Tribochemical Behavior of Pure Magnesium During Sliding Friction
Abstract
:1. Introduction
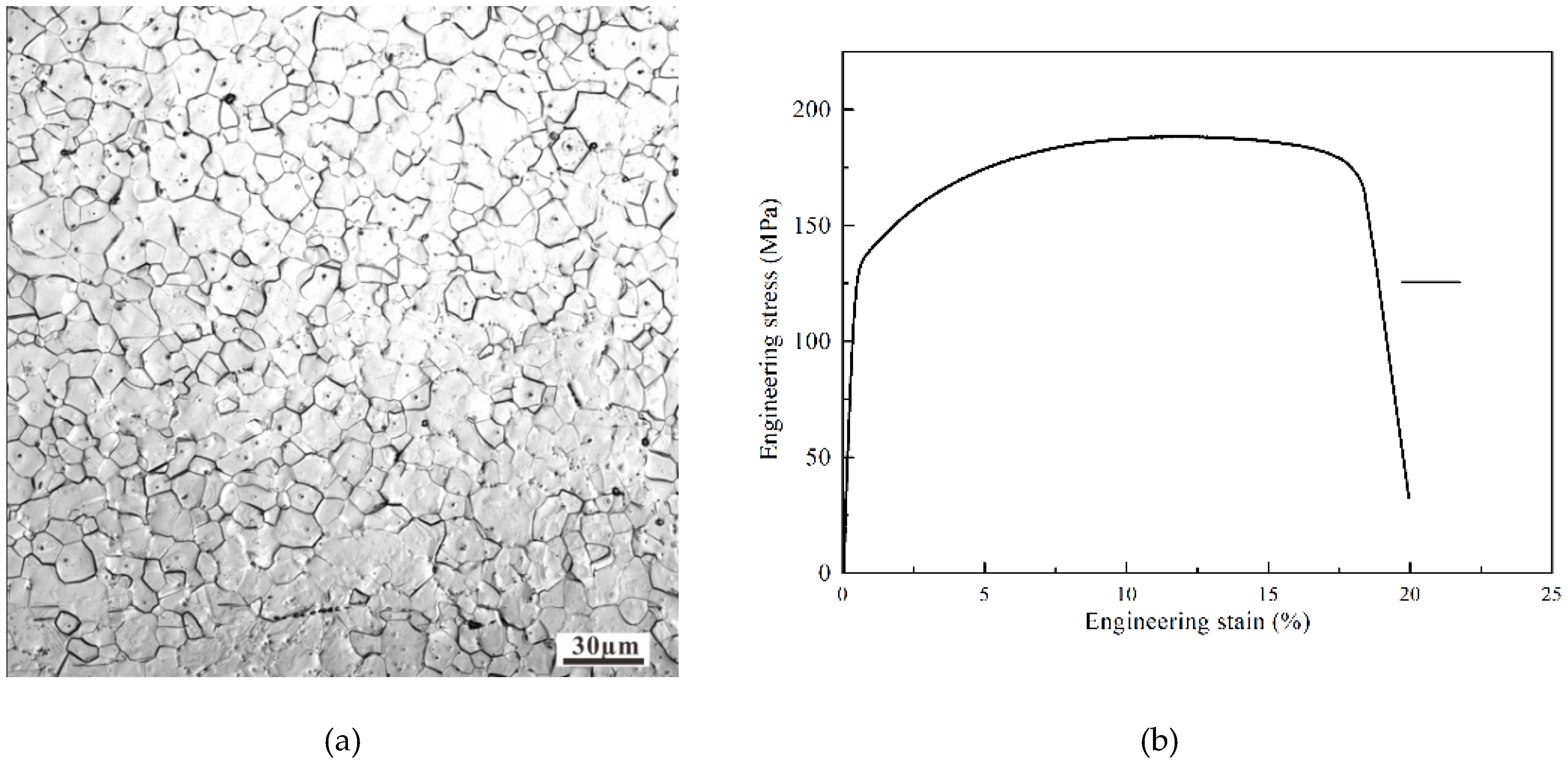
2. Material and Methods
3. Results and Discussion
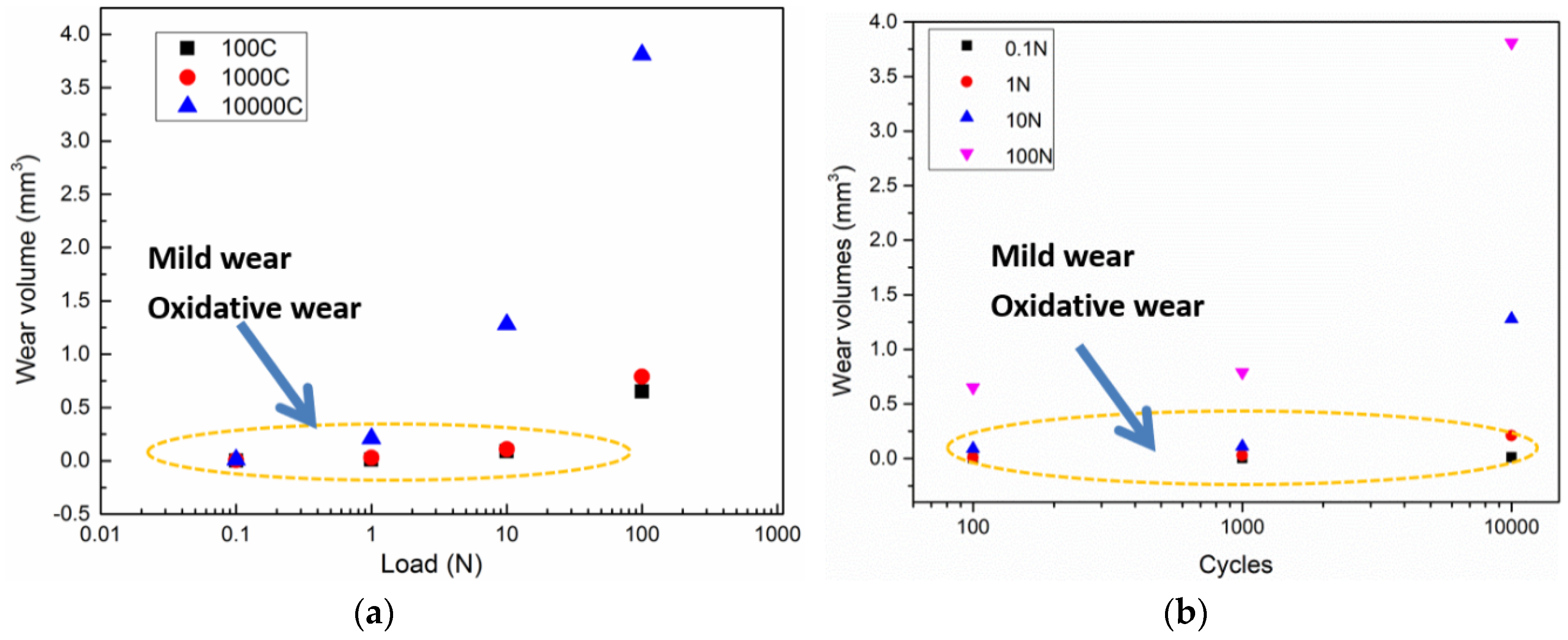
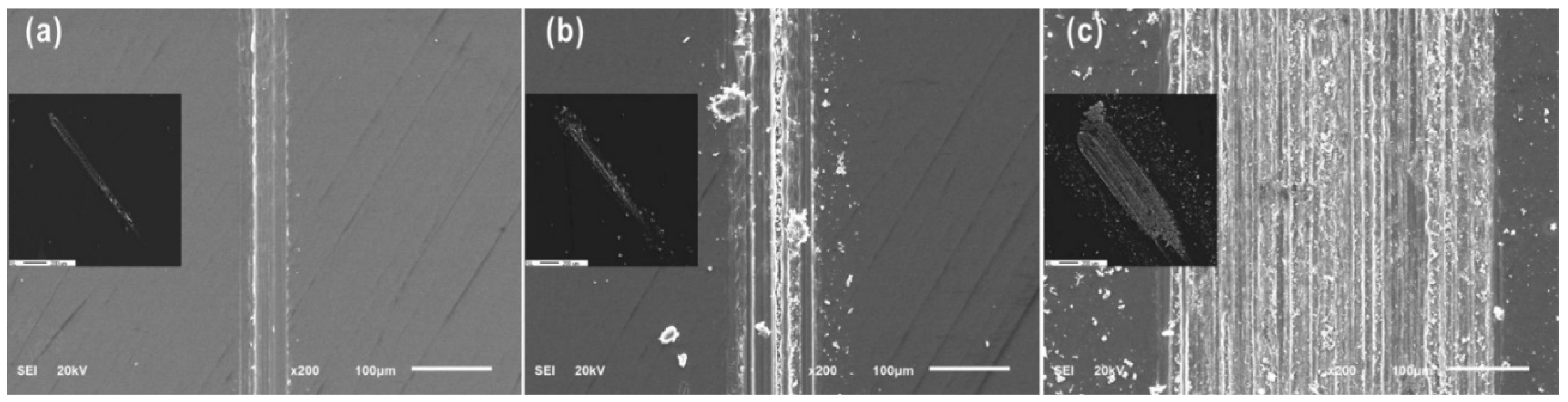
