Mineralogical Characteristics and Isothermal Oxidation Kinetics of Ironsand Pellets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Oxidation Experiments
2.2. Characterization
3. Results and Discussion
3.1. Mineralogical Characteristics of Ironsand
3.2. Effect of Oxidation Temperature on the Microstructure of the Pellets
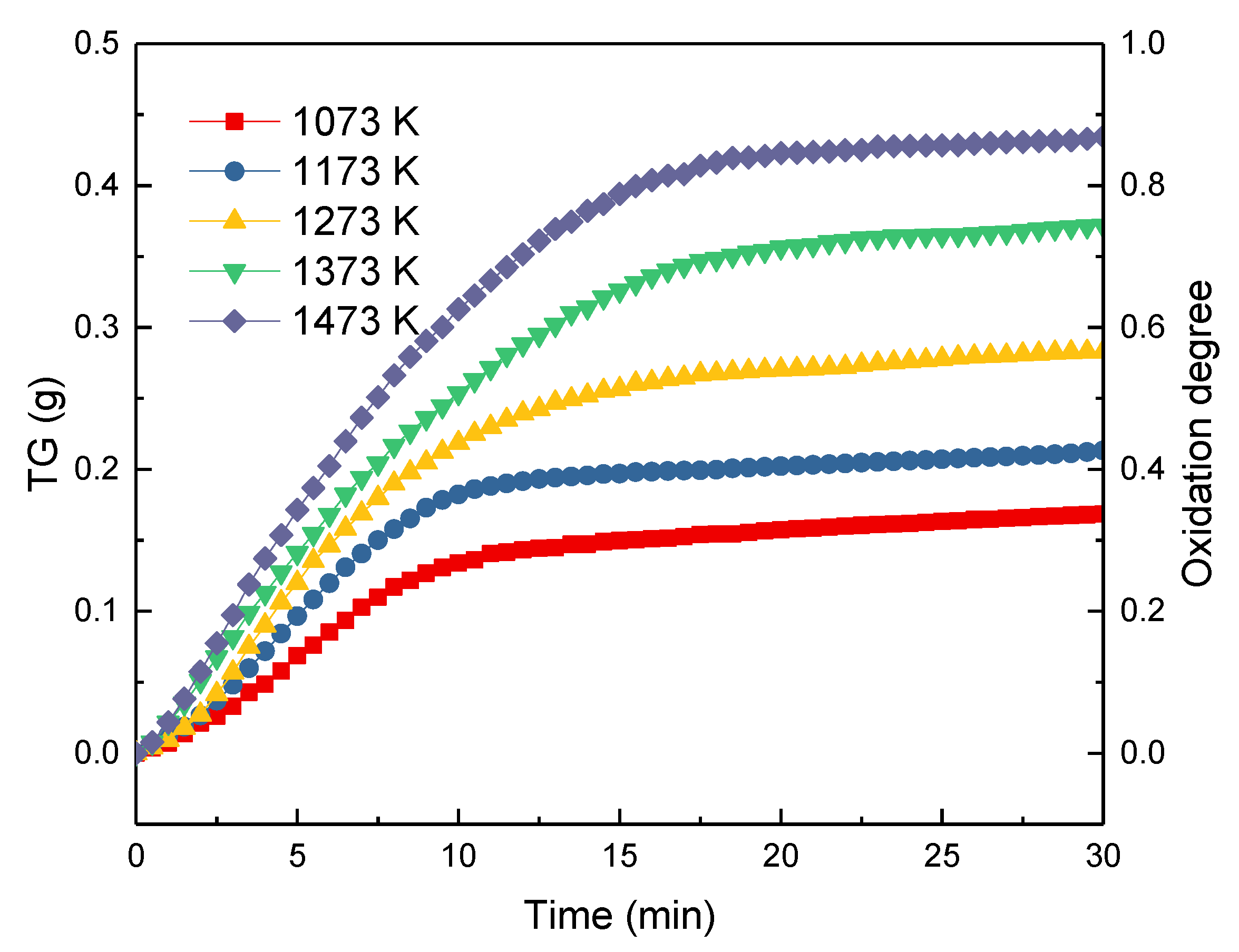
3.3. Thermogravimetric Analysis and Reaction Mechanism
3.3.1. Thermogravimetric Analysis
3.3.2. Reaction Mechanism
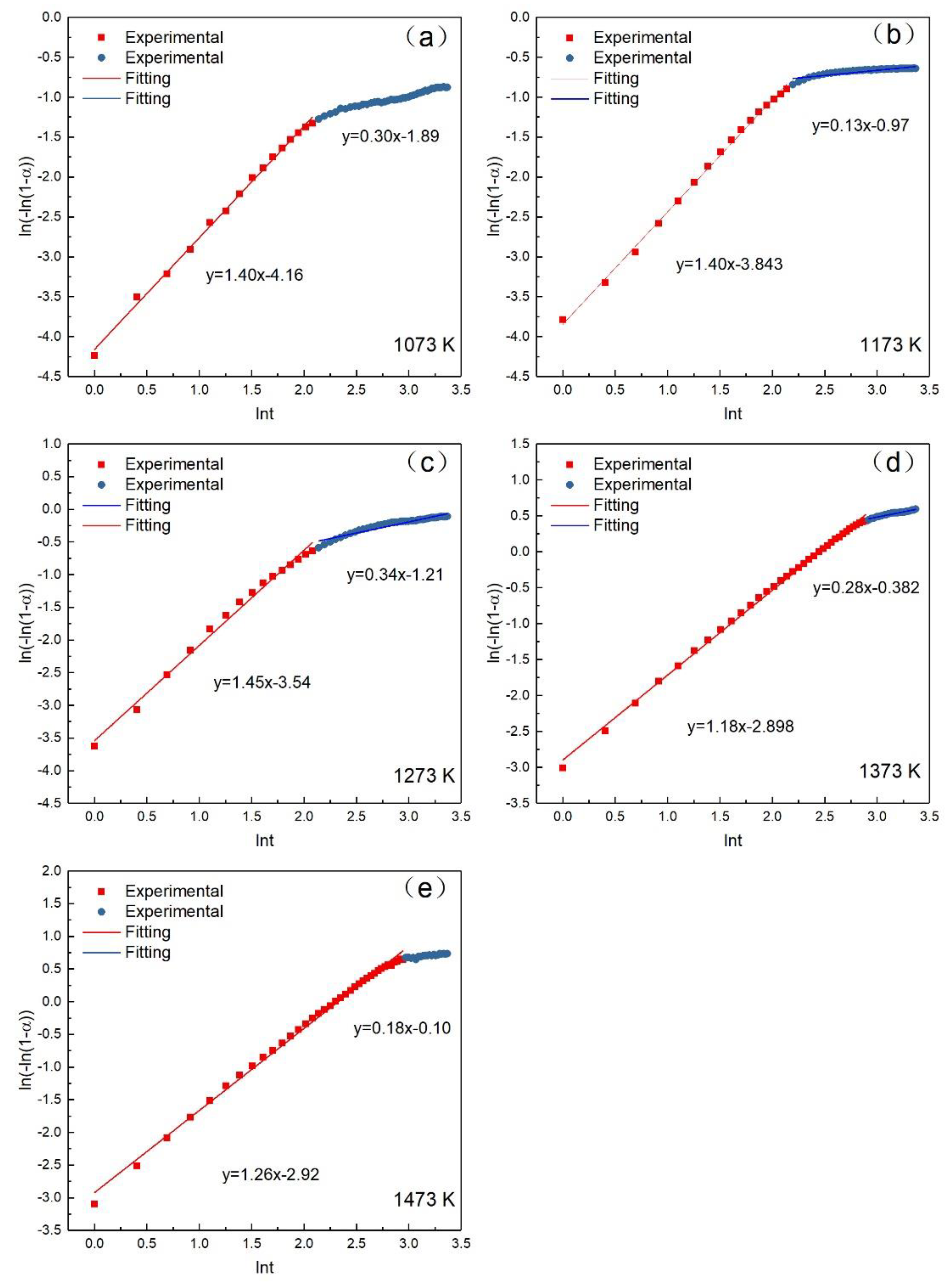
3.4. Oxidation Reaction Kinetics Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, X.; Zhong, Y.; Zhu, S.; Zhu, J.; Yuan, P.; He, H.; Zhang, J. The decolorization of Acid Orange II in non-homogeneous Fenton reaction catalyzed by natural vanadium–titanium magnetite. J. Hazard. Mater. 2010, 181, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, G.; Liu, Z.; Chu, M.; Xue, X. Reduction process of pellet containing high chromic vanadium–titanium magnetite in cohesive zone. Steel Res. Int. 2015, 86, 808–816. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Y.; Qiu, G.; Jiang, T. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium–titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, J.-L.; Liu, Z.