Material Removal Characteristic of Laser Cladding Cobalt-Based Alloy in the Photochemical Process
Abstract
:1. Introduction
2. Materials and Methods
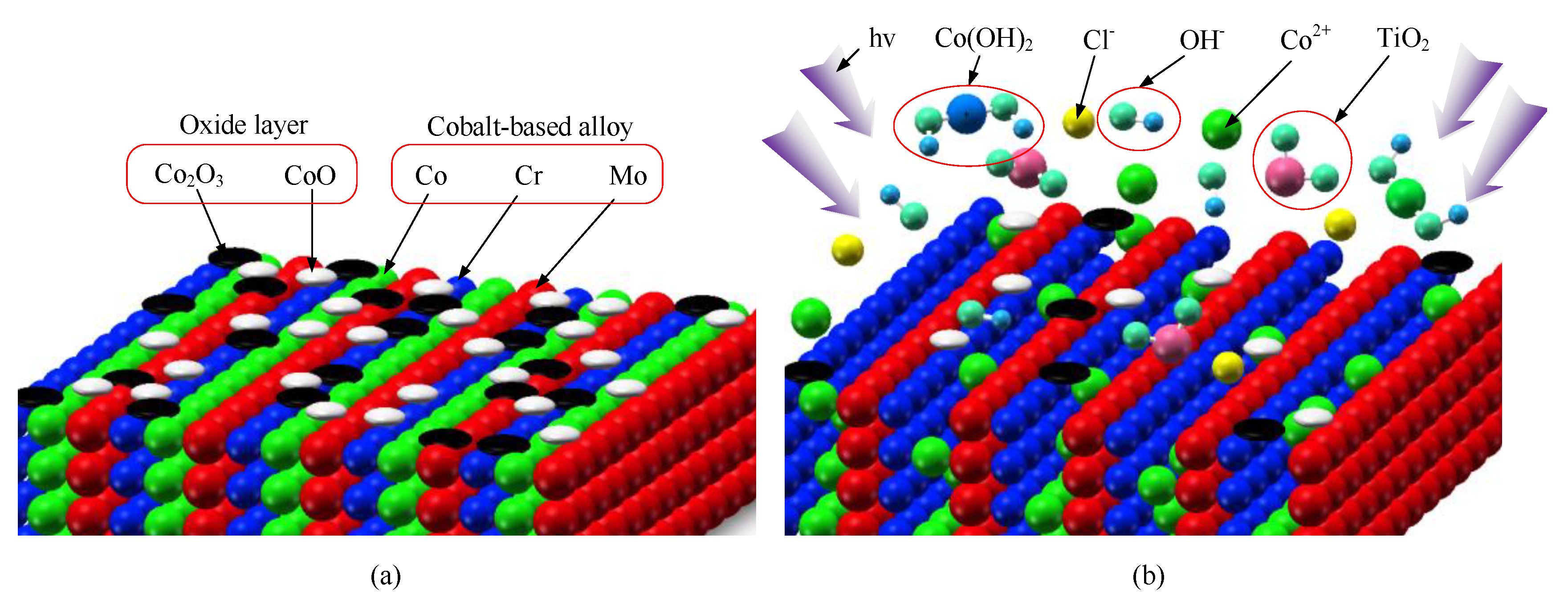
2.1. Photocatalytic Material Removal Principle
2.2. Photocatalytic Cobalt-Based Alloy Material Removal Test
Chemical Corrosion Solution Configuration Test
2.3. Pneumatic Wheel Processing Test
3. Results and Discussion
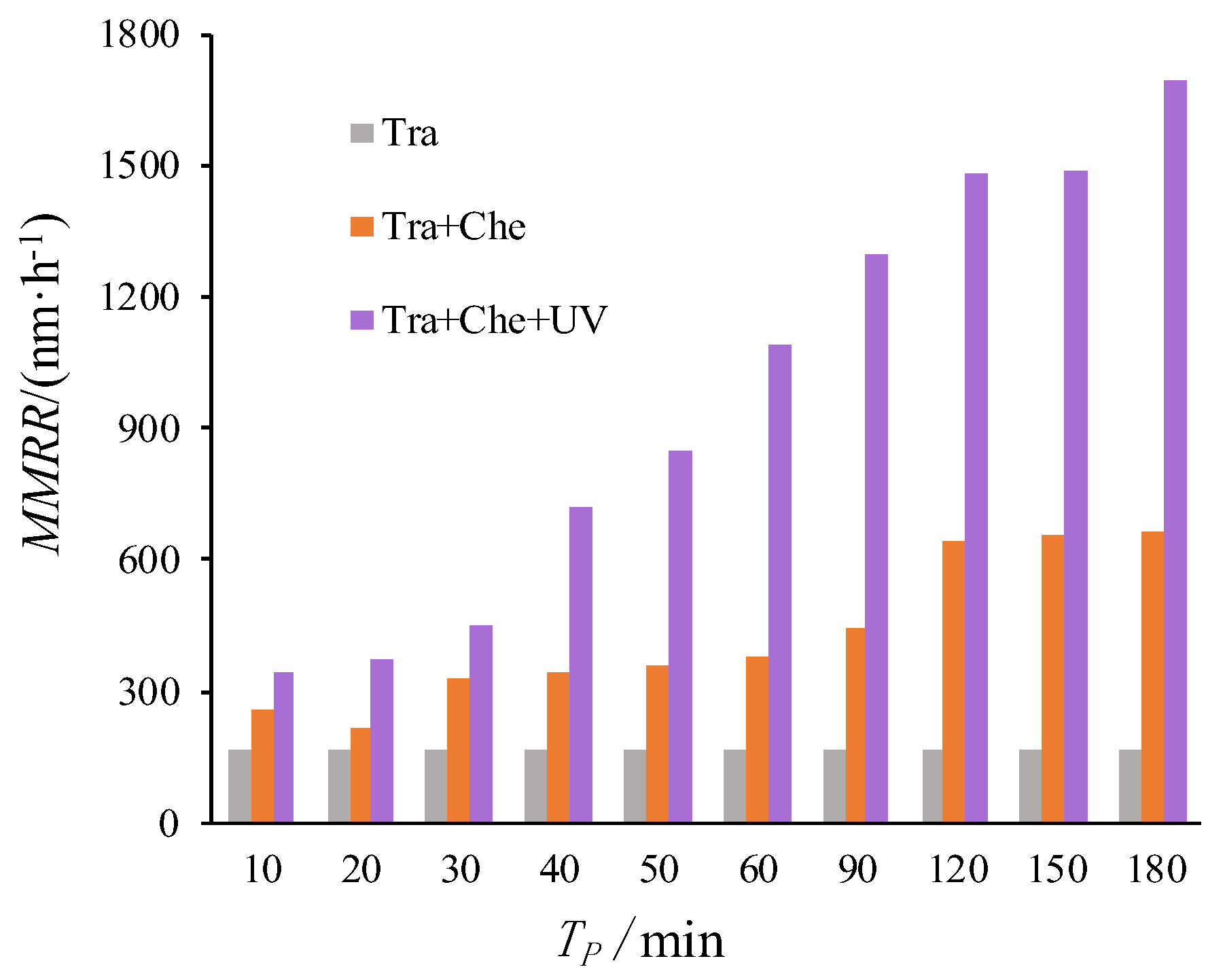
3.1. Chemical Corrosion Solution Configuration Test
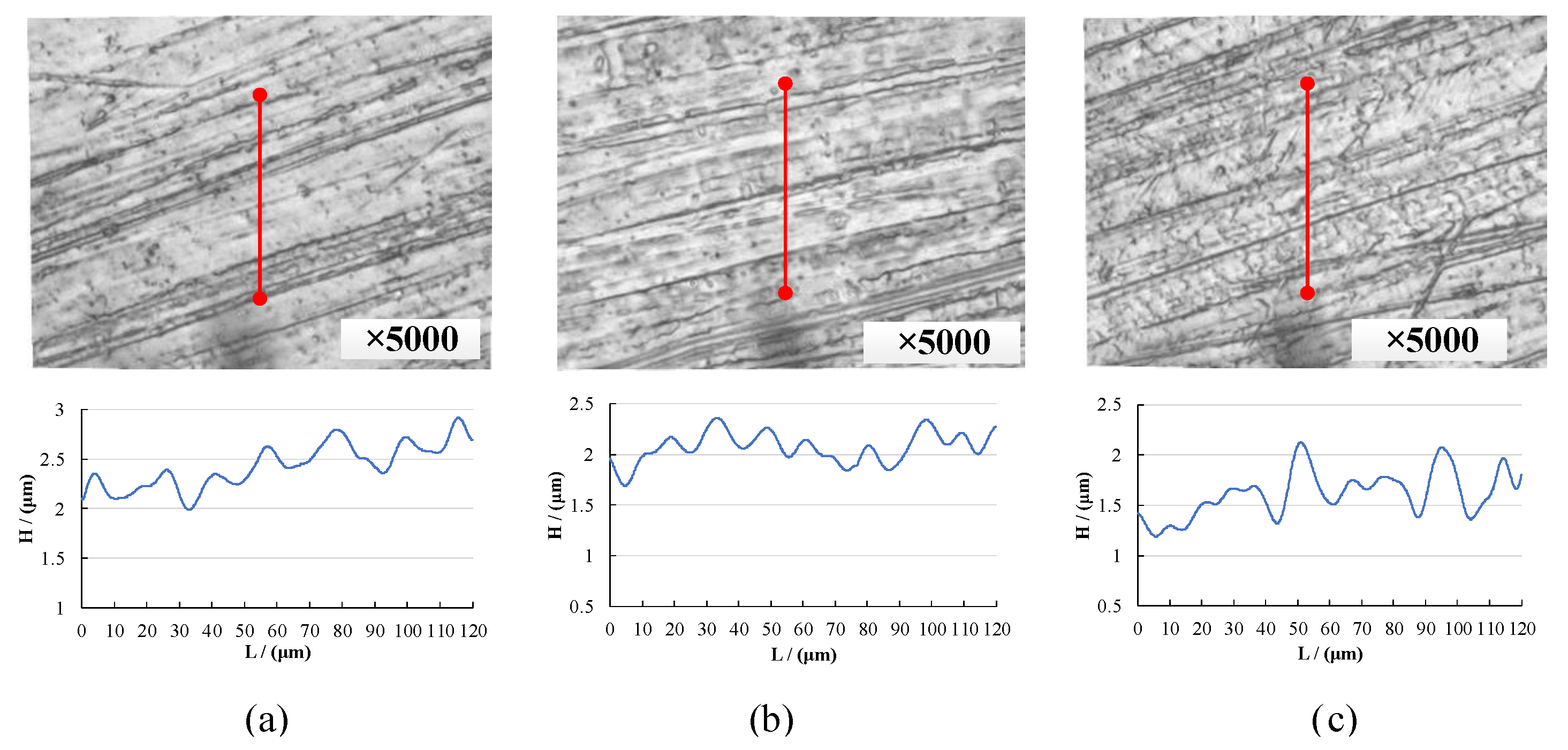
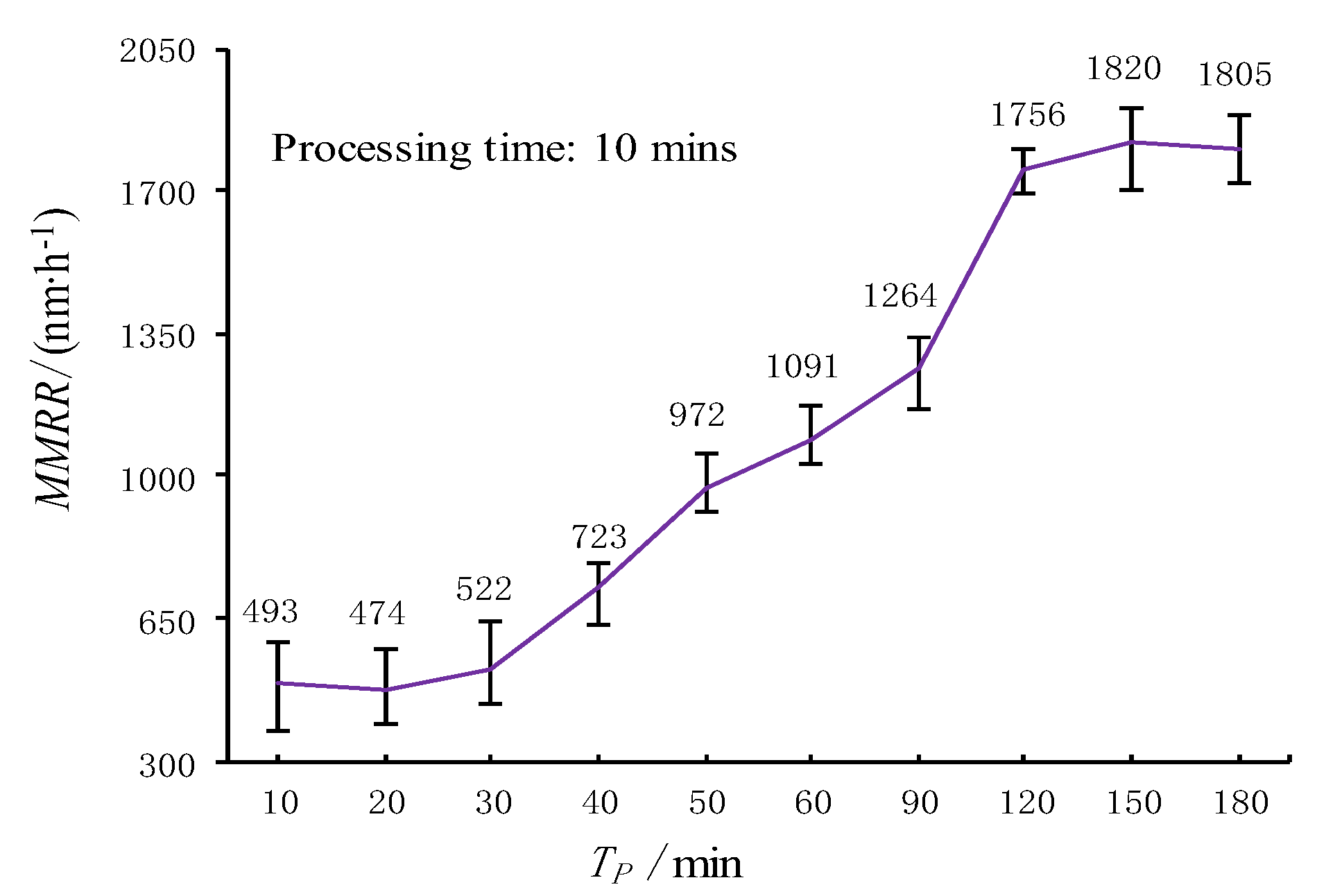
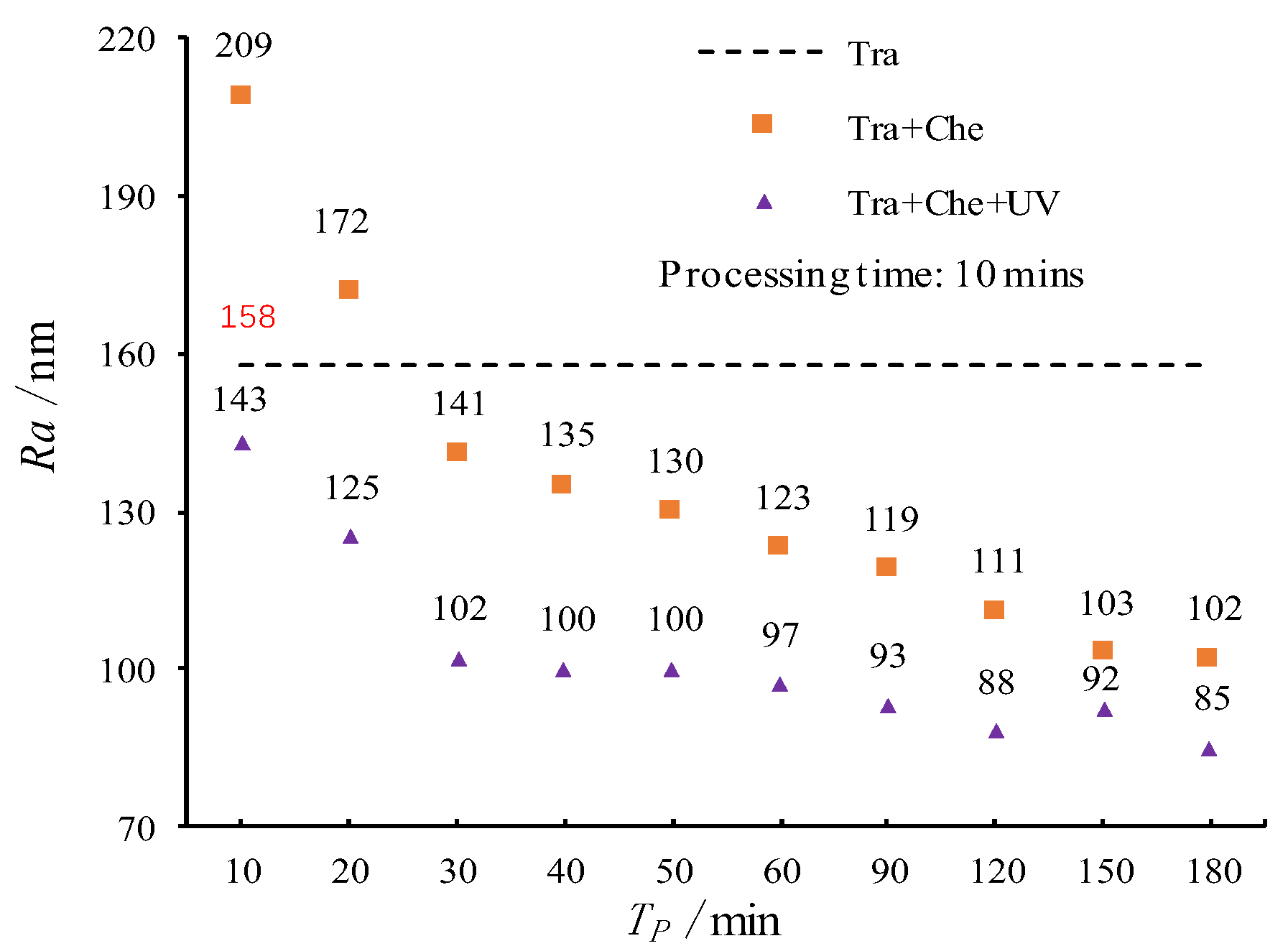
3.2. Pneumatic Wheel Processing Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.R.; Lian, G.F.; Jiang, J.B.; Wang, Q.T. Simplification and experimental investigation of geometrical surface smoothness model for multi-track laser cladding processes. J. Manuf. Process. 2018, 36, 621–628. [Google Scholar] [CrossRef]
- Zhang, L.B.; Lv, H.P.; Tan, D.P.; Xu, F.; Chen, J.L.; Bao, G.J.; Cai, S.B. An adaptive quantum genetic algorithm for task sequence planning of complex assembly systems. Electron. Lett. 2018, 54, 870–871. [Google Scholar] [CrossRef]
- Li, C.; Ji, S.M.; Tan, D.P. Softness abrasive flow method oriented to tiny scale mold structural surface. Int. J. Adv. Manuf. Technol. 2012, 61, 975–987. [Google Scholar] [CrossRef]
- Tan, D.P.; Chen, S.T.; Bao, G.J.; Zhang, L.B. An embedded lightweight GUI component library and the ergonomics optimization method for industry process monitoring. Front. Inform. Technol. Electron. Eng. 2018, 19, 604–625. [Google Scholar] [CrossRef]