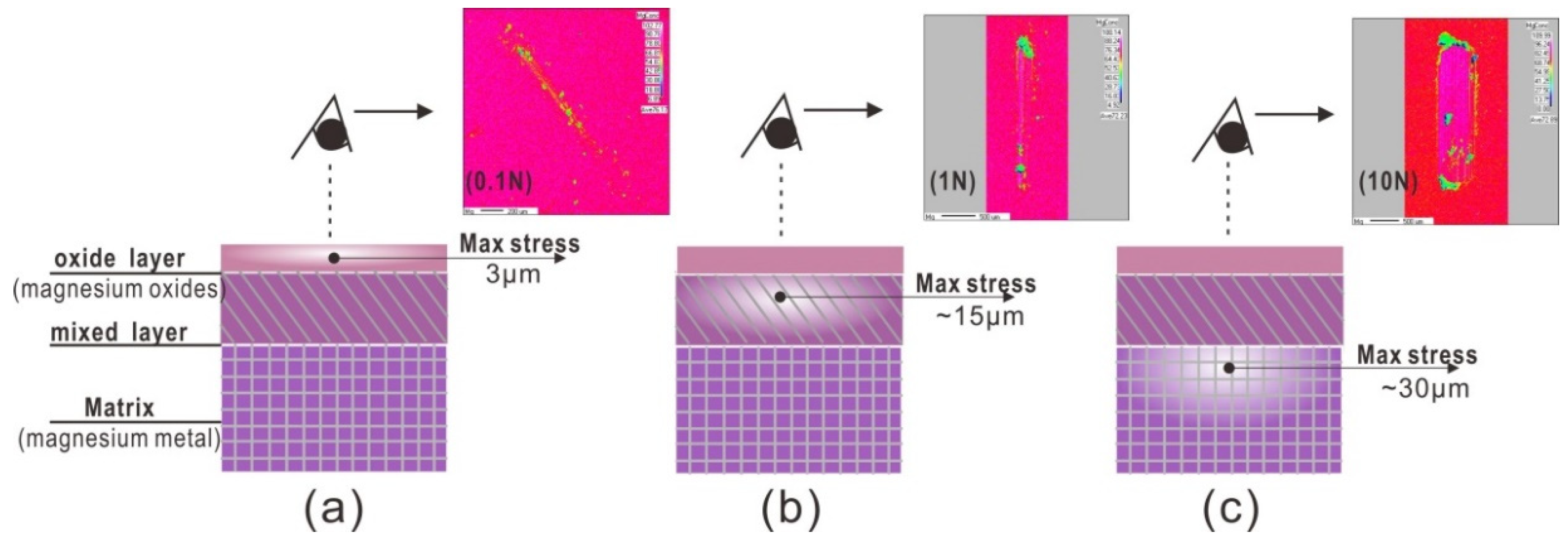
3.1. Wear Behavior
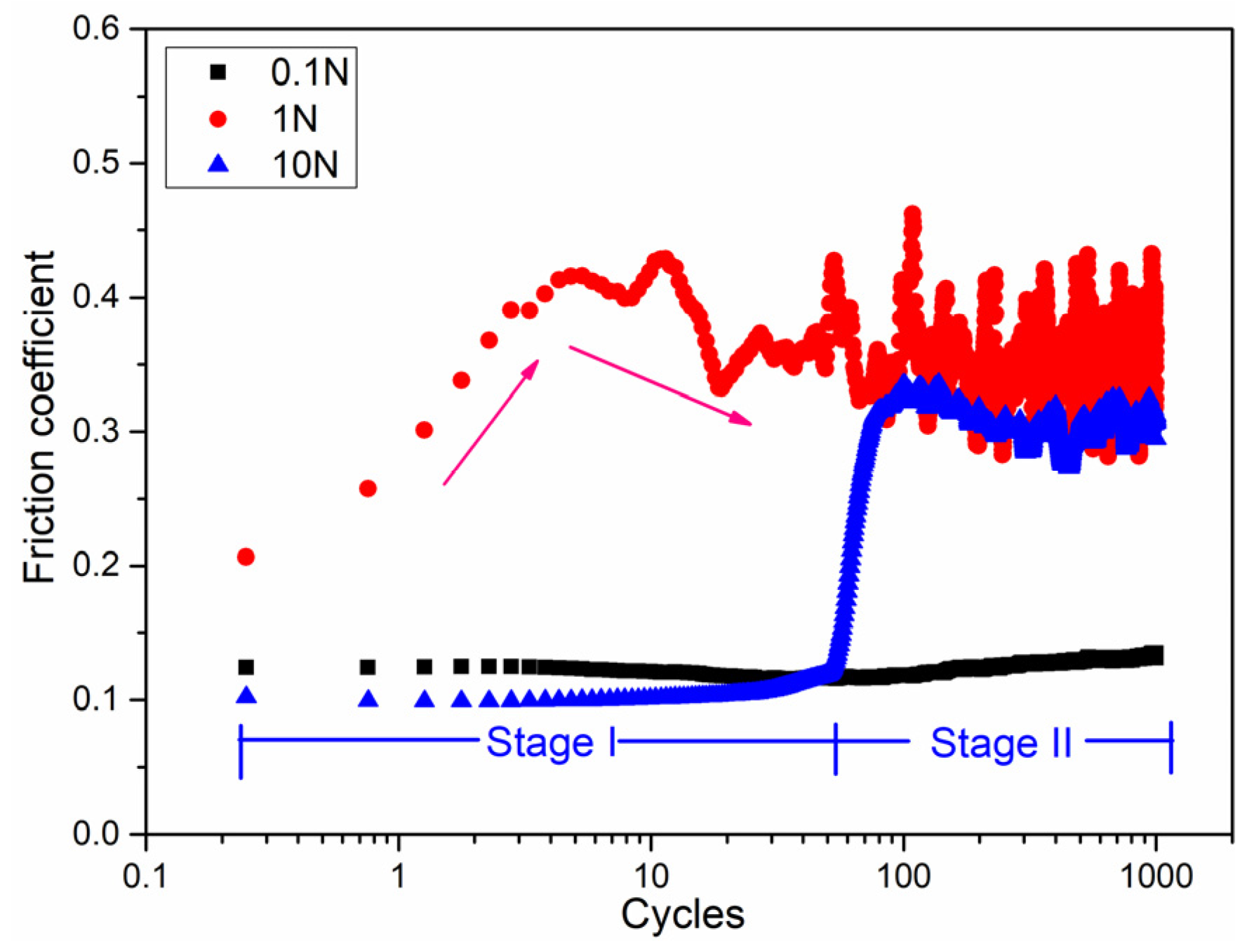
3.2. Tribochemical Behavior
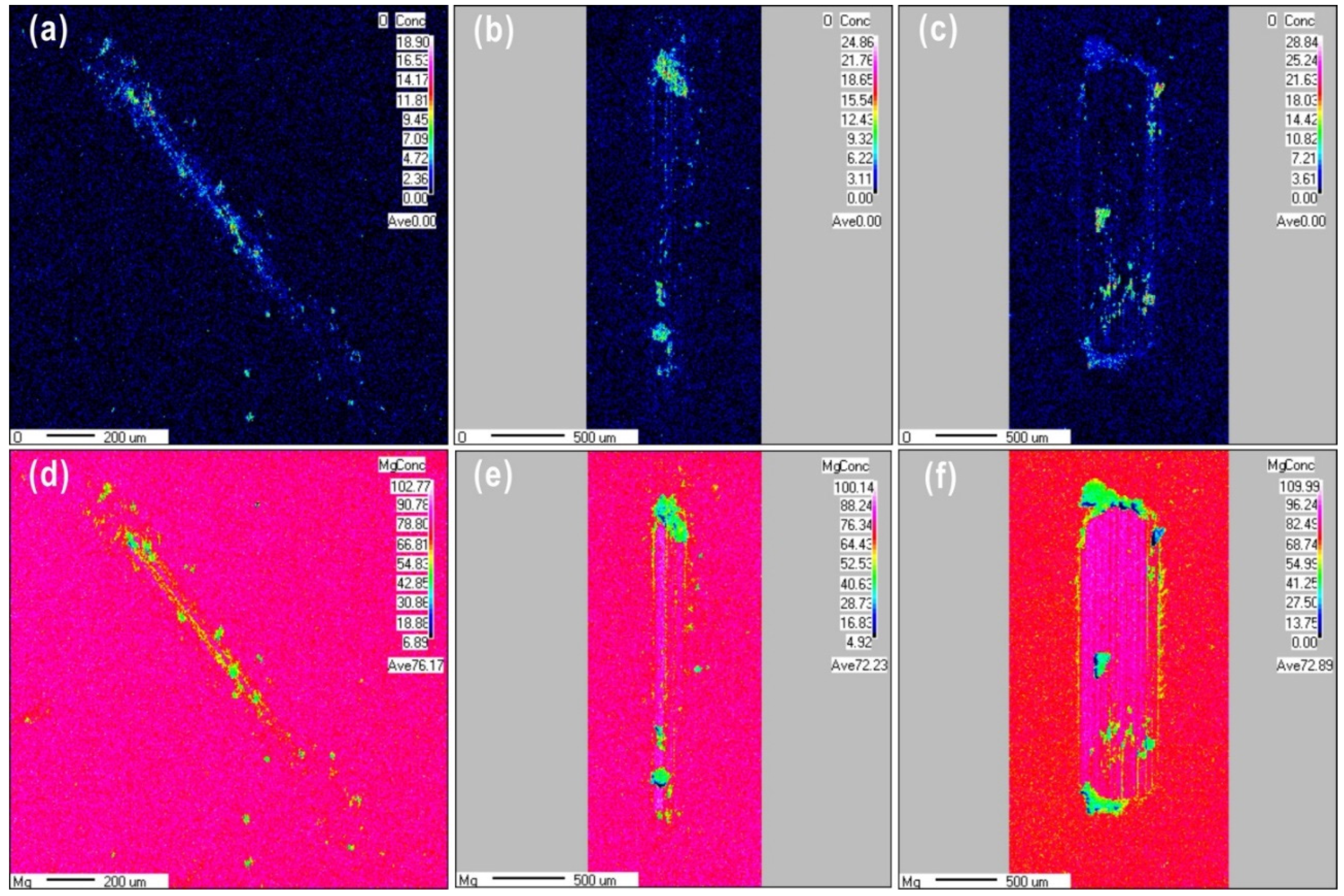
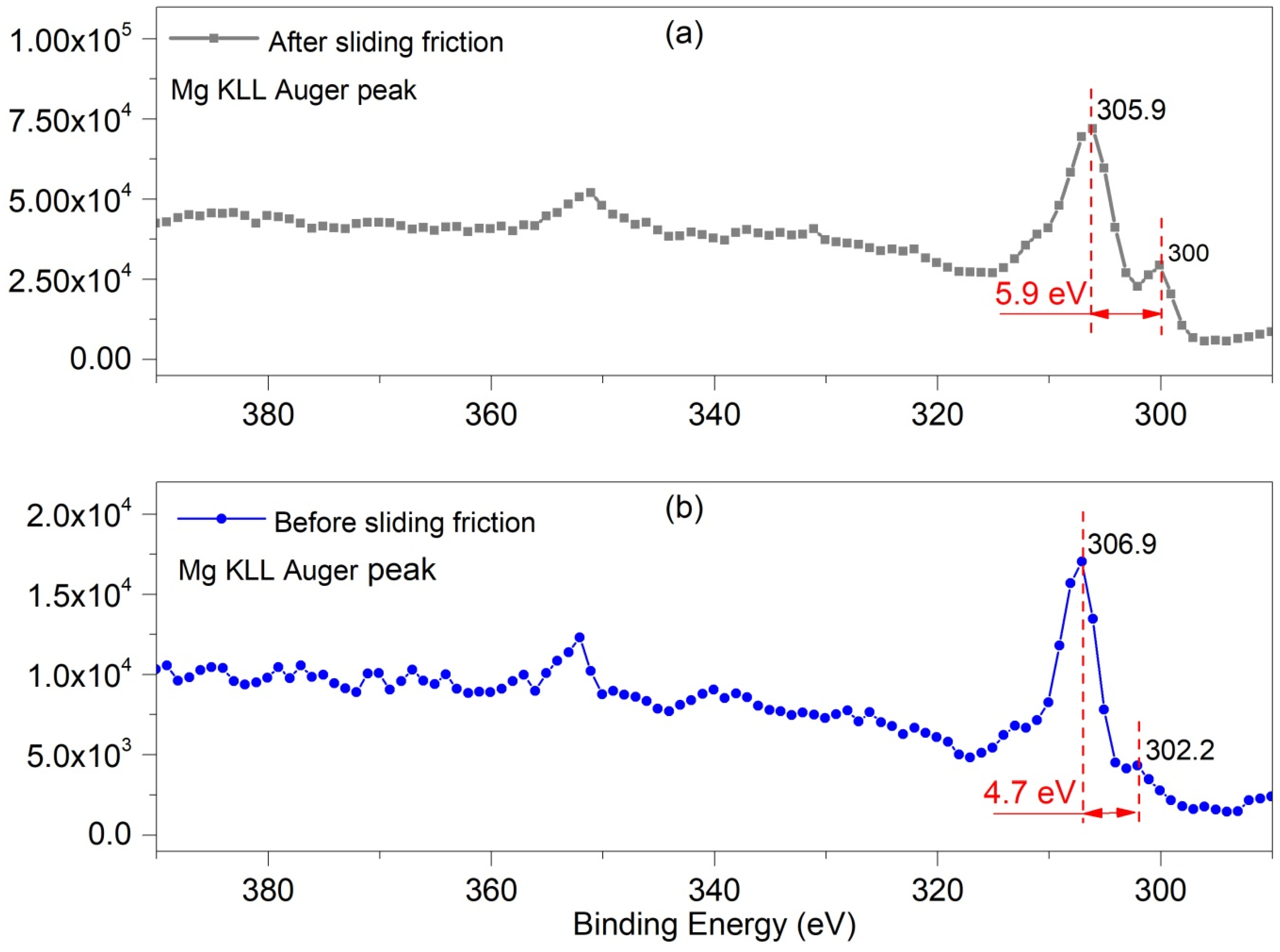
3.2.1. Surface Chemical Composition