-J.; Du, C.-B. Carbothermic reduction reactions at the metal–slag interface in Ti-bearing slag from a blast furnace. JOM 2017, 69, 2397–2403. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B. Occurrence and recycling of main elements in Panxi vanadium-titanium magnetite. Conserv. Util. Miner. Resour. 2008, 5, 12. [Google Scholar]
- Liang, X.; Zhu, S.; Zhong, Y.; Zhu, J.; Yuan, P.; He, H.; Zhang, J. The remarkable effect of vanadium doping on the adsorption and catalytic activity of magnetite in the decolorization of methylene blue. Appl. Catal. B Environ. 2010, 97, 151–159. [Google Scholar] [CrossRef]
- Qin, J.; Xue, X.; Deng, J. Study on CO2 emission in the process of dealing with vanadium-titanium magnetite. In Proceedings of the 2011 International Conference on Materials and Products Manufacturing Technology, Chengdu, China, 28–30 October 2011; Trans Tech Publications: Zurich, Switzerland, 2011; pp. 125–132. [Google Scholar]
- Li, H.; Wang, H.; Qing, S.; Hu, J.; Li, Y.; Hou, Y.; Hu, L.; Li, L. Energy conservation and emissions reduction in iron and steel industry. In Proceedings of the Third International Conference on Measuring Technology and Mechatronics Automation, Shangshai, China, 6–7 January 2011; pp. 41–44. [Google Scholar]