- Ge, J.Q.; Tan, D.P.; Ji, S.M. A gas-liquid-solid three-phase abrasive flow processing method based on bubble collapsing. Int. J. Adv. Manuf. Technol. 2018, 95, 1069–1085. [Google Scholar] [CrossRef]
- Tan, D.P.; Zhang, L.B. A WP-based nonlinear vibration sensing method for invisible liquid steel slag detection. Sens. Actuators B Chem. 2014, 202, 1257–1269. [Google Scholar] [CrossRef]
- Tan, D.P.; Ji, S.M.; Fu, Y.Z. An improved soft abrasive flow finishing method based on fluid collision theory. Int. J. Adv. Manuf. Technol. 2016, 85, 1261–1274. [Google Scholar] [CrossRef]
- Shi, S.H.; Xu, A.Q.; Fan, J.W.; Wei, H.P. Study of cobalt-free, Fe-based alloy powder used for sealing surfaces of nuclear valves by laser cladding. Nucl. Eng. Des. 2012, 245, 8–12. [Google Scholar] [CrossRef]
- Huebner, J.; Kata, D.; Kusiński, J.; Rutkowski, P.; Lis, J. Microstructure of laser cladded carbide reinforced Inconel 625 alloy for turbine blade application. Ceram. Int. 2017, 43, 8677–8684. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, J.; Xiong, Y.B.; Zhang, J.S.; Meng, Y. Analysis of service condition of large hot forging die and refabrication of die by bimetal-layer weld surfacing technology with a cobalt-based superalloy and a ferrous alloy. J. Manuf. Process. 2018, 31, 731–743. [Google Scholar] [CrossRef]
- Tan, D.P.; Li, L.; Zhu, Y.L.; Zheng, S.; Ruan, H.J.; Jiang, X.Y. An embedded cloud database service method for distributed industry monitoring. IEEE Trans. Ind. Inform. 2018, 14, 2881–2893. [Google Scholar] [CrossRef]