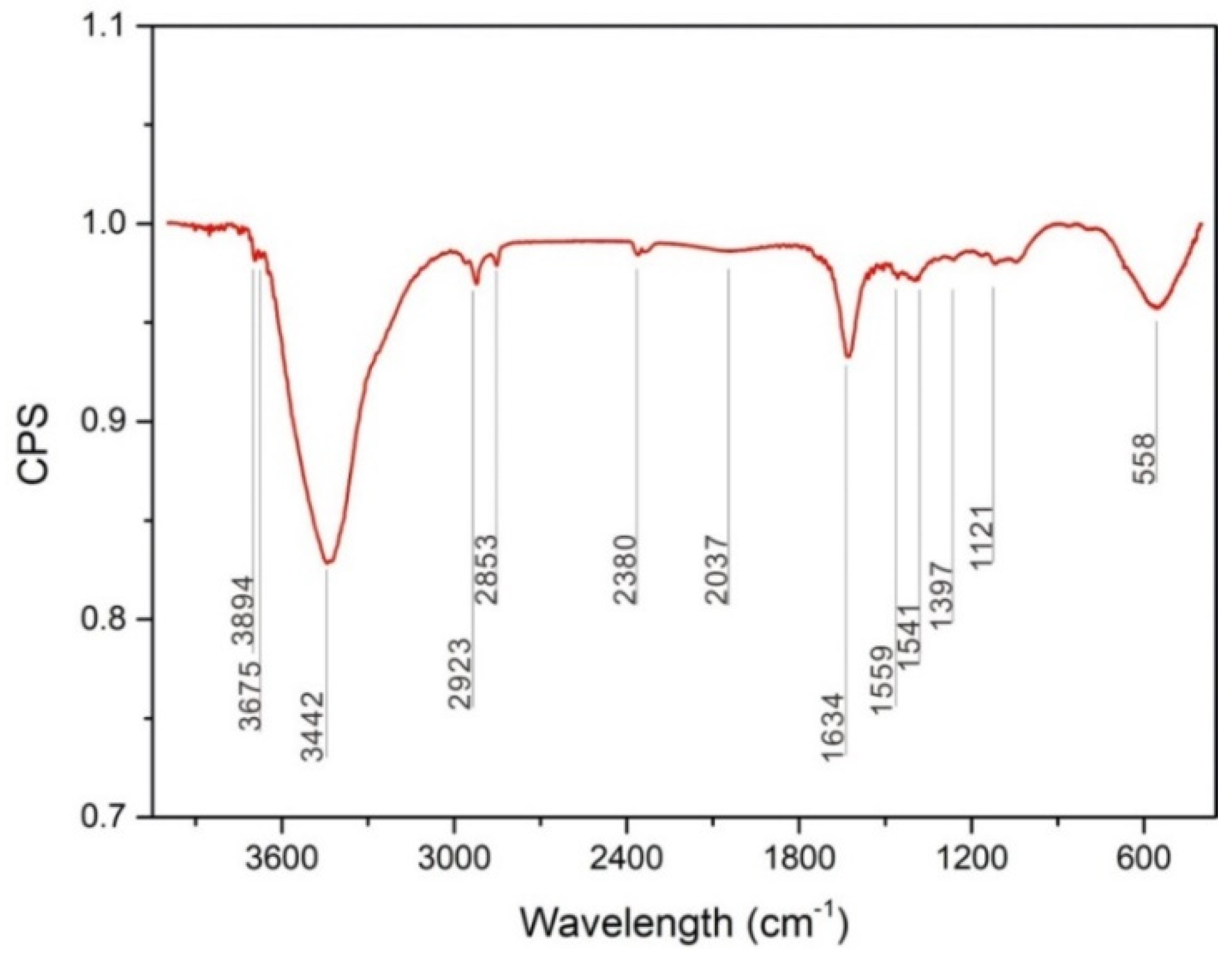
3.2.2. FT-IR spectra
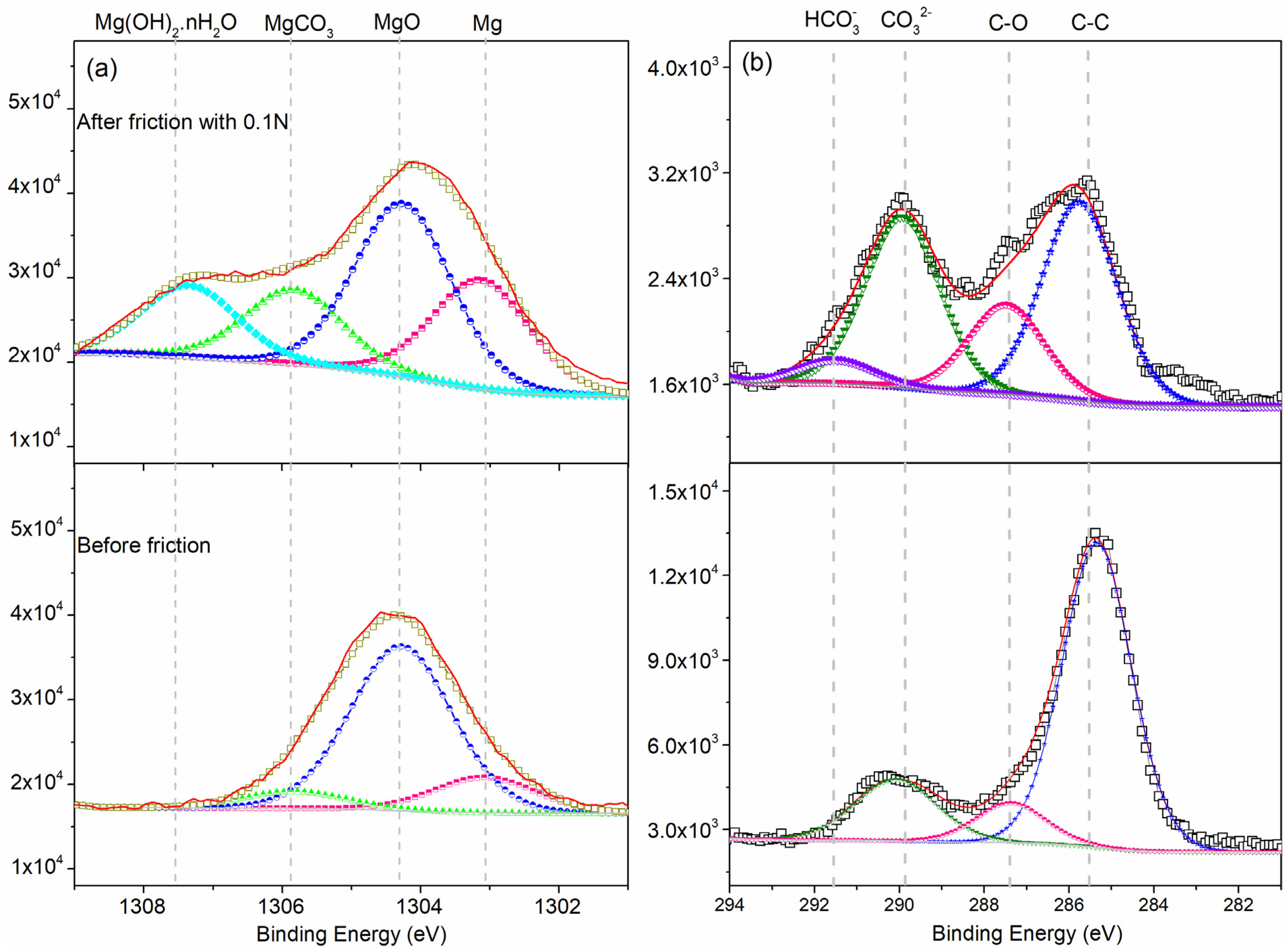
3.2.3. XPS
4. Conclusions
- (1)
- Under an air atmosphere, the typical tribochemical behavior was tribo-oxidation, which frequently observed in the region of mild wear corresponding to the oxidative wear mechanism. As the load increased, the wear mechanism transformed from oxidative to abrasive wear.
- (2)
- During tribo-oxidation, the tribofilm on the worn zone of the pure Mg acted as a third body layer and fine particle debris was observed. The tribofilm had shown a stable low friction coefficient. As the tribo-film was damaged with increasing load or number of cycles, the friction coefficient became unstable and increased steeply due to wear mechanism transition to abrasive wear.
- (3)
- The third body layer formed at the load of 0.1 N consisted of Mg(OH)2·nH2O, Mg(OH)2, MgO, MgCO3, and metallic Mg. The reciprocating sliding friction exposed fresh magnesium metal, resulting in the formation of Mg(OH)2·nH2O, while MgCO3 decomposed.