- Yu, C.; Li, H.; Jia, X.; Li, Q. Improving resource utilization efficiency in China’s mineral resource-based cities: A case study of Chengde, Hebei province. Resour. Conserv. Recycl. 2015, 94, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Huang, J.; Zhang, S.; Zhao, X. Growth kinetics of cubic carbide free layers in graded cemented carbides. Int. J. Miner. Metall. Mater. 2012, 19, 64–71. [Google Scholar] [CrossRef]
- Smolik, J.; Zdunek, K.; Larisch, B. Investigation of adhesion between component layers of a multi-layer coating TiC/Ti (Cx, N1−x)/TiN by the scratch-test method. Vacuum 1999, 55, 45–50. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Liu, Z.; Zhang, Y.P.; Liu, D.H.; Du, C.B. Effects of w(TiO2) on mineral structure and softening-melting property of sinter. Iron Steel 2017, 52, 20–28. [Google Scholar]
- Dehghan-Manshadi, A.; Manuel, J.; Hapugoda, S.; Ware, N. Sintering characteristics of titanium containing iron ores. ISIJ Int. 2014, 54, 2189–2195. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.; Jiang, T.; Xue, X. Influence of basicity on high-chromium vanadium-titanium magnetite sinter properties, productivity, and mineralogy. JOM 2015, 67, 1203–1213. [Google Scholar] [CrossRef]
