Oxide-Inclusion Evolution in the Steelmaking Process of 304L Stainless Steel for Nuclear Power
Abstract
1. Introduction
2. Experiments
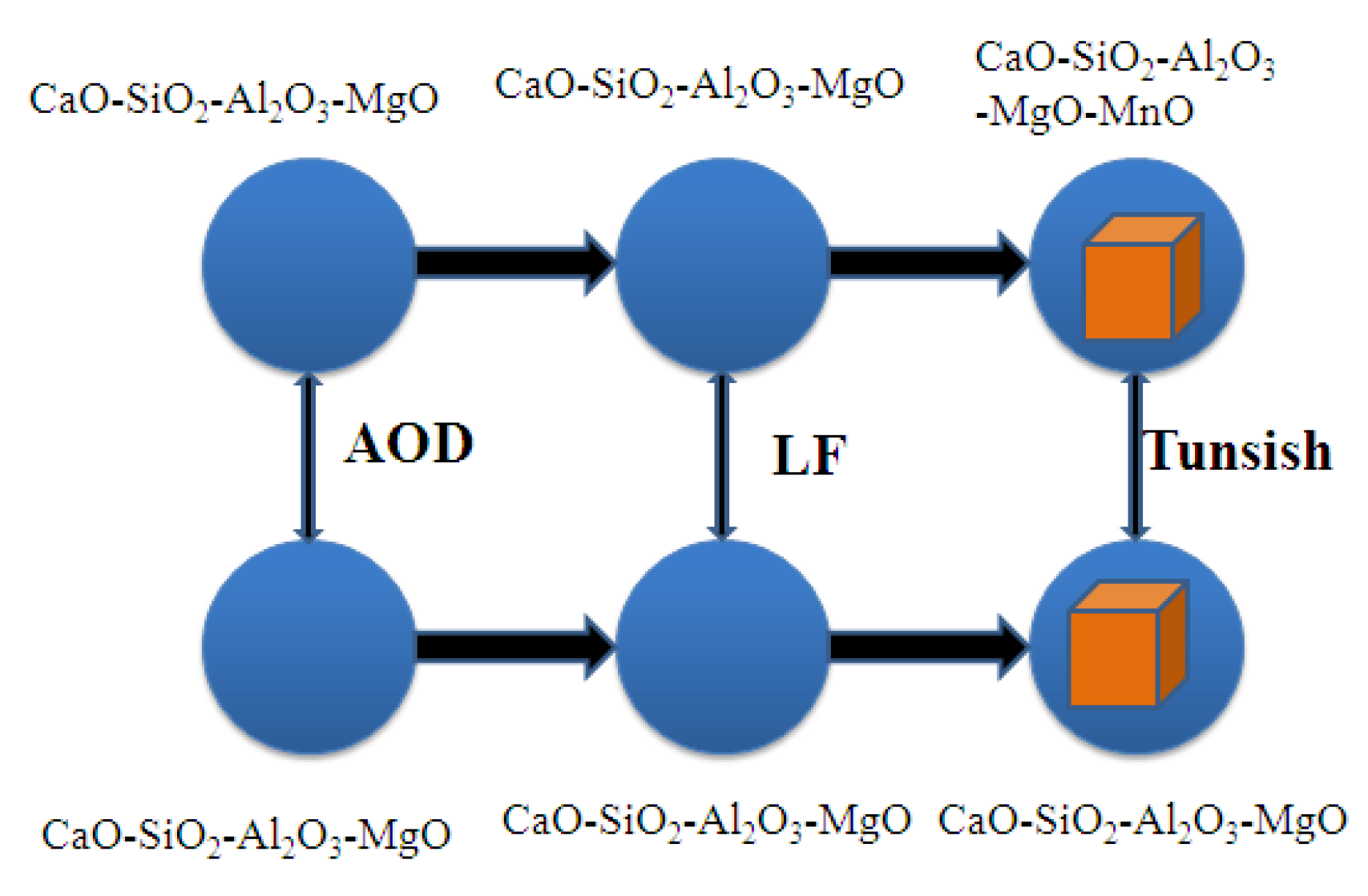
2.1. Experimental Procedure and Sampling
2.2. Analysis of Samples
3. Results
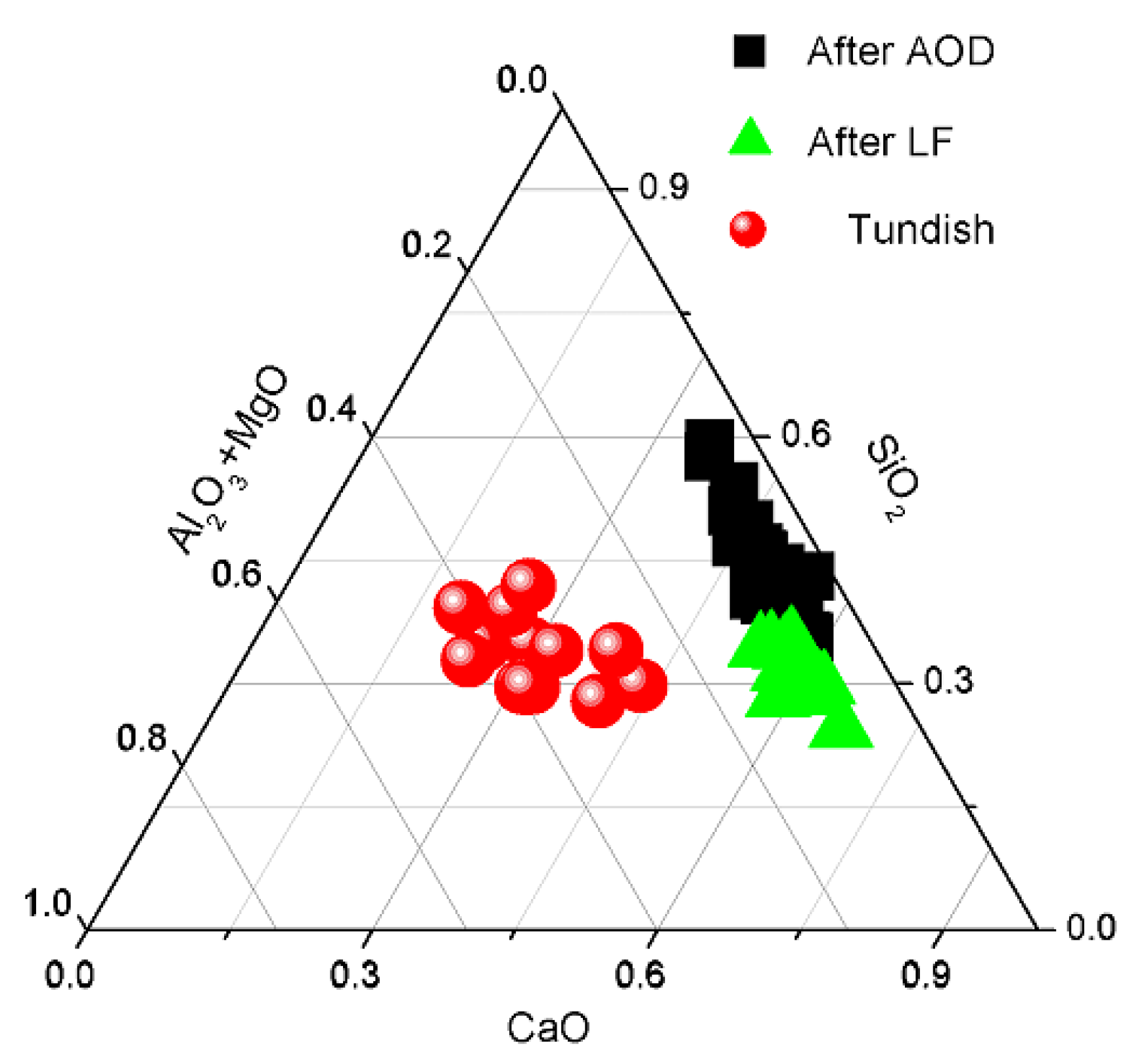
3.1. Composition of the Molten Steel and Slag
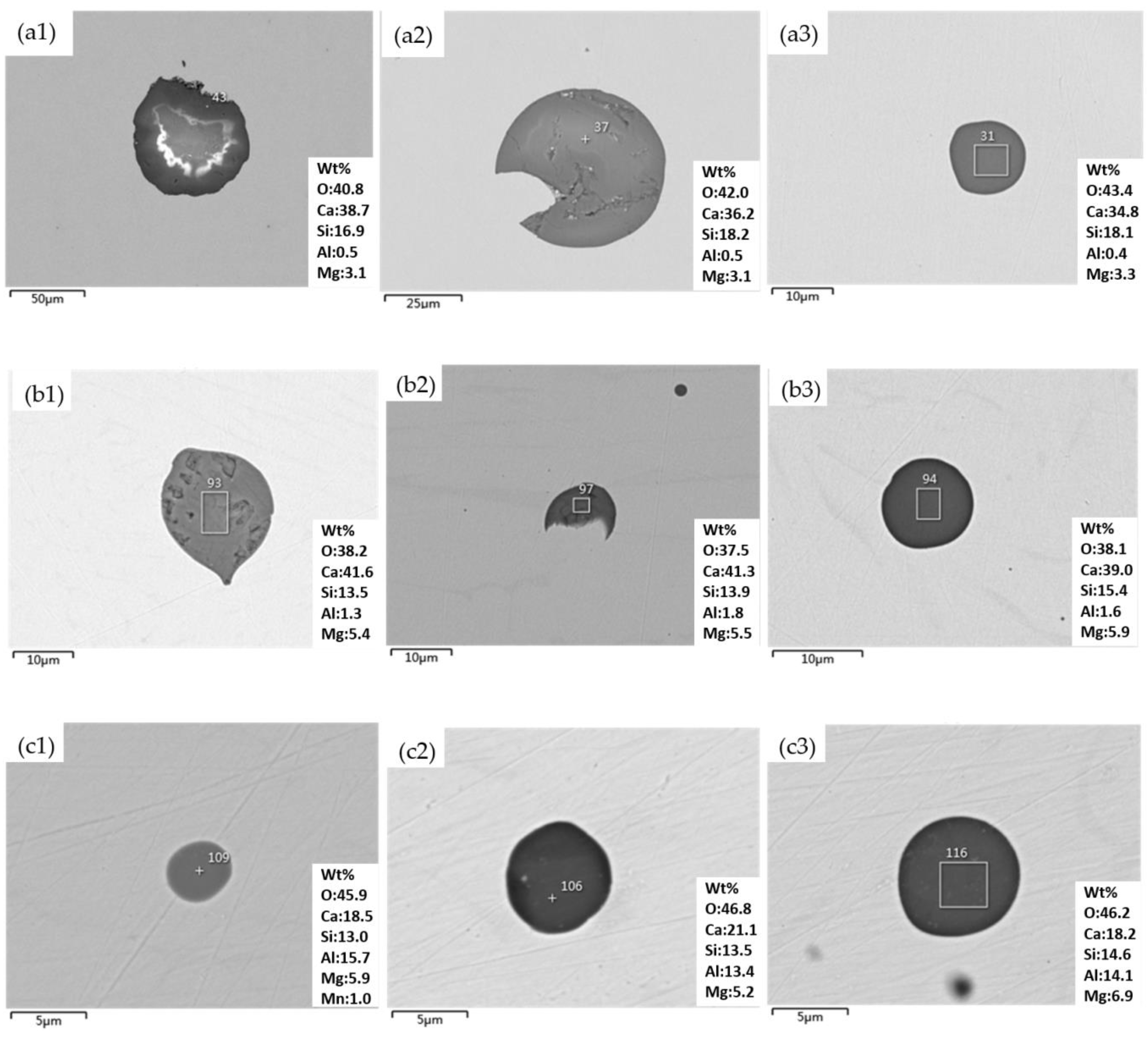
3.2. Type of Inclusions in the Smelting Process
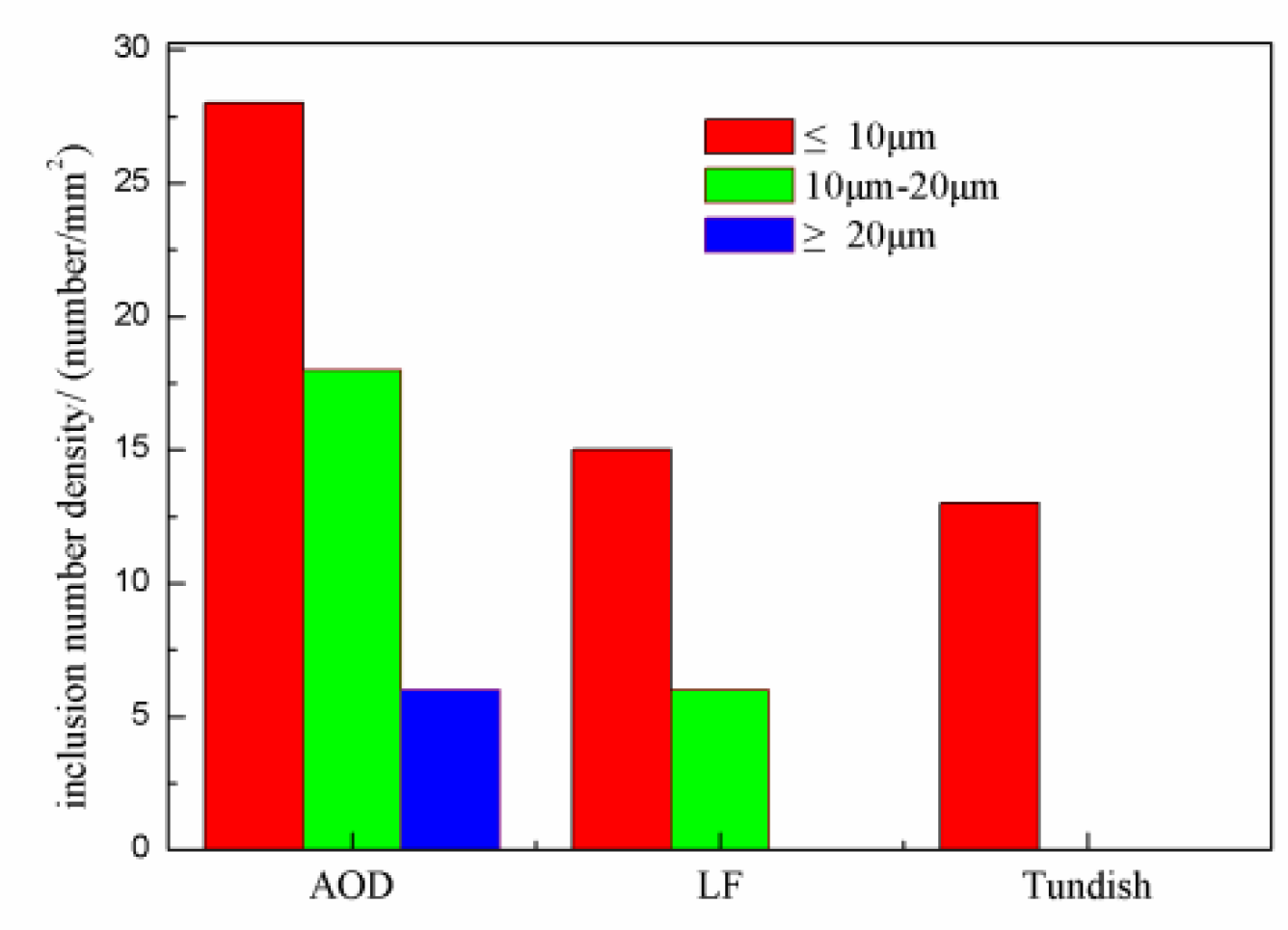
3.3. Number Density and Size of Inclusions in the Smelting Process
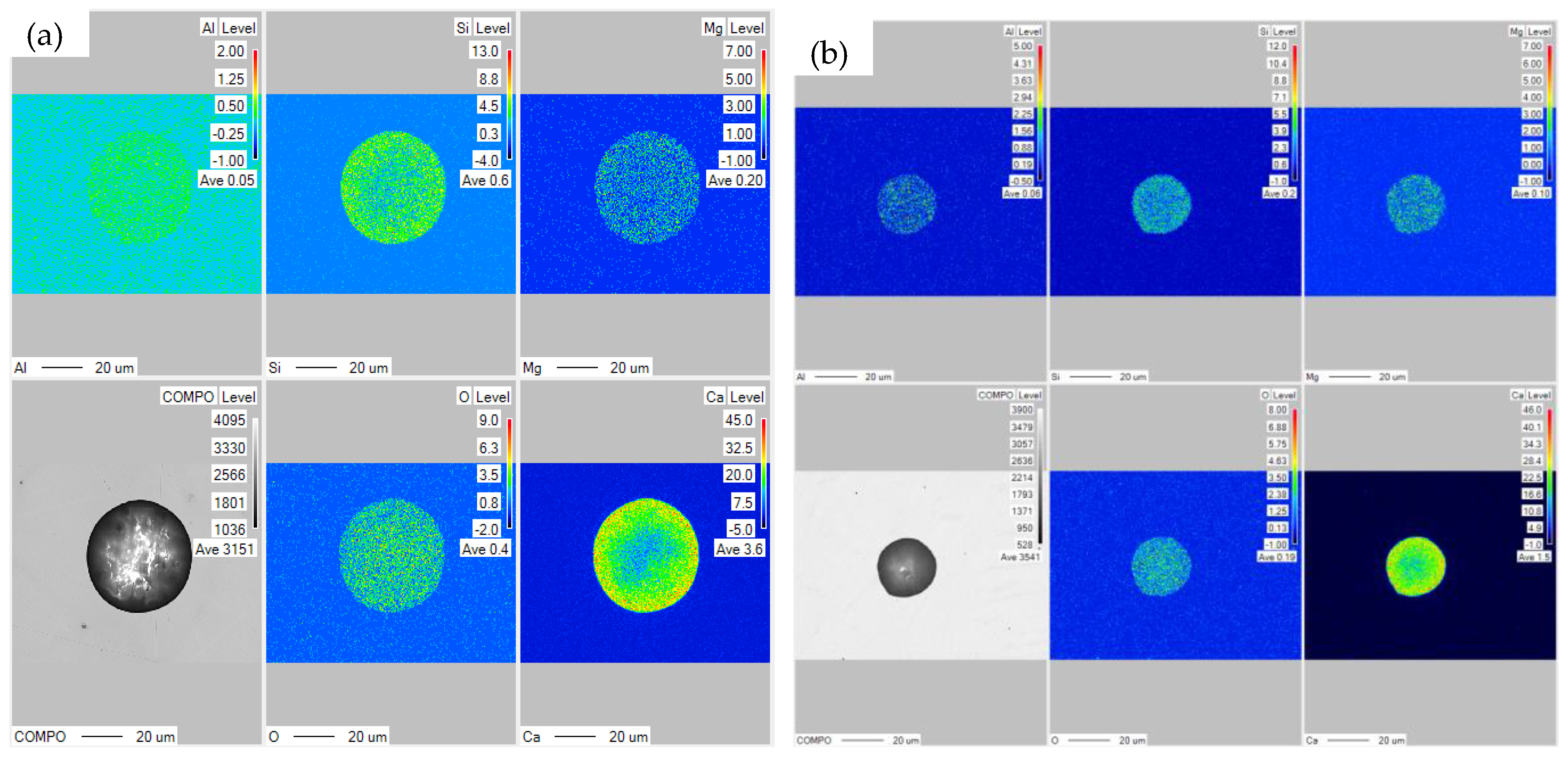
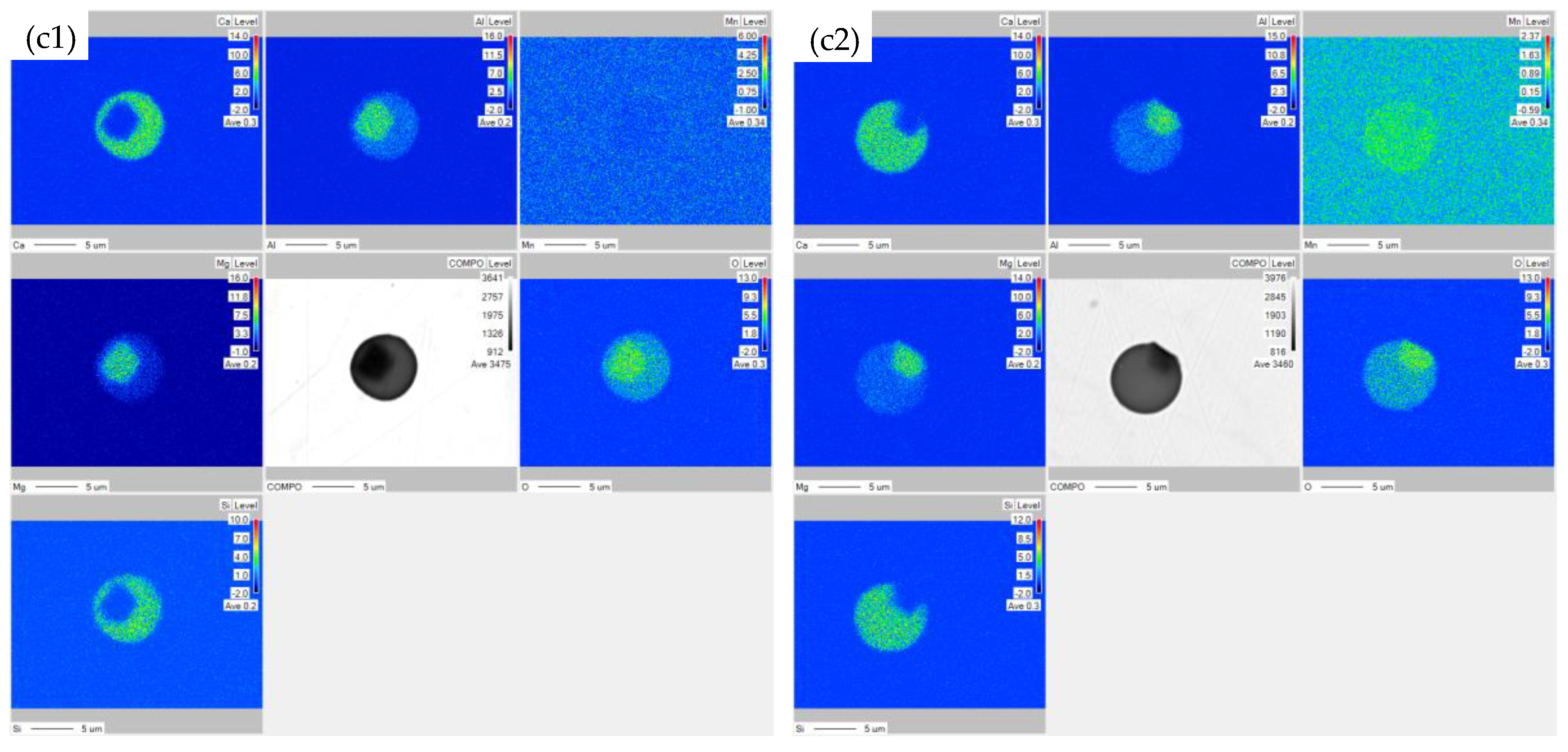
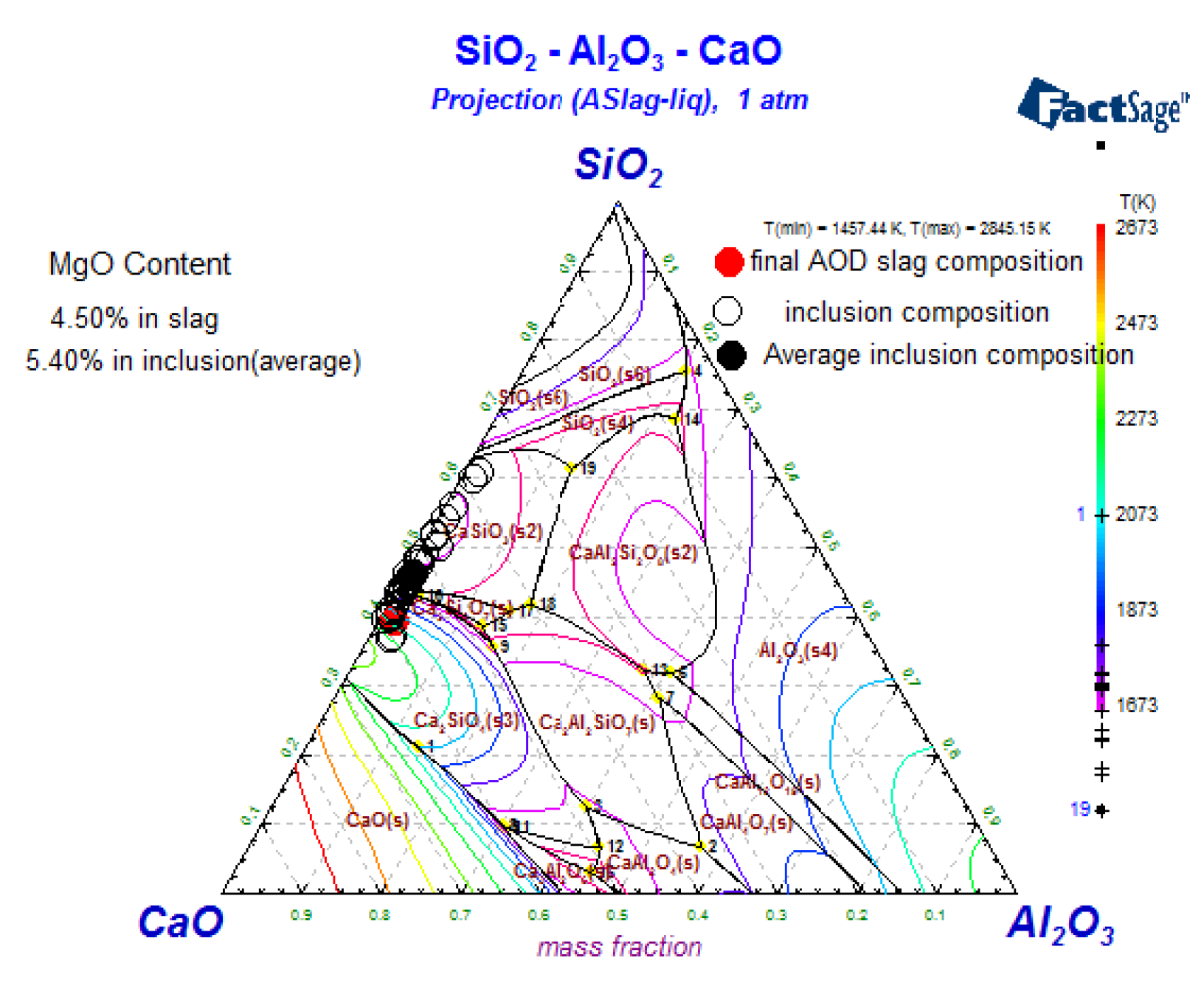
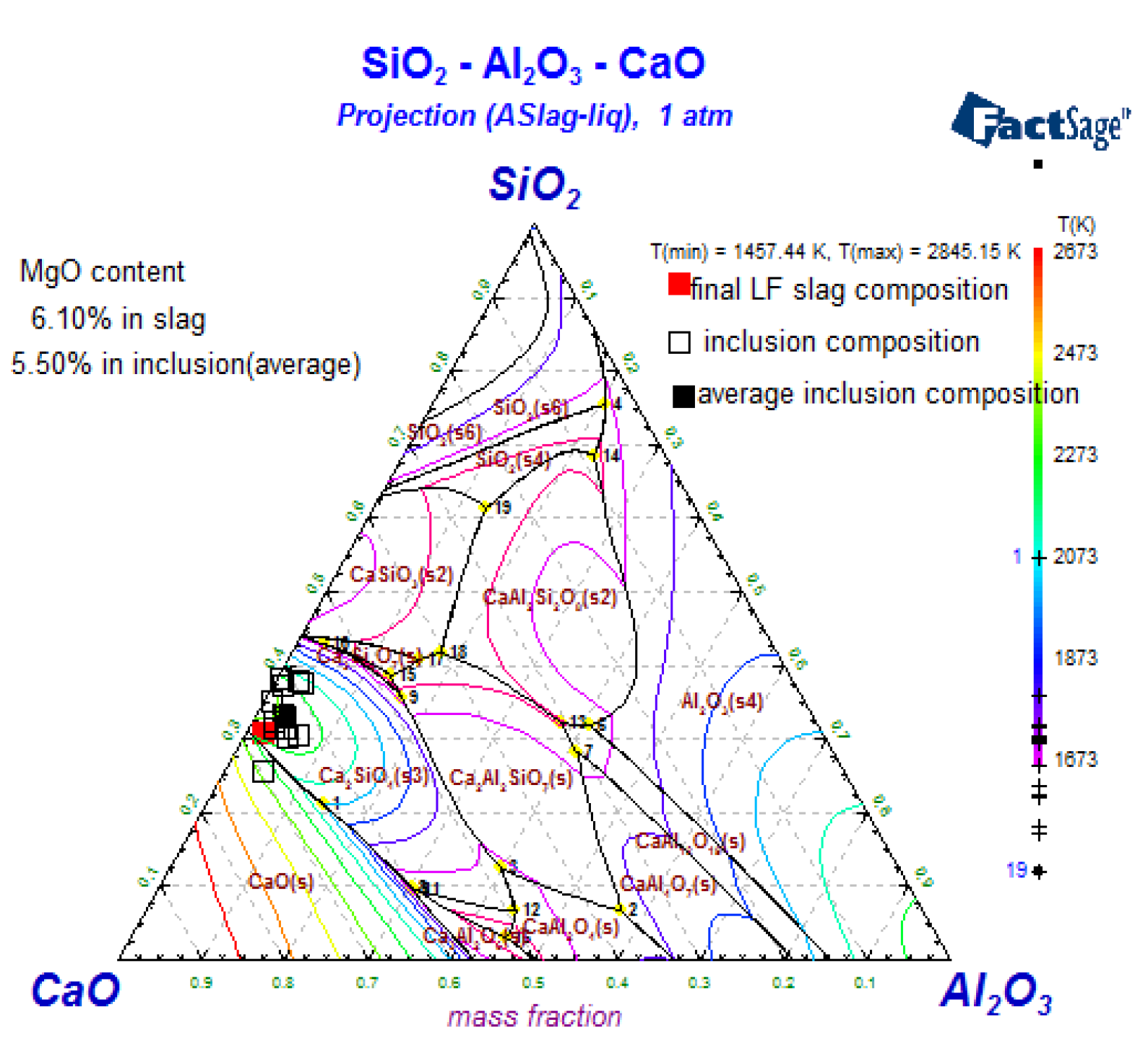
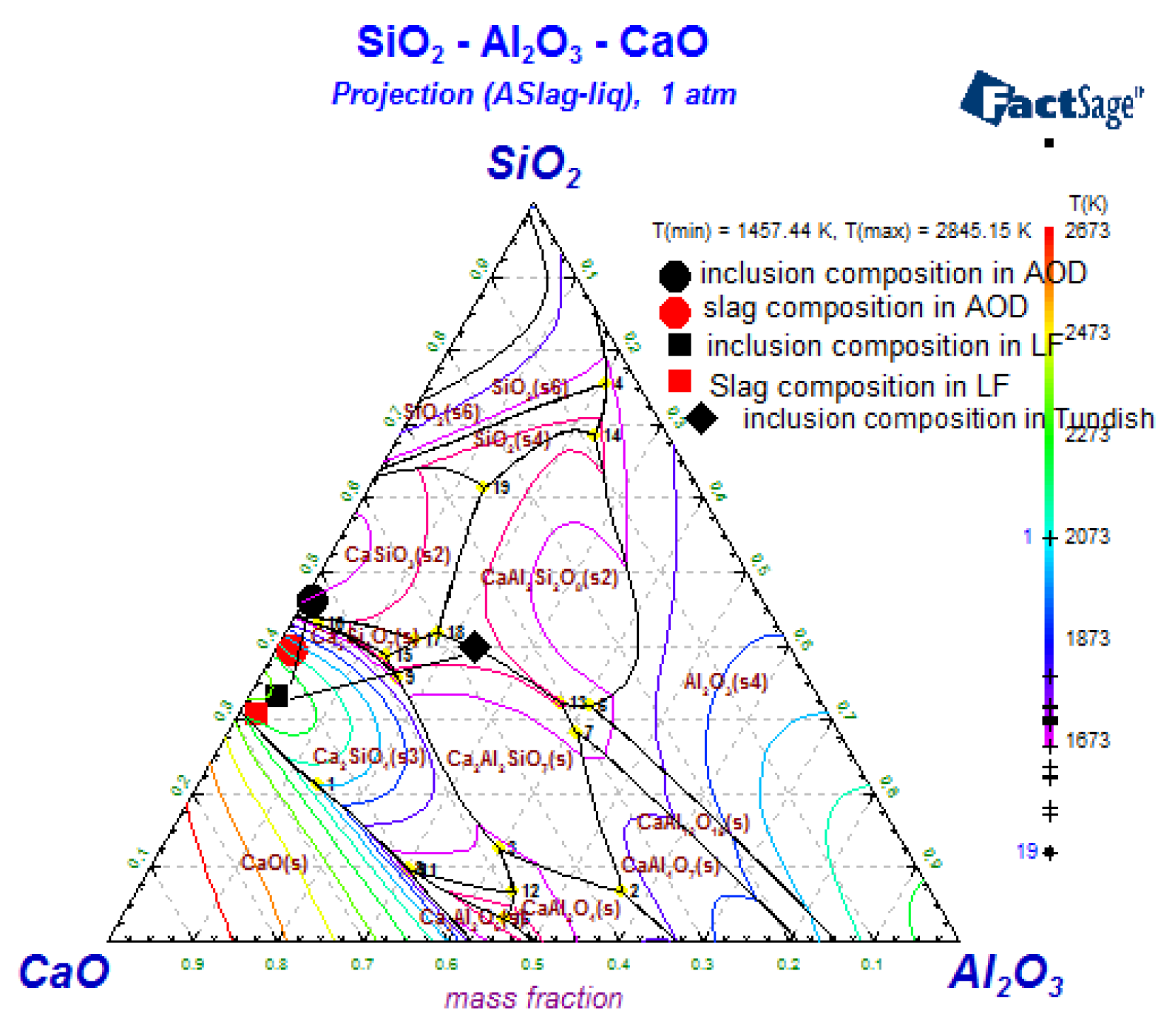
3.4. The Composition of Inclusions
4. Discussion
4.1. Oxide-Inclusion Evolution in the Steelmaking Process