Author Contributions
Funding
Conflicts of Interest
References
- Jost, H.P. Tribology—Origin and future. Wear 1990, 136, 1–7. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Akgul, Y.; Turen, Y.; Ahlatci, H. The effect of GNPs on wear and corrosion behaviors of pure magnesium. J. Alloy Compd. 2017, 724, 14–23. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- López, A.D.F.; Lehr, I.L.; Brugnoni, L.I.; Saidman, S.B. Improvement in the corrosion protection and bactericidal properties of AZ91D magnesium alloy coated with a microstructured polypyrrole film. J. Magnes. Alloys 2018, 6, 15–22. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Zuo, J.; Li, M. An AZ31 magnesium alloy coating for protecting polyimide from erosion-corrosion by atomic oxygen. Corros. Sci. 2018, 138, 170–177. [Google Scholar] [CrossRef]
- Sikdar, K.; Shekhar, S.; Balani, K. Fretting wear of Mg–Li–Al based alloys. Wear 2014, 318, 177–187. [Google Scholar] [CrossRef]
- Saini, S.P.; Kumar, S.; Barman, R.; Dixit, A.; Kumar, V. Oxidation Study of Mg-Li-Al based Alloy. Mater. Today Proc. 2016, 3, 3035–3044. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.-H. Investigation of surface oxide film on magnesium lithium alloy. J. Alloys Compd. 2009, 484, 585–590. [Google Scholar] [CrossRef]
- Newberg, J.T.; Starr, D.E.; Yamamoto, S.; Kaya, S.; Kendelewicz, T.; Mysak, E.R.; Porsgaard, S.; Salmeron, M.B.; Brown, G.E.; Nilsson, A.; et al. Formation of hydroxyl and water layers on MgO films studied with ambient pressure XPS. Surf. Sci. 2011, 605, 89–94. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.U.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferr. Metal Soc. 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and localized corrosion of magnesium alloys: A critical review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Wang, X.M.; Zeng, X.Q.; Zhou, Y.; Wu, G.S.; Yao, S.S.; Lai, Y.J. Early oxidation behaviors of Mg–Y alloys at high temperatures. J. Alloys Compd. 2008, 460, 368–374. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Sim, Y.H.M.; Gupta, M.; Lim, C.Y.H. Tribology characteristics of magnesium alloy AZ31B and its composites. Tribol. Int. 2015, 82, 464–471. [Google Scholar] [CrossRef]
- Hiratsuka, K.I.; Muramoto, K.I. Role of wear particles in severe-mild wear transition. Wear 2005, 259, 467–476. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, Z.B.; Peng, J.F.; Cao, B.B.; Jin, X.S.; Zhu, M.H. Tribo-chemical behavior of eutectoid steel during rolling contact friction. Appl. Surf. Sci. 2016, 388, 40–48. [Google Scholar] [CrossRef]
- Johnson, K.L.; Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Chang, L.; Cao, F.; Cai, J.; Liu, W.; Zhang, J.; Cao, C. Formation and transformation of Mg(OH)2 in anodic coating using FTIR mapping. Electrochem. Commun. 2009, 11, 2245–2248. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J.H.; Jang, E.; Lee, K.B.; Kim, J.H. MgCO3-crystal-containing mixed matrix membranes with enhanced CO2 permselectivity. Chem. Eng. J. 2017, 307, 503–512. [Google Scholar] [CrossRef]
- Thoms, H.; Epple, M.; Reller, A. Magnesium diolates as precursors for MgO: A low-temperature route. Thermochim. Acta 1997, 302, 195–200. [Google Scholar] [CrossRef]