- Paananen, T.; Kinnunen, K. Effect of TiO2-content on reduction of iron ore agglomerates. Steel Res. Int. 2009, 80, 408–414. [Google Scholar]
- Liu, Z.; Chu, M.; Wang, H.; Zhao, W.; Xue, X. Effect of MgO content in sinter on the softening–melting behavior of mixed burden made from chromium-bearing vanadium–titanium magnetite. Int. J. Miner. Metall. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]
- Han, G.; Jiang, T.; Zhang, Y.; Huang, Y.; Li, G. High-temperature oxidation behavior of vanadium, titanium-bearing magnetite pellet. J. Iron Steel Res. Int. 2011, 18, 14–19. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.-Q.; Chu, M.-S.; Zhu, M.-Y. Oxidation induration process and kinetics of Hongge vanadium titanium-bearing magnetite pellets. Ironmak. Steelmak. 2017, 44, 294–303. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Y.; Chu, M.; Xue, X. Preparation of oxidized pellets with high Chromium Vanadium-Titanium Magnetite. J. Northeast. Univ. Nat. Sci. 2013, 4, 22. [Google Scholar]
- Wang, Y.; Liu, Z.; Zhang, J.; Du, C.; Niu, L. Central band structures: New insights into the coupling effects between the pores and minerals of sinter. Metall. Mater. Trans. B 2019. [Google Scholar] [CrossRef]