- Li, L.; Qi, H.; Yin, Z.; Li, D.; Zhu, Z.; Tangwarodomnukun, V.; Tan, D. Investigation on the multiphase sink vortex Ekman pumping effects by CFD-DEM coupling method. Powder Technol. 2019, in press. [Google Scholar] [CrossRef]
- Xu, G.J.; Yang, W.Q.; Hang, Z.X.; Liu, X.Y.; Chi, H.L.; Ou, X.S. Performance of Clad Layer Using Mixed Powder of Stellite 6 and VC. J. Mech. Eng. 2017, 53, 165–170. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.H.; Ji, S.M.; Pan, Y.; Hang, W.; Chen, G.D. Research on machining characteristic of double-layer elastomer in pneumatic wheel method. Int. J. Adv. Manuf. Technol. 2017, 92, 1329–1338. [Google Scholar] [CrossRef]
- Hang, W.; Zhou, L.B.; Shimizu, J.; Yuan, J.L. A robust procedure of data analysis for micro/nano indentation. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 2013, 37, 408–414. [Google Scholar] [CrossRef]
- Li, C.; Ji, S.M.; Tan, D.P. Multiple-loop Digital Control Method for a 400-Hz Inverter System Based on Phase Feedback. IEEE Trans. Power Electron. 2013, 28, 408–417. [Google Scholar] [CrossRef]
- Zeng, S.; Blunt, L. Experimental investigation and analytical modelling of the effects of process parameters on material removal rate for bonnet polishing of cobalt chrome alloy. Precis. Eng. J. Int. Soc. Precis. Eng. Nanotechnol. 2014, 38, 348–355. [Google Scholar] [CrossRef]
- Zeng, X.; Ji, S.M.; Jin, M.S.; Tan, D.P.; Li, J.H.; Zeng, W.T. Mechanical Characteristic Analysis of Softness Consolidation Abrasives Pneumatic Wheel. J. Mech. Eng. 2014, 50, 170–177. [Google Scholar] [CrossRef]