- Wagner, C.; Muilenberg, G. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Waltham, MA, USA, 1979. [Google Scholar]
- Kantia, T.; Partanen, L.; Aksela, S.; Aksela, H. High resolution KLL Auger spectra of free sodium and magnesium atoms. J. Electron Spectrosc. Relat. Phenom. 2010, 180, 58–65. [Google Scholar] [CrossRef]
- Dubecký, F.; Kindl, D.; Hubík, P.; Mičušík, M.; Dubecký, M.; Boháček, P.; Vanko, G.; Gombia, E.; Nečas, V.; Mudroň, J. A comparative study of Mg and Pt contacts on semi-insulating GaAs: Electrical and XPS characterization. Appl. Surf. Sci. 2017, 395, 131–135. [Google Scholar] [CrossRef]
- Hoogewijs, R.; Fiermans, L.; Vennik, J. Electronic relaxation processes in the KLL′ auger spectra of the free magnesium atom, solid magnesium and MgO. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 171–183. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Liang, W.; Yin, Y.; Wang, L.; Chen, L.; Li, H. A new method of preparing anhydrous magnesium carbonate (MgCO3) under high pressure and its thermal property. J. Alloys Compd. 2017, 702, 346–351. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]

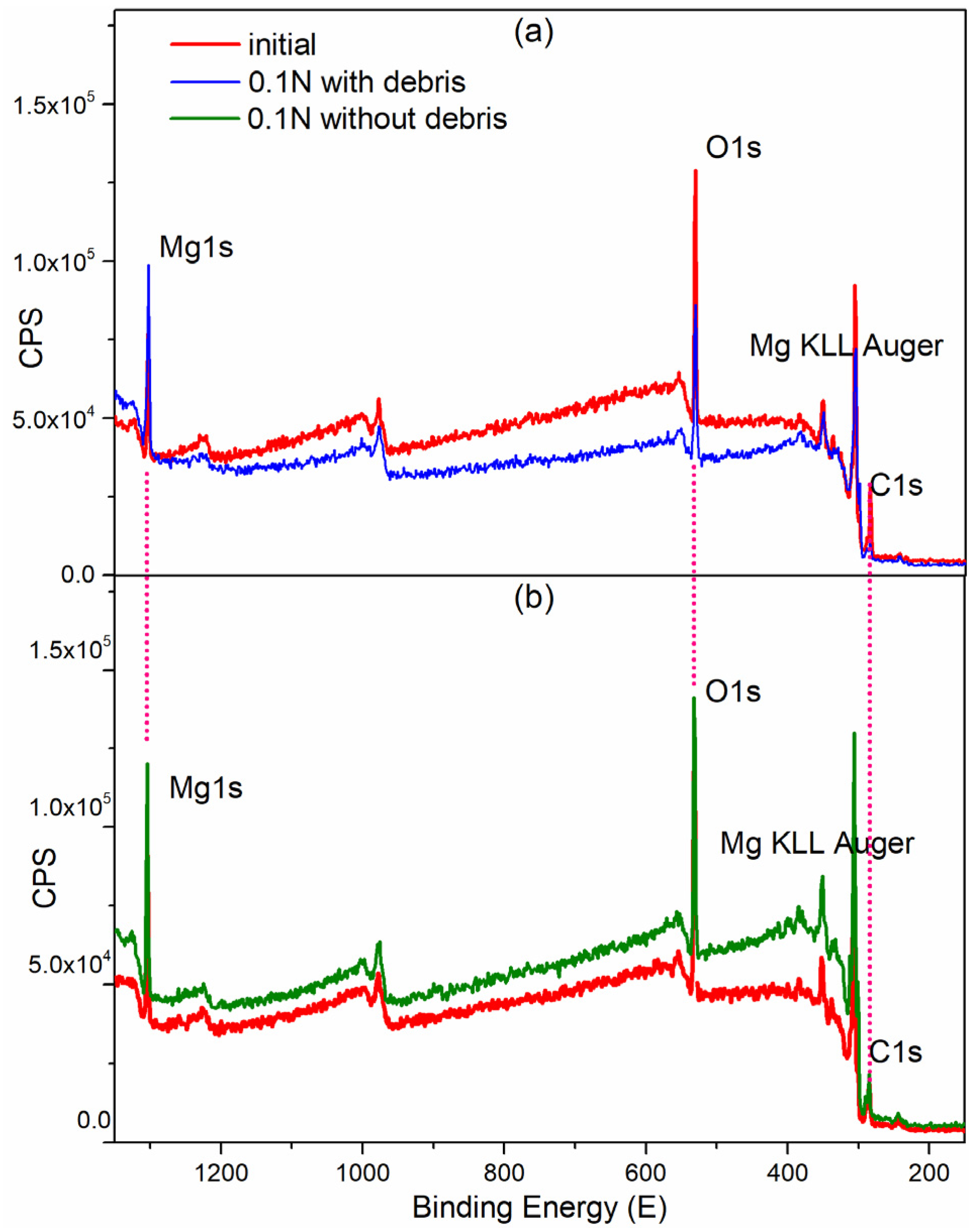

| XPS Zone | C 1s | Mg 1s | O 1s | |||
|---|---|---|---|---|---|---|
| Peak | FWHM | Peak | FWHM | Peak | FWHM | |
| Matrix-clear | 284.85 | 3.72 | 1303.7 | 3.28 | 531.67 | 3.4 |
| 0.1N-with debris | 284.82 | 3.95 | 1303.1 | 3.31 | 532.05 | 3.88 |
| 0.1N-without debris | 284.85 | 3.30 | 1303.9 | 3.09 | 531.61 | 3.4 |
| Atomic % | C 1s | Mg 1s | O 1s | Ratio O/C | Ratio Mg/C | Ratio O/Mg |
|---|---|---|---|---|---|---|
| Matrix-clear | 33.43 | 16.44 | 50.13 | 1.50 | 0.49 | 3.05 |
| 0.1N-with debris | 16.11 | 25.96 | 57.93 | 3.59 | 1.61 | 2.23 |
| 0.1N-without debris | 30.85 | 18.40 | 50.75 | 1.65 | 0.59 | 2.76 |
| Mg 1s peak | O 1s Peak | |||||||
|---|---|---|---|---|---|---|---|---|
| Mg(OH)2·nH2O | Mg2+(CO32−) | Mg2+(O2−) | Mg | Hydrocarbonate | Carbonate | C–O | C–C | |
| Matrix | - | 1304.78 | 1303.74 | 1302.53 | - | 286.8 | 285.5 | 284.8 |
| Worn-surface | 1306.5 | 1304.98 | 1303.41 | 1302.27 | 291.6 | 286.9 | 285.7 | 284.8 |
| Zone | MgCO3 | MgO | Mg | Mg(OH)2·nH2O |
|---|---|---|---|---|
| 0.1 N | 17.39 | 40.52 | 25.32 | 16.76 |
| Matrix | 21.90 | 64.29. | 13.81 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Peng, J.; Wang, M.; Mo, J.; Deng, C.; Zhu, M. Tribochemical Behavior of Pure Magnesium During Sliding Friction. Metals 2019, 9, 311. https://doi.org/10.3390/met9030311
Zhou Y, Peng J, Wang M, Mo J, Deng C, Zhu M. Tribochemical Behavior of Pure Magnesium During Sliding Friction. Metals. 2019; 9(3):311. https://doi.org/10.3390/met9030311
Chicago/Turabian StyleZhou, Yan, Jinfang Peng, Mengjie Wang, Jiliang Mo, Changguang Deng, and Minhao Zhu. 2019. "Tribochemical Behavior of Pure Magnesium During Sliding Friction" Metals 9, no. 3: 311. https://doi.org/10.3390/met9030311
APA StyleZhou, Y., Peng, J., Wang, M., Mo, J., Deng, C., & Zhu, M. (2019). Tribochemical Behavior of Pure Magnesium During Sliding Friction. Metals, 9(3), 311. https://doi.org/10.3390/met9030311