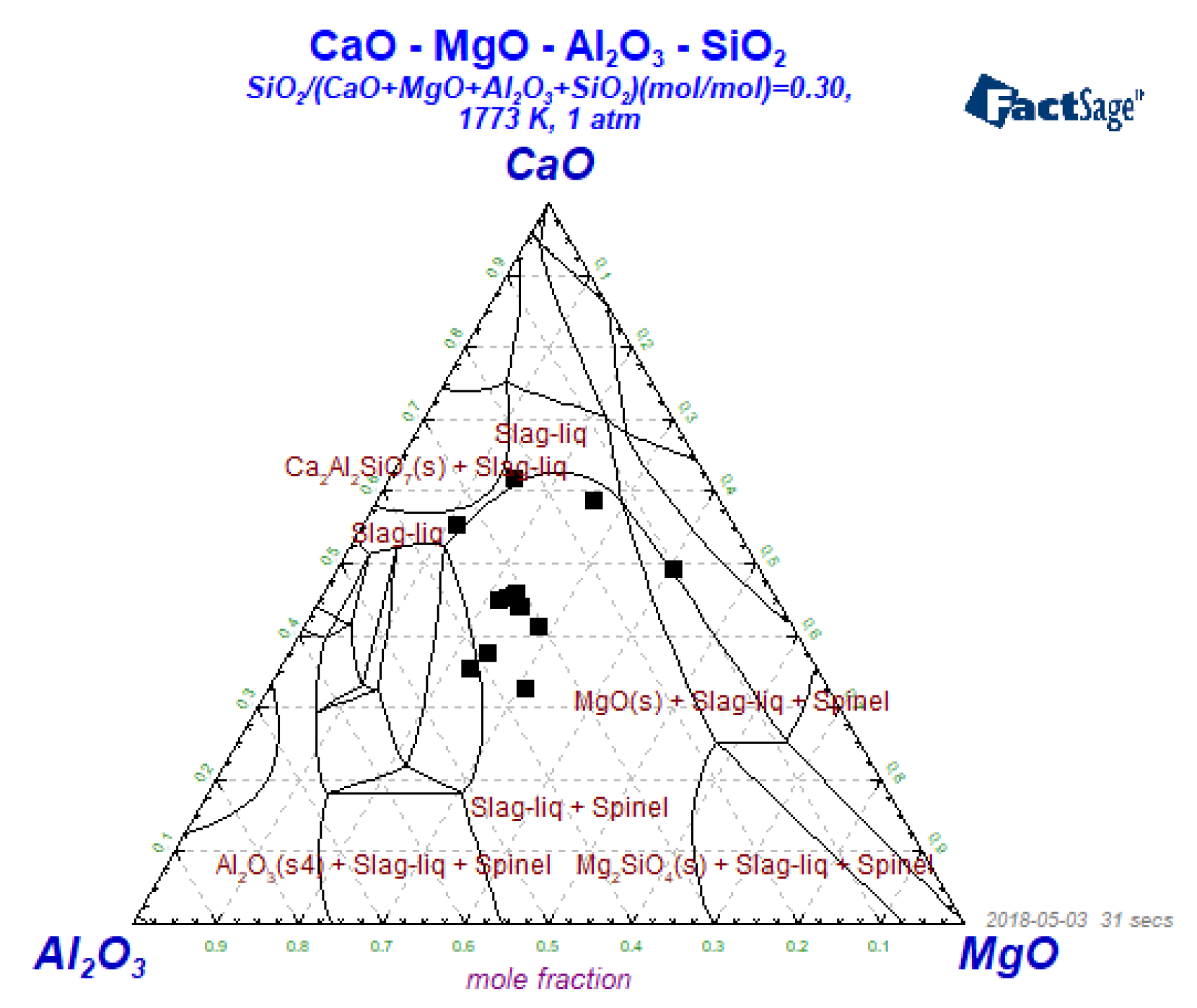
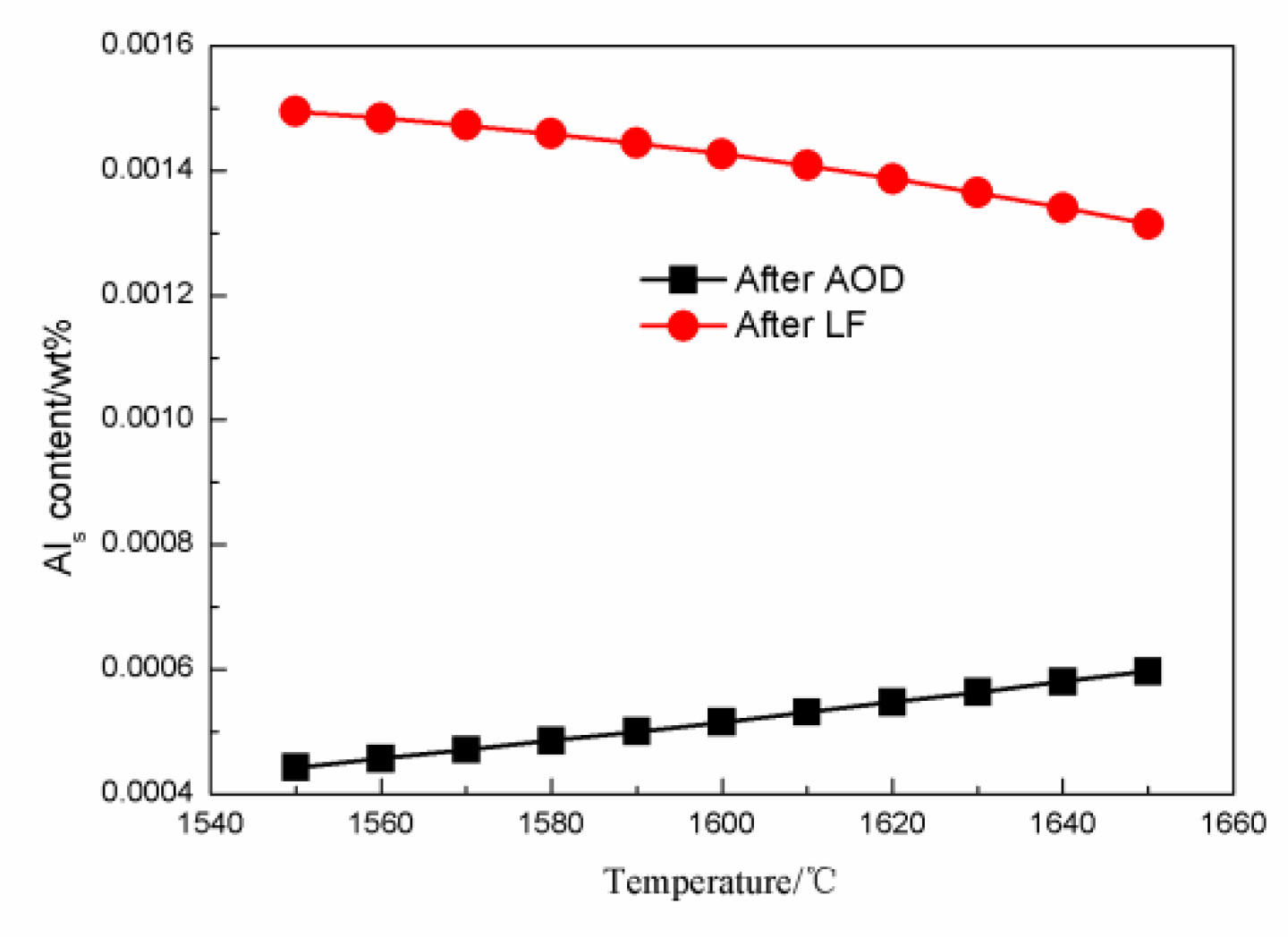
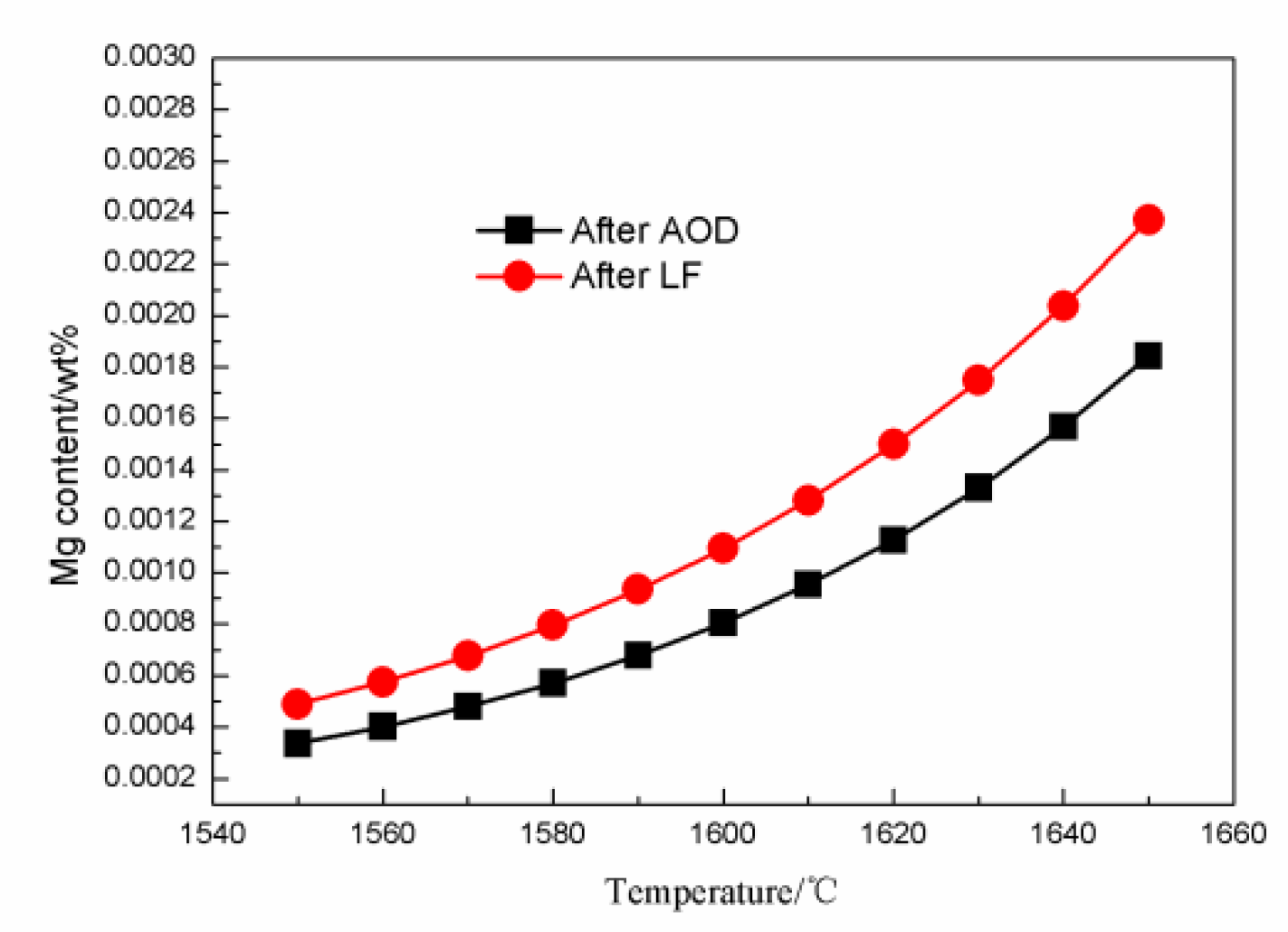
4.2. The Effect of Slag on the Als and Mg Content in Steel during the AOD-LF Process
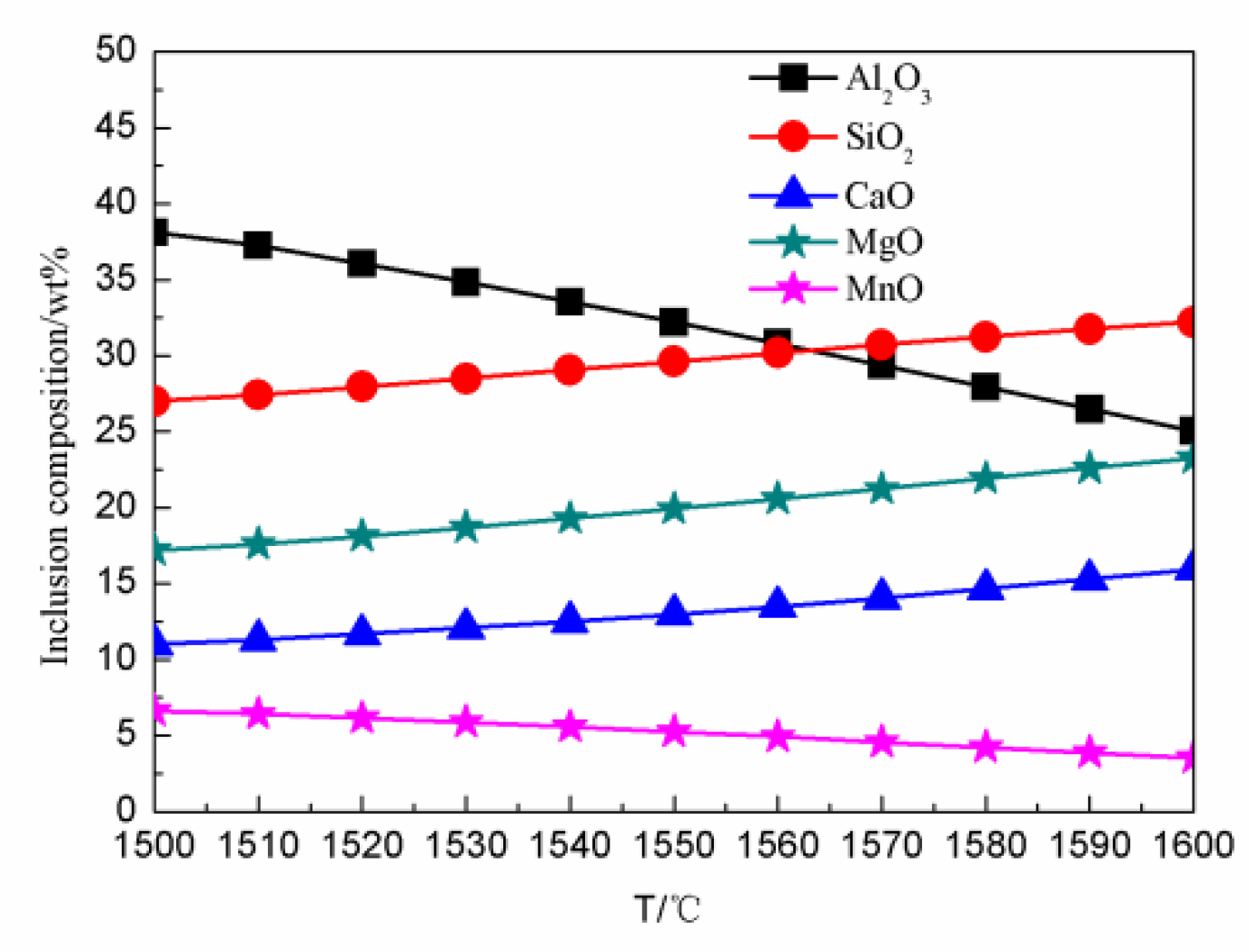
4.3. The Effect of Temperature on the Inclusion Contents in the Tundish
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momeni, A.; Abbasi, S.M. Repetitive thermomechanical processing towards ultra-fine grain structure in 301, 304 and 304L stainless steels. J. Mater. Sci. Technol. 2011, 27, 338–343. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Z.; Shi, J.-B.; Tao, L.; Song, S.-Z. Characterization of stress corrosion crack growth of 304 stainless steel by electrochemical noise and scanning kelvin probe. J. Cent. South Univ. T. 2010, 17, 13–18. [Google Scholar] [CrossRef]