- Tan, D.P.; Li, P.Y.; Ji, Y.X.; Wen, D.H.; Li, C. SA-ANN-based slag carry-over detection method and the embedded WME platform. IEEE Trans. Ind. Electron. 2013, 60, 4702–4713. [Google Scholar] [CrossRef]
- Ji, S.M.; Ge, J.Q.; Tan, D.P. Wall contact effects of particle-wall collision process in two-phase particle fluid. J. Zhejiang Univ.-Sci. A 2017, 18, 958–973. [Google Scholar] [CrossRef]
- Lee, C.; Park, S. Damage visualization of pipeline structures using laser-induced ultrasonic waves. Struct. Health Monit. 2015, 14, 475–488. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, P.F.; Zhao, W.; Luo, Y.Y.; Xie, Y.H. Experimental Study of Laser Cladding Methods on Water Erosion Resistance to Low Pressure Blades Materials of Steam Turbine. Adv. Condens. Matter Phys. 2014, 2014, 318–327. [Google Scholar] [CrossRef]
- Tan, D.; Li, L.; Zhu, Y.; Zheng, S.; Yin, Z.; Li, D. Critical penetration condition and Ekman suction-extraction mechanism of sink vortex. J. Zhejiang Univ.-Sci. A 2019, 20, 61–72. [Google Scholar] [CrossRef]
- Chen, S.T.; Tan, D.P. A SA-ANN-based modeling method for human cognition mechanism and the PSACO cognition algorithm. Complexity 2018, 2018, 6264124. [Google Scholar] [CrossRef]
- Kato, T.; Higashijima, G.; Yazawa, T.; Otsubo, T.; Tanaka, K. Proposal of Disturb-ance-Compensating and Energy-Saving Control Method of Air Turbine Spindle and Evaluation of Its Energy Consumption. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 2015, 43, 439–447. [Google Scholar] [CrossRef]