- Bartels, W.J.; Hornstra, J.; Lobeek, D.J.W. X-ray diffraction of multilayers and superlattices. Acta Crystallogr. Sect. A 1986, 42, 539–545. [Google Scholar] [CrossRef]
- Tan, S.; Su, A.; Li, W.; Zhou, E. New insight into melting and crystallization behavior in semicrystalline poly (ethylene terephthalate). J. Polym. Sci. B Polym. Phys. 2000, 38, 53–60. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.; Zhang, Y.; Li, S.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar] [CrossRef]
- Rekoske, J.E.; Barteau, M.A. Isothermal reduction kinetics of titanium dioxide-based materials. J. Phys. Chem. B 1997, 101, 1113–1124. [Google Scholar] [CrossRef]
- Zhang, Y.; Lü, W.; Lü, X.; Li, S.; Bai, C.; Song, B.; Han, K. Isothermal reduction kinetics of Panzhihua ilmenite concentrate under 30vol% CO–70vol% N 2 atmosphere. Int. J. Miner. Metall. Mater. 2017, 24, 240–248. [Google Scholar] [CrossRef]


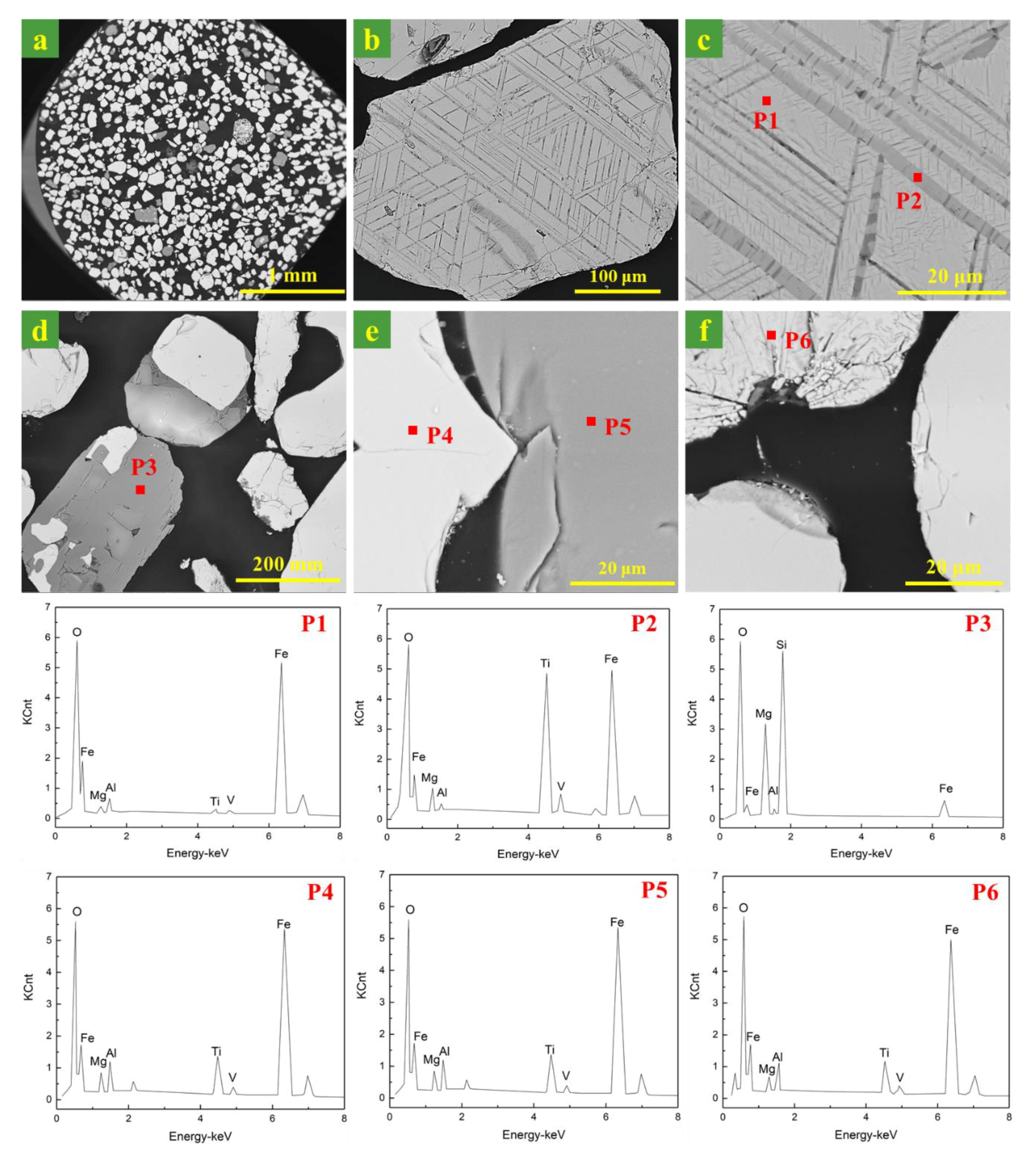
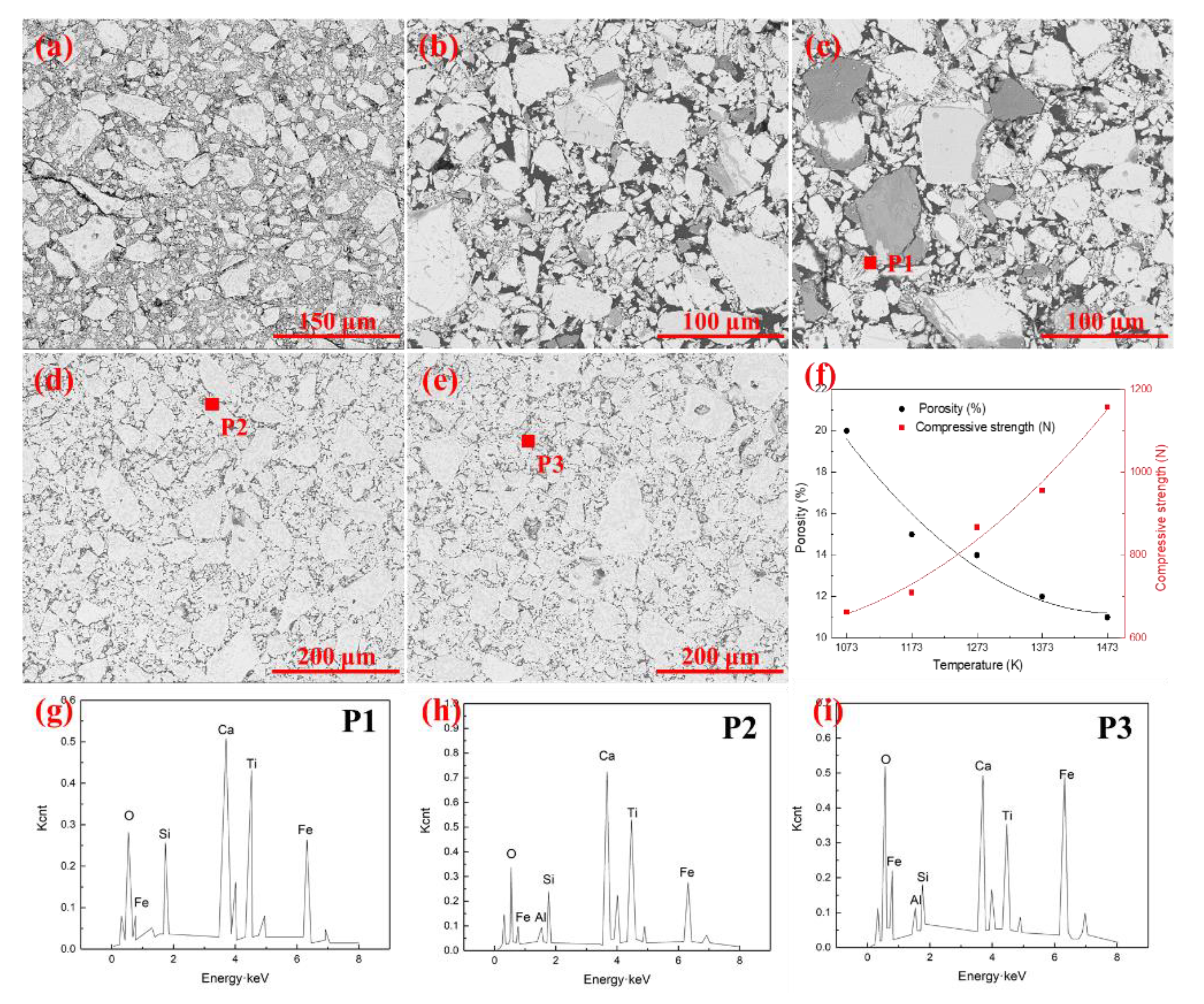
| Item | Total Fe | FeO | SiO2 | Al2O3 | CaO | MgO | TiO2 | V2O5 | P | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ironsand | 55.63 | 29.60 | 4.13 | 3.38 | 0.60 | 3.74 | 11.41 | 0.76 | 0.03 | 0.02 | 0.02 |
| Phase | Chemical Formula | Quantitative Analysis |
|---|---|---|
