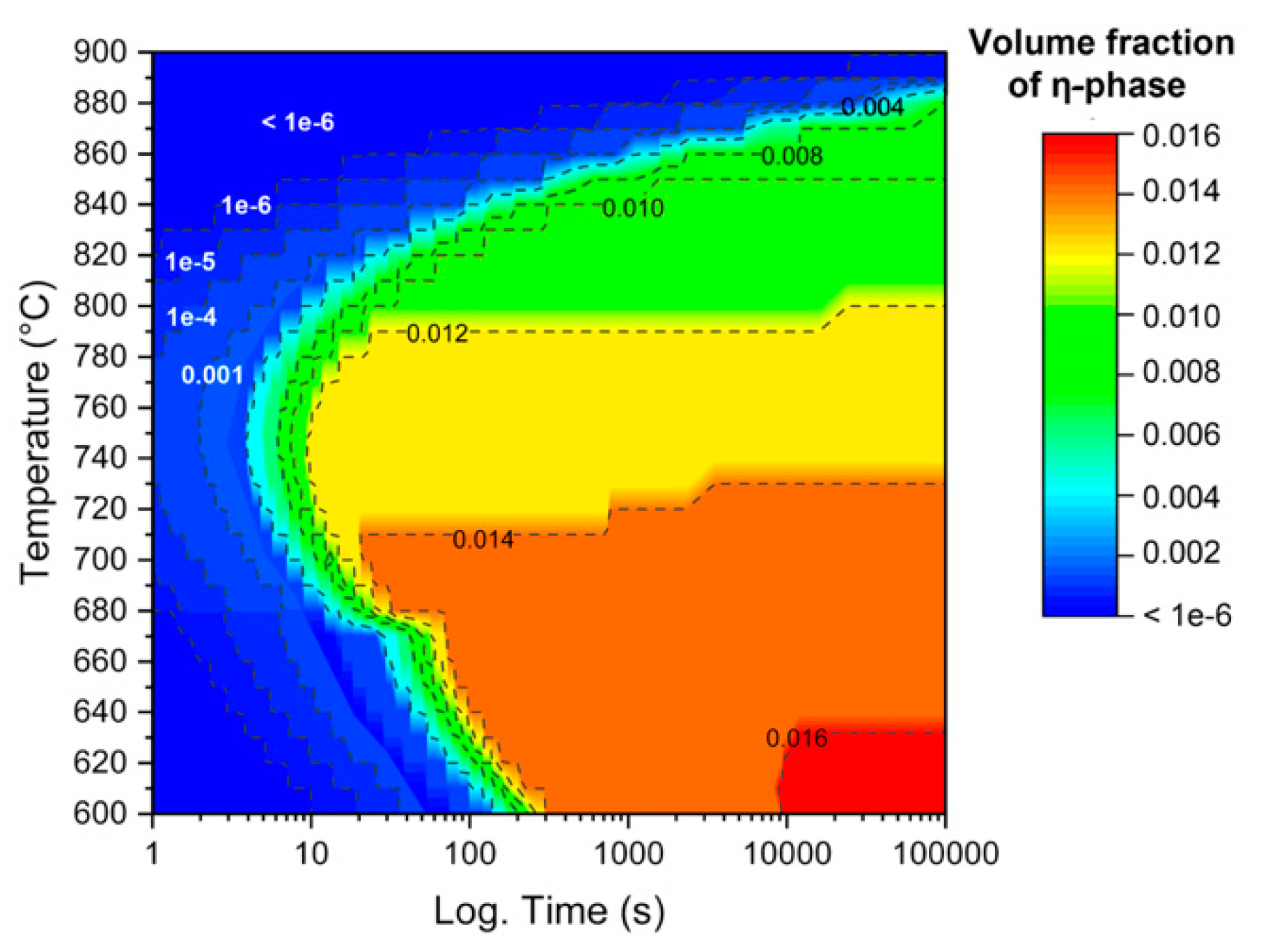
3.1. Thermodynamic Simulations
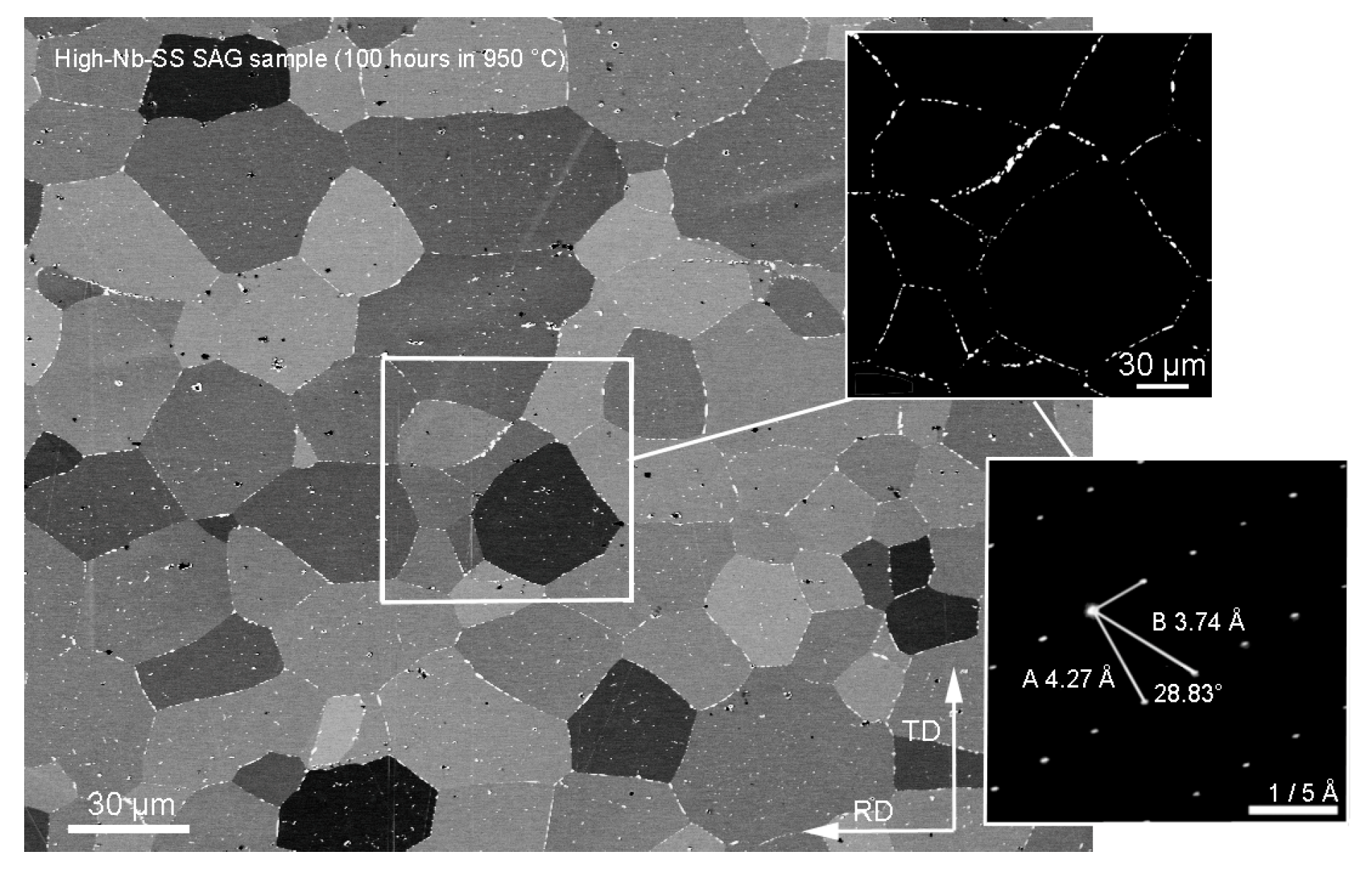
3.2. Annealing Microstructures
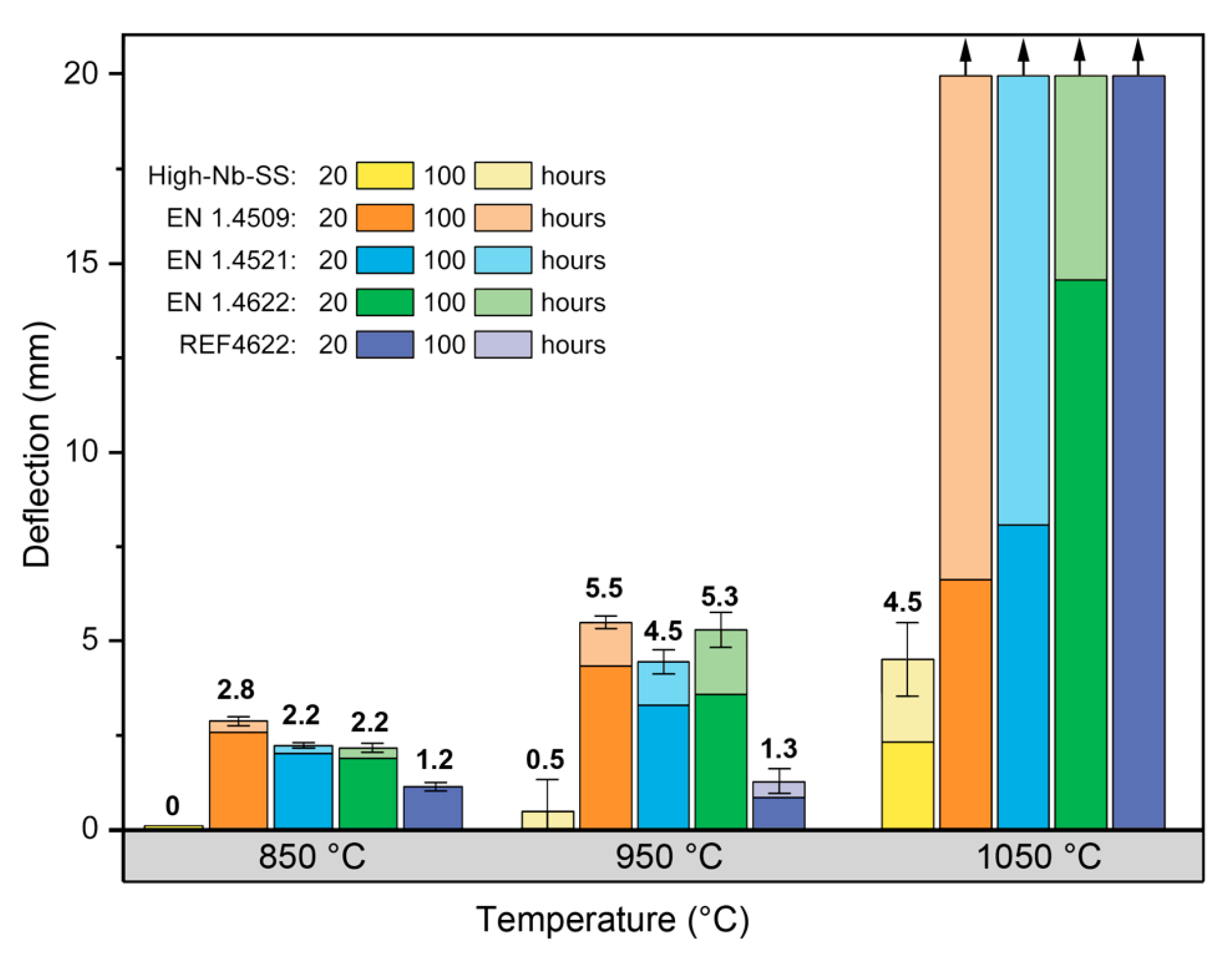
3.3. Creep Resistance
4. Discussion
5. Conclusions
- 1)
- When alloying 0.8 wt.% of Nb and Si, the equilibrium solvus temperature of the η-phase in High-Nb-SS was calculated as 1026 °C. The solvus temperature is 175 °C higher than in EN1.4509 which is a commonly used steel in high temperature applications. When exposed to temperatures above the solvus temperature, the rapid grain growth begins, limiting the service temperature of the steel.