- Tan, D.P.; Zhang, L.B.; Ai, Q.L. An embedded self-adapting network service framework for networked manufacturing system. J. Intell. Manuf. 2019, 30, 539–556. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.S.; Tan, D.P.; Yuan, Z.M. Gas compensation-based abrasive flow processing method for complex titanium alloy surfaces. Int. J. Adv. Manuf. Technol. 2017, 92, 3385–3397. [Google Scholar] [CrossRef]
- Tang, C.B.; Zhang, C.; Ma, A.M.; Liu, D.X.; Liu, C.S.; Yu, S.M. Effect of Cobalt-based Alloy Coating on Wear Resistance and Corrosion Resistance of Titanium Alloy. Rare Met. 2017, 41, 620–628. [Google Scholar] [CrossRef]
- Tong, W.H.; Zhao, Z.L.; Zhang, X.Y.; Wang, J.; Guo, X.M.; Duan, X.H.; Liu, Y. Microstructure and Properties of Laser Cladding Cobalt Based Alloy Coating on Ductile Iron Surface. J. Met. 2017, 53, 472–478. [Google Scholar]
- Xu, J.L.; Zhou, J.Z.; Tan, W.S.; Huang, S.; Meng, X.K.; He, W.Y. Thermal Corrosion Resistance of Electromagnetic Stirring Assisted Laser Cladding Cobalt-Based Alloy Coatings. J. Opt. 2019, 39, 355–363. [Google Scholar]
- Zhou, Y.; Pan, G.; Shi, X.; Gong, H.; Luo, G.; Gu, Z. Chemical mechanical planarization (CMP) of on-axis Si-face SiC wafer using catalyst nanoparticles in slurry. Surf. Coat. Technol. 2014, 251, 48–55. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.Q.; Pan, G.S.; Lu, X.C. Mechanism of GaN CMP Based on H2O2 Slurry combined with UV Light. ECS J. Solid State Sci. Technol. 2015, 4, 112–117. [Google Scholar] [CrossRef]
- Ye, Z.F.; Zhou, Y.; Xu, L.; Pan, G. UV LED Assisted 4H-SiC Chemical Mechanical Polishing. Nanotechnol. Precis. Eng. 2017, 15, 342–346. [Google Scholar] [CrossRef]