| Magnetite | Fe3O4 | 21.7 |
| Titanomagnetite | Fe2.814Ti0.186O4 | 40.9 |
| Enstatite | Mg1.56Fe0.44Si2O6 | 17.1 |
| Hematite–ilmenite solid solution | Fe1.696Ti0.228O3 | 14.5 |
| Magnesium iron aluminum silicon oxide | Al0.205Fe0.281Mg0.719O3Si0.795 | 5.8 |
| Period | Temp (K) | 1073 | 1173 | 1273 | 1373 | 1473 |
| First stage | Slope (n) | 1.397 ± 0.020 | 1.383 ± 0.018 | 1.341 ± 0.037 | 1.180 ± 0.010 | 1.256 ± 0.013 |
| Intercept (lnkr) | −4.159 ± 0.030 | −3.821 ± 0.027 | −3.428 ± 0.054 | −2.898 ± 0.020 | −2.923 ± 0.030 | |
| α | 0–0.209 | 0–0.333 | 0–0.467 | 0–0.774 | 0–0.844 | |
| R-Square | 0.997 | 0.997 | 0.982 | 0.998 | 0.996 | |
| Standard deviation | 0.047 | 0.043 | 0.085 | 0.041 | 0.060 | |
| Second stage | Slope (n) | 0.303 ± 0.006 | 0.103 ± 0.010 | 0.254 ± 0.016 | 0.289 ± 0.013 | 0.188 ± 0.021 |
| Intercept (lnkr) | −1.889 ± 0.020 | −0.971 ± 0.030 | −0.949 ± 0.045 | −0.382 ± 0.041 | −0.104 ± 0.065 | |
| α | 0.280–0.340 | 0.333–0.410 | 0.468–0.591 | 0.774–0.835 | 0.844–0.875 | |
| R-Square | 0.981 | 0.925 | 0.955 | 0.960 | 0.813 | |
| Standard deviation | 0.015 | 0.021 | 0.036 | 0.001 | 0.011 |
| Mechanism | Code | Differential Form | Integral Form | R-square | |||
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | |||||