- Jiao, Z.; Hesterberg, J.; Was, G.S. Effect of post-irradiation annealing on the irradiated microstructure of neutron-irradiated 304L stainless steel. J. Nucl. Mater. 2018, 500, 220–234. [Google Scholar] [CrossRef]
- Chikhi, N.; Fouquart, P.; Delacroix, J.; Piluso, P. Measurement of type 304L stainless steel and 16MND5 ferritic steel density and surface tension: possible impact for stratified molten pool. Nucl. Technol. 2019, 205, 200–212. [Google Scholar] [CrossRef]
- Gupta, J.; Hure, J.; Tanguy, B.; Laffont, L.; Lafont, M.C.; Andrieu, E. Evaluation of stress corrosion cracking of irradiated 304L stainless steel in PWR environment using heavy ion irradiation. J. Nucl. Mater. 2016, 476, 82–92. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Raja, V.S.; Tewari, R.; Dey, G.K. Low temperature thermal ageing embrittlement of austenitic stainless steel welds and its electrochemical assessment. Corros. Sci. 2012, 54, 278–290. [Google Scholar] [CrossRef]
- Garnier, J.; Bréchet, Y.; Delnondedieu, M.; Pokor, C.; Dubuisson, P.; Renault, A.; Averty, X.; Massoud, J.P. Irradiation creep of SA 304L and CW 316 stainless steels: Mechanical behaviour and microstructural aspects. Part i: Experimental results. J. Nucl. Mater. 2011, 413, 63–69. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Deng, X.; Barati, M.; McLean, A. Effect of alloy addition on inclusion evolution in stainless steels. Ironmak. Steelmak. 2017, 44, 152–158. [Google Scholar] [CrossRef]
- Yoshioka, T.; Nakahata, K.; Kawamura, T.; Ohba, Y. Factors to determine inclusion compositions in molten steel during the secondary refining process of case-hardening steel. ISIJ Int. 2016, 56, 1973–1981. [Google Scholar] [CrossRef]