- Chizhova, N.V.; Mal’Tseva, O.V.; Zav’Yalov, A.V.; Mamardashvili, N.Zh. Copper(II), cobalt(II), cobalt(III), and tin(IV) 5,10,15,20-tetraphenyl tetrabenzoporphyrinates: Synthesis and properties. Russ. J. Inorg. Chem. 2017, 62, 683–687. [Google Scholar] [CrossRef]
- Sun, L.; Zhai, J.L.; Li, H.Y.; Zhao, Y.; Yang, H.J.; Yu, H.W. Study of Homologous Elements: Fe, Co, and Ni Dopant Effects on the Photoreactivity of TiO2 Nanosheets. ChemCatChem 2014, 6, 339–347. [Google Scholar] [CrossRef]
- Dan, Xu.; Yao, K.L.; Gao, G.Y.; Yang, L. Electronic and optical properties of N-doped, Co-doped as well as N,Co-codoped rutile TiO2. J. Magn. Magn. Mater. 2013, 335, 118–124. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, X.; Wei, Z.; Li, X.; Xi, X.; Hu, R.; Tang, B.; Wang, R.; Wang, S.; Wang, T.; et al. Photocatalytic Hydrogen Production Performance of Cobalt-Doped Titanium Dioxide. J. Phys. Chem. 2011, 27, 2406–2410. [Google Scholar]
- Li, J.B.; Xiao, Q.S.; Li, L.C.; Shen, J.; Hu, D. Novel ternary composites: Preparation, performance and application of ZnFe2O4/TiO2/polyaniline. Appl. Surf. Sci. 2015, 331, 108–114. [Google Scholar] [CrossRef]
- Wang, W.S.; Wang, D.H.; Qu, W.G.; Lu, L.Q.; Xu, A.W. Large Ultrathin Anatase TiO2 Nanosheets with Exposed {001 Facets on Graphene for Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. C. 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Li, S.T.; Zhou, G.W.; Wei, Y.Q. Study on preparation and photocatalytic properties of Co2+–TiO2. Appl. Chem. Ind. 2005, 34, 737–740. [Google Scholar] [CrossRef]