- 2)
- The dominant creep mechanism during the sag test is Cobble creep, which is related to the grain boundary diffusion and can therefore be suppressed by grain boundary precipitation.
- 3)
- Grain boundary precipitation of η-phase resulted in significantly lower deflections in Sag test, i.e. enhanced the creep resistance of High-Nb-SS compared to commercially available steels used in high temperatures.
- 4)
- Creep rate in the Cobble mechanism is inversely proportional to the cube of the grain size. However, the η-phase precipitation mitigated the negative effect of a finer grain size in High-Nb-SS steel. Avoiding grain growth at higher temperature will better maintain other mechanical properties of the steel.
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hua, M.; Garcia, C.I.; DeArdo, A.J.; Tither, G. Dual-Stabilized Ferritic Stainless Steels for Demanding Applications Such as Automotive Exhaust Systems. Iron Steelmak. 1997, 24, 41–44. [Google Scholar]
- Inoue, Y.; Kikuchi, M. Present and future trends of stainless steel for automotive exhaust system. Nippon Steel Tech. Rep. 2003, 28, 62–69. [Google Scholar]
- Ali-Löytty, H.; Jussila, P.; Juuti, T.; Karjalainen, L.P.; Zakharov, A.A.; Valden, M. Influence of precipitation on initial high-temperature oxidation of Ti-Nb stabilized ferritic stainless steel SOFC interconnect alloy. Int. J. Hydrogen Energy 2012, 37, 14528–14535. [Google Scholar] [CrossRef]
- Ishii, K.; Miyazaki, A.; Satoh, S. Stainless Steels for Automotive Exhaust Systems. Kawasaki Steel Tech. Rep. 1999, 40, 39–41. [Google Scholar]
- Fujita, N.; Ohmura, K.; Sato, E.; Yamamoto, A. Development of Ferritic Stainless Steel YUS 450 with High Heat Resistance for Automotive Exhaust System Components. Nippon Steel Tech. Rep. 1996, 71, 25–30. [Google Scholar]
- Fujita, N. New Ferritic stainless steels in automotive exhaust system. Nippon Steel Tech. Rep. 2000, 81, 29–33. [Google Scholar]
- Yan, H.; Bi, H.; Li, X.; Xu, Z. Effect of two-step cold rolling and annealing on texture, grain boundary character distribution and r-value of Nb + Ti stabilized ferritic stainless steel. Mater. Charact. 2009, 60, 65–68. [Google Scholar] [CrossRef]
- Juuti, T.; Rovatti, L.; Mäkelä, A.; Karjalainen, L.P.; Porter, D. Influence of Long Heat Treatments on the Laves Phase Nucleation in a type 444 Ferritic Stainless Steel. J. Alloys Compd. 2014, 616, 250–256. [Google Scholar] [CrossRef]
- Juuti, T.; Rovatti, L.; Porter, D.; Angella, G.; Kömi, J. Factors controlling ambient and high temperature yield strength of ferritic stainless steel susceptible to intermetallic phase formation. Mater. Sci. Eng. A 2018, 726, 45–55. [Google Scholar] [CrossRef]
- Hald, J. Microstructure and long-term creep properties of 9–12% Cr steels. Int. J. Press. Vessel. Pip. 2008, 85, 30–37. [Google Scholar] [CrossRef]
- Abe, F. Effect of fine precipitation and subsequent coarsening of Fe2W laves phase on the creep deformation behavior of tempered martensitic 9Cr-W steels. Metall. Mater. Trans. A 2005, 36, 321–332. [Google Scholar] [CrossRef]