| Chemical reaction | n = 1 | F1 | (1 − α) | −ln(1 − α) | −0.57 | 0.90 | 0.75 |
| n = 2 | F2 | (1 − α)2 | (1 − α)−1 − 1 | −1.85 | 0.51 | 0.91 | |
| n = 3 | F3 | (1 − α)3 | [(1 − α)−1 − 1]/2 | −2.60 | 0.06 | 0.89 | |
| Diffusion | The two- dimension Diffusion control | D2 | [−ln(1 − α)]−1 | (1 − α)ln(1 − α)+α | −4.11 | −0.11 | 0.96 |
| The two- dimension Diffusion control | D3 | 1.5(1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]2 | −4.21 | −0.42 | 0.88 | |
| The three- dimension Diffusion control | D4 | 1.5[(1 − α)1/3 − 1]−1 | (1 − 2α/3) − (1 − α)2/3 | −4.21 | 0.02 | 0.97 | |
| Random nucleation and nuclei growth | Two dimension | A2 | 2(1 − α) [−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 | 0.91 | 0.56 | 0.20 |
| Three dimension | A3 | 3(1 − α) [−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 | 0.88 | 0.33 | 0.02 | |
| Exponential nucleation | Power series law, n = 1/2 | P2 | 2α1/2 | α1/2 | −0.72 | −0.60 | −0.40 |
| Power series law, n = 1/3 | P3 | 3α2/3 | α1/3 | −1.05 | −0.76 | −0.48 | |
| Power series law, n = 1/4 | P4 | 3α3/4 | α1/4 | −1.20 | −0.83 | −0.52 | |
| Phase boundary reaction | Cylindrical symmetry | R2 | 2(1 − α)1/2 | 1 − (1 − α)1/2 | 0.27 | 0.83 | 0.48 |