- Zeng, X.; Ji, S.M.; Jin, M.S.; Tan, D.P.; Ge, J.Q. Research on dynamic characteristic of softness con-solidation abrasives in machining process. Int. J. Adv. Manuf. Technol. 2016, 82, 1115–1125. [Google Scholar] [CrossRef]
- Zeng, X.; Ji, S.M.; Tan, D.P.; Jin, M.S.; Wen, D.H.; Zhang, L. Softness consolidation abrasives material removal characteristic oriented to laser hardening surface. Int. J. Adv. Manuf. Technol. 2013, 69, 2323–2332. [Google Scholar] [CrossRef]
- Zeng, X.; Ji, S.M.; Jin, M.S.; Tan, D.P.; Li, J.H.; Zeng, W.T. Investigation on Machining Characteristic of Pneumatic Wheel Based on Softness Consolidation Abrasives. Int. J. Precis. Eng. Manuf. 2014, 15, 2031–2039. [Google Scholar] [CrossRef]
- Tao, Y.J.; Gan, X.J.; Zhang, X.D.; Hu, L.F.; Peng, J.; Wang, J.; Feng, H. Effects of UV Irradiation on the Molecular Structure and Tensile Properties of Nucleated Polypropylene. Adv. Mater. Res. 2014, 905, 82–87. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, H.D.; Dornfeld, D.A. Semi-empirical material removal rate distribution model for SiO2, chemical mechanical polishing (CMP) processes. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 2013, 37, 483–490. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.Q.; Pan, G.S.; Lu, X.C. Effect of photocatalytic oxidation technology on GaN CMP. Appl. Surf. Sci. 2016, 361, 18–24. [Google Scholar] [CrossRef]



| Group | H2O | TiO2 | Basic Yellow 40 |
|---|---|---|---|
| (g) | (g) | (g) | |
| A | 245 | 5 | 0 |
| B | 245 | 0 | 5 |
| C | 245 | 5 | 5 |
| Balloon Diameter | Airbag Elastic Modulus | Wheel Abrasive Material | Abrasive Diameter | Air Pressure | |||