- Li, Q. Precipitation of Fe2W laves phase and modeling of its direct influence on the strength of a 12Cr-2W steel. Metall. Mater. Trans. A 2006, 37, 89–97. [Google Scholar] [CrossRef]
- Miyazaki, A.; Hirasawa, J.; Furukimi, O.; Kobayashi, M.; Kihara, S. Development of High Heat—Resistant Ferritic Stainless Steel with High Formability, “RMH-1”, for Automotive Exhaust Gas Systems. Mater. Jpn. 2003, 42, 157–159. [Google Scholar] [CrossRef]
- Sim, G.M.; Ahn, J.C.; Hong, S.C.; Lee, K.J.; Lee, K.S. Effect of Nb Precipitate Coarsening on the High Temperature Strength in Nb Containing Ferritic Stainless Steels. Mater. Sci. Eng. A 2005, 396, 159–165. [Google Scholar] [CrossRef]
- Fujita, N.; Ohmura, K.; Kikuchi, M.; Suzuki, T.; Funaki, S.; Hiroshige, I. Effect of Nb on High-temperature Properties for Ferritic Stainless Steel. Scr. Mater. 1996, 35, 705–710. [Google Scholar] [CrossRef]
- Froitzheim, J.; Meier, G.H.; Niewolak, L.; Ennis, P.J.; Hattendorf, H.; Singheiser, L.; Quadakkers, W.J. Development of high strength ferritic steel for interconnect application in SOFCs. J. Power Sources 2008, 178, 163–173. [Google Scholar] [CrossRef]
- Andrews, K.W.; Dyson, D.J.; Keown, S.R. Interpretation of Electron. Diffraction Patterns; Springer US: Boston, MA, USA, 1967; ISBN 978-1-4899-6228-7. [Google Scholar]
- Brown, E.L.; Burnett, M.E.; Purtscher, P.T.; Krauss, G. Intermetallic phase formation in 25Cr-3Mo-4Ni ferritic stainless steel. Metall. Trans. A 1983, 14, 791–800. [Google Scholar] [CrossRef]
- Speich, G. Precipitation of Laves Phases from Iron-niobium (Columbium) and Iron-Titanium Solid Solutions. Trans. Met. Soc. AIME 1962, 224, 850–858. [Google Scholar]
- Chen, Q.; Jou, H.J.; Sterner, G. Thermodynamic and Mobility Databases Overview; Thermo-Calc Software AB: Stockholm, Sweden, 2018; pp. 1–20. [Google Scholar]
- Chen, Q.; Jou, H.J.; Sterner, G. TC-PRISMA User’s Guide; Thermo-Calc Software AB: Stockholm, Sweden, 2015. [Google Scholar]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics, 1st ed.; Pergamon Press: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps for Pure Iron, Two Austenitic Stainless Steels, and a Low-Alloy Ferritic Steel. In Fundamental Aspects of Structural Alloy Design; Jaffee, R.I., Wilcox, B.A., Eds.; Springer US: Boston, MA, USA, 1977; pp. 27–65. ISBN 978-1-4684-2421-8. [Google Scholar]
- Puchi-Cabrera, E.S.; Guérin, J.D.; Barbier, D.; Dubar, M.; Lesage, J. Plastic Deformation of Structural Steels Under Hot-working Conditions. Mater. Sci. Eng. A 2013, 559, 268–275. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Heilmaier, M. Hall & Petch Breakdown at Elevated Temperatures. Mater. Trans. 2014, 55, 44–51. [Google Scholar]
- Nabiran, N.; Klein, S.; Weber, S.; Theisen, W. Evolution of the Laves Phase in Ferritic Heat-Resistant Steels During Long-term Annealing and its Influence on the High-Temperature Strength. Metall. Mater. Trans. A 2014, 46, 102–114. [Google Scholar] [CrossRef]