| Spherical symmetry | R3 | 3(1 − α)2/3 | 1 − (1 − α)1/3 | 0.02 | 0.89 | 0.59 | |
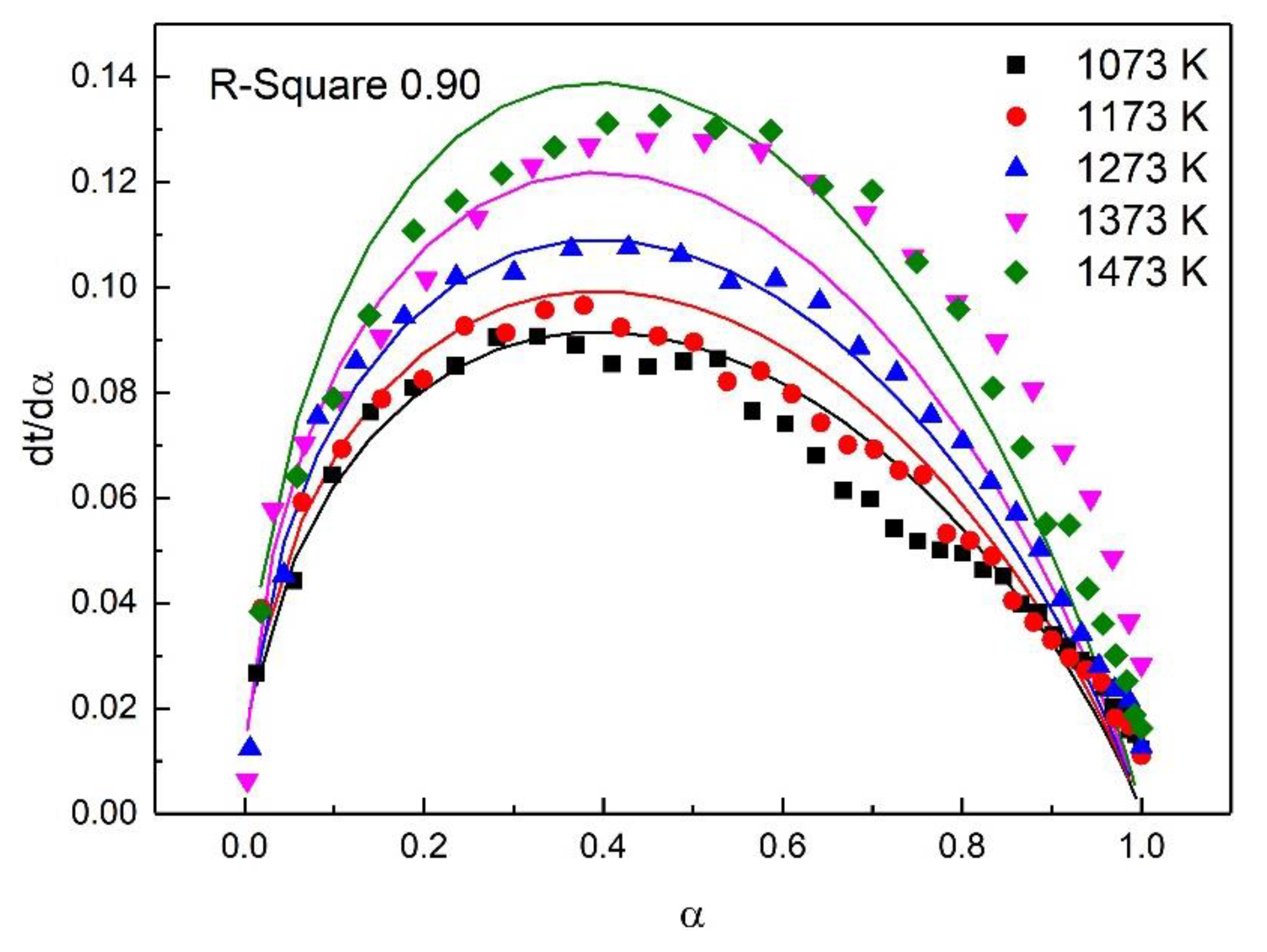
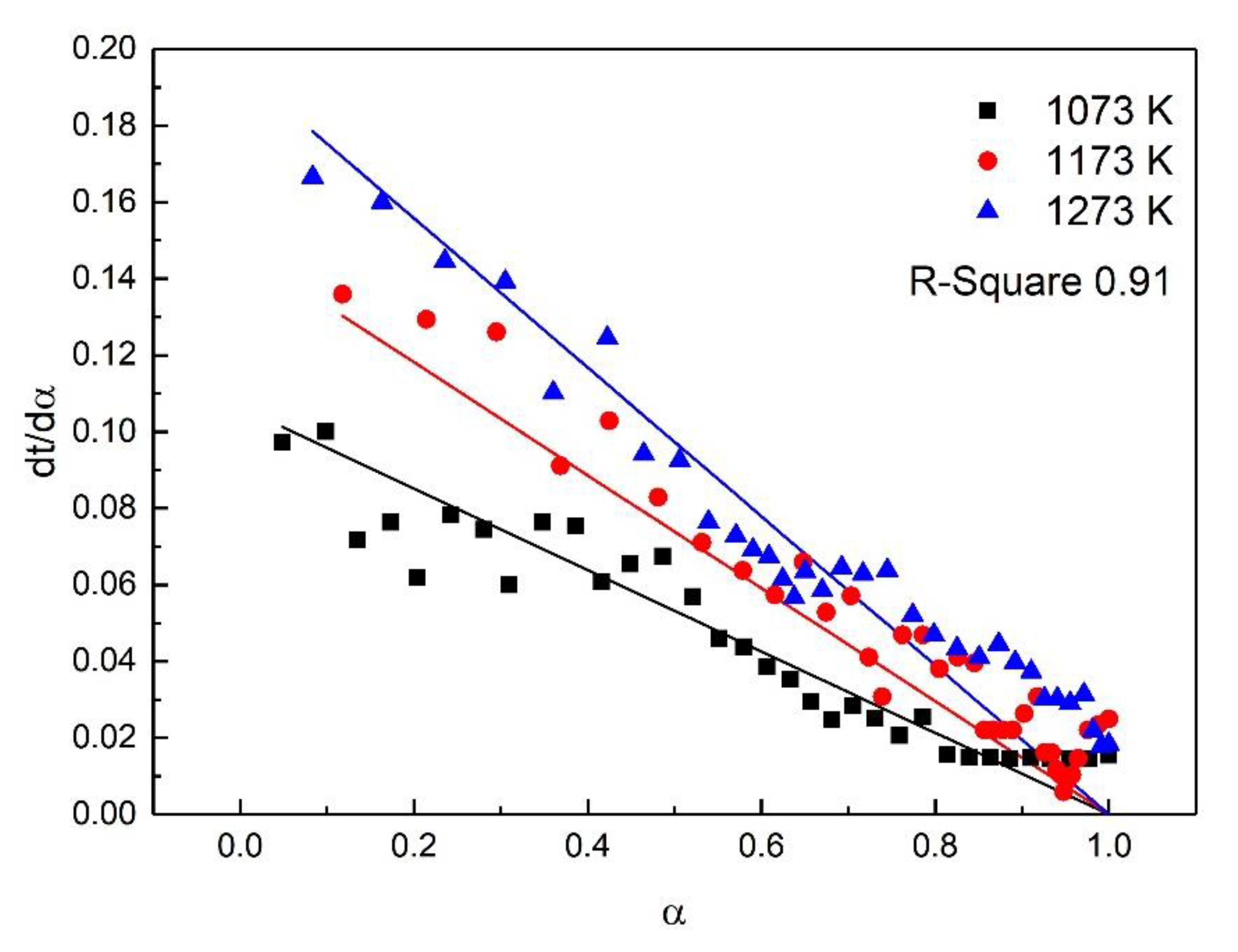
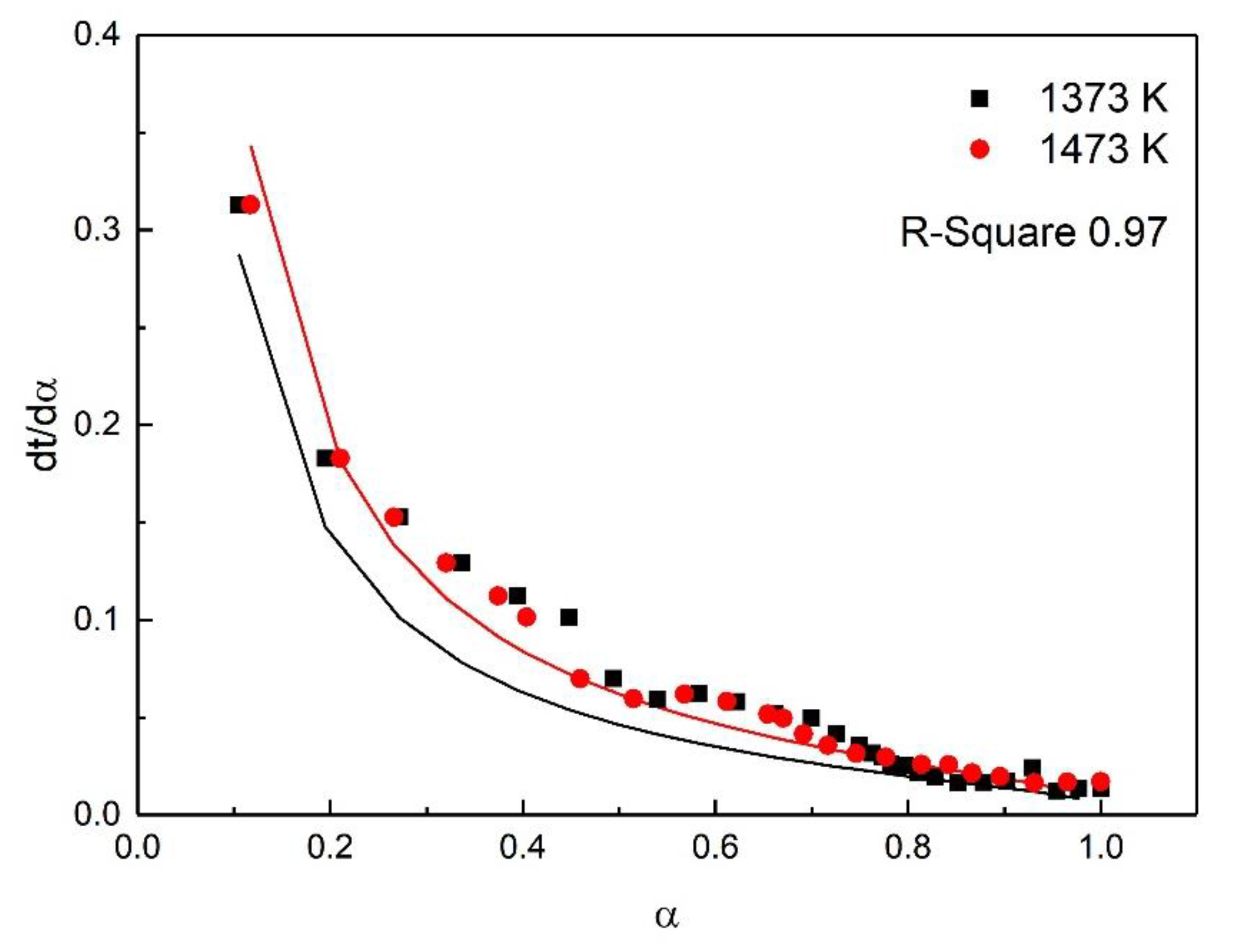
| Stage | Early Stage of the Oxidation | Post-Oxidation | Post-Oxidation |
|---|---|---|---|
| Fitting Model | A2 | F2 | D4 |
| Temp (K) | 1073–1473 | 1073–1273 | 1373–1473 |
| E (KJ/mol) | 15.39 | 34.29 | 13.88 |
| A | 0.77 | 4.97 | 0.23 |
| R-square | 0.90 | 0.91 | 0.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Liu, Z. Mineralogical Characteristics and Isothermal Oxidation Kinetics of Ironsand Pellets. Metals 2019, 9, 265. https://doi.org/10.3390/met9020265
Wang Y, Zhang J, Liu Z. Mineralogical Characteristics and Isothermal Oxidation Kinetics of Ironsand Pellets. Metals. 2019; 9(2):265. https://doi.org/10.3390/met9020265
Chicago/Turabian StyleWang, Yaozu, Jianliang Zhang, and Zhengjian Liu. 2019. "Mineralogical Characteristics and Isothermal Oxidation Kinetics of Ironsand Pellets" Metals 9, no. 2: 265. https://doi.org/10.3390/met9020265
APA StyleWang, Y., Zhang, J., & Liu, Z. (2019). Mineralogical Characteristics and Isothermal Oxidation Kinetics of Ironsand Pellets. Metals, 9(2), 265. https://doi.org/10.3390/met9020265