|---|---|---|---|---|---|---|---|
| (mm) | (MPa) | - | (μm) | (MPa) | |||
| 50 | 11.9 | Al2O3 | 75 | 0.2 | |||
| Pressing Amount | Wheel Inclination | Wheel Speed | Processing Time | ||||
| (mm) | (Rad) | (r·min−1) | (min) | ||||
| 0.5 | 0.5 | 0.4 | 10 | ||||
| Scheme | Corrosion Liquid | UV | Time of Reaction | Content of Co2+ |
|---|---|---|---|---|
| /h | /mg·L−1 | |||
| 1 | A | Off | 3 | 0.39 |
| 2 | A | On | 3 | 0.05 |
| 3 | B | Off | 3 | 12 |
| 4 | B | On | 3 | 15 |
| 5 | C | Off | 3 | 23 |
| 6 | C | On | 3 | 64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Li, Z.; Xi, F.; Ji, S.; Qiu, L.; Shi, M.; Zheng, Q.; Qiu, W. Material Removal Characteristic of Laser Cladding Cobalt-Based Alloy in the Photochemical Process. Metals 2019, 9, 657. https://doi.org/10.3390/met9060657
Zeng X, Li Z, Xi F, Ji S, Qiu L, Shi M, Zheng Q, Qiu W. Material Removal Characteristic of Laser Cladding Cobalt-Based Alloy in the Photochemical Process. Metals. 2019; 9(6):657. https://doi.org/10.3390/met9060657
Chicago/Turabian StyleZeng, Xi, Zhuo Li, Fengfei Xi, Shiming Ji, Lei Qiu, Meng Shi, Qianqian Zheng, and Wenbin Qiu. 2019. "Material Removal Characteristic of Laser Cladding Cobalt-Based Alloy in the Photochemical Process" Metals 9, no. 6: 657. https://doi.org/10.3390/met9060657
APA StyleZeng, X., Li, Z., Xi, F., Ji, S., Qiu, L., Shi, M., Zheng, Q., & Qiu, W. (2019). Material Removal Characteristic of Laser Cladding Cobalt-Based Alloy in the Photochemical Process. Metals, 9(6), 657. https://doi.org/10.3390/met9060657