- Grubb, J.; Wrigth, R.; Farrar, P.J. Toughness of Ferritic Stainless Steels; Lula, R., Ed.; ASTM International: West Conshohocken, PA, USA, 1982; ISBN 978-0-8031-0792-2. [Google Scholar]
- Redmond, J. Toughness of 18Cr-2Mo Stainless Steel. In Toughness of Ferritic Stainless Steels; ASTM International: West Conshohocken, PA, USA, 1980; pp. 123–144. [Google Scholar]
- Suehiro, M.; Liu, Z.-K.; Ågren, J. Effect of niobium on massive transformation in ultra low carbon steels: A solute drag treatment. Acta Mater. 1996, 44, 4241–4251. [Google Scholar] [CrossRef]
- Suehiro, M. Recrystallization and Related Phenomena. An Analysis of the Solute Drag Effect of Nb on Recrystallization of Ultra Low Carbon Steel. ISIJ Int. 1998, 38, 547–552. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Lucas, G.E. Deformation and Fracture Mechanisms in Irradiated FCC and BCC Metals; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2003. [Google Scholar]
- Hertzberg, R.W. Deformation and Fracture Mechanics of Engineering Materials, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Čadek, J. Creep in Metallic Materials; Elsevier: Amsterdam, The Netherlands, 1988; ISBN 0444989161. [Google Scholar]
- Erneman, J.; Schwind, M.; Andren, H.; Nilsson, J.; Wilson, A.; Agren, J. The evolution of primary and secondary niobium carbonitrides in AISI 347 stainless steel during manufacturing and long-term ageing. Acta Mater. 2006, 54, 67–76. [Google Scholar] [CrossRef]



| Steel Product | C | N | Cr | Ti | Mo | Nb | Si | Mn | GS in RT (ASTM) | GS in Sag (ASTM) | η-phase solvus T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EN 1.4509 | 0.01 | 0.02 | 18.0 | 0.12 | 0.03 | 0.36 | 0.51 | 0.53 | 6.0 | 2.0 | 851 |
| EN 1.4521 | 0.01 | 0.02 | 17.9 | 0.13 | 2.1 | 0.39 | 0.48 | 0.49 | 7.8 | <0 | 906 |
| EN 1.4622 | 0.02 | 0.02 | 20.8 | 0.17 | 0.03 | 0.36 | 0.48 | 0.40 | 8.8 | 4.3 | 846 |
| REF4622 | 0.02 | 0.02 | 21.0 | 0.10 | 0.12 | 0.40 | 0.43 | 0.33 | 6.1 | 4.5 | 831 |
| High-Nb-SS | 0.02 | 0.02 | 21.0 | 0.20 | 0.02 | 0.80 | 0.80 | 0.32 | 5.0 | 4.0 | 1026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juuti, T.; Manninen, T.; Uusikallio, S.; Kömi, J.; Porter, D. New Ferritic Stainless Steel for Service Temperatures up to 1050 °C Utilizing Intermetallic Phase Transformation. Metals 2019, 9, 664. https://doi.org/10.3390/met9060664
Juuti T, Manninen T, Uusikallio S, Kömi J, Porter D. New Ferritic Stainless Steel for Service Temperatures up to 1050 °C Utilizing Intermetallic Phase Transformation. Metals. 2019; 9(6):664. https://doi.org/10.3390/met9060664
Chicago/Turabian StyleJuuti, Timo, Timo Manninen, Sampo Uusikallio, Jukka Kömi, and David Porter. 2019. "New Ferritic Stainless Steel for Service Temperatures up to 1050 °C Utilizing Intermetallic Phase Transformation" Metals 9, no. 6: 664. https://doi.org/10.3390/met9060664
APA StyleJuuti, T., Manninen, T., Uusikallio, S., Kömi, J., & Porter, D. (2019). New Ferritic Stainless Steel for Service Temperatures up to 1050 °C Utilizing Intermetallic Phase Transformation. Metals, 9(6), 664. https://doi.org/10.3390/met9060664