- Kaushik, P.; Lehmann, J.; Nadif, M. State of the art in control of inclusions, their characterization, and future requirements. Metall. Mater. Trans. B 2012, 43, 710–725. [Google Scholar] [CrossRef]
- Kaushik, P.; Pielet, H.; Yin, H. Inclusion characterisation – tool for measurement of steel cleanliness and process control: Part 1. Ironmak. Steelmak. 2009, 36, 561–571. [Google Scholar] [CrossRef]
- Bi, Y.; Karasev, A.V.; Jönsson, P.G. Evolution of different inclusions during ladle treatment and continuous casting of stainless steel. ISIJ Int. 2013, 53, 2099–2109. [Google Scholar] [CrossRef]
- Ehara, Y.; Yokoyama, S.; Kawakami, M. Formation mechanism of inclusions containing MgO-Al2O3 spinel in type 304 stainless steel. Tetsu-to-Hagane 2007, 93, 208–214. [Google Scholar] [CrossRef]
- Park, J.H. Thermodynamic investigation on the formation of inclusions containing MgAl2O4 spinel during 16Cr–14Ni austenitic stainless steel manufacturing processes. Mater. Sci. Eng. A 2008, 472, 43–51. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Deng, X.X.; Barati, M.; McLean, A. Inclusion evolution during refining and continuous casting of 316L stainless steel. Ironmak. Steelmak. 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.-K.; Kang, Y.-B.; Lee, H.-G. Compositional evolution of oxide inclusions in austenitic stainless steel during continuous casting. Steel Res. Int. 2015, 86, 284–292. [Google Scholar] [CrossRef]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef]
- Yan, P.; Huang, S.; Van dyck, J.; Guo, M.; Blanpain, B. Desulphurisation and inclusion behaviour of stainless steel refining by using CaO-Al2O3 based slag at low sulphur levels. ISIJ Int. 2014, 54, 72–81. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Fang, W.; Shao, S.; Yang, J.; Mao, W. Effect of slag composition on inclusions in Si-deoxidized 18Cr-8Ni stainless steels. Metall. Mater. Trans. B 2016, 47, 1024–1034. [Google Scholar] [CrossRef]
- Sakata, K. Technology for production of austenite type clean stainless steel. ISIJ Int. 2006, 46, 1795–1799. [Google Scholar] [CrossRef]
- Park, J.H. Formation mechanism of spinel-type inclusions in high-alloyed stainless steel melts. Metall. Mater. Trans. B 2007, 38, 657–663. [Google Scholar] [CrossRef]
| Stage | C | Si | Mn | Ni | Cr | Al | Mg | Ca | T.O |
|---|---|---|---|---|---|---|---|---|---|
| After AOD | 0.011 | 0.14 | 1.50 | 8.12 | 17.99 | 0.003 | 0.0006 | 0.0011 | 0.0152 |
| After LF | 0.015 | 0.37 | 1.59 | 8.21 | 18.14 | 0.004 | 0.0008 | 0.0006 | 0.0047 |
| Tundish | 0.015 | 0.38 | 1.58 | 8.27 | 18.10 | 0.004 | 0.0008 | 0.0006 | 0.0040 |
| Stage | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | FeO |
|---|---|---|---|---|---|---|
| After AOD | 55.97 | 30.21 | 4.47 | 1.37 | 1.49 | 2.36 |
| After LF | 58.69 | 26.63 | 6.08 | 1.89 | 0.07 | 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cheng, G.; Hou, Y.; Li, J. Oxide-Inclusion Evolution in the Steelmaking Process of 304L Stainless Steel for Nuclear Power. Metals 2019, 9, 257. https://doi.org/10.3390/met9020257
Chen X, Cheng G, Hou Y, Li J. Oxide-Inclusion Evolution in the Steelmaking Process of 304L Stainless Steel for Nuclear Power. Metals. 2019; 9(2):257. https://doi.org/10.3390/met9020257
Chicago/Turabian StyleChen, Xingrun, Guoguang Cheng, Yuyang Hou, and Jingyu Li. 2019. "Oxide-Inclusion Evolution in the Steelmaking Process of 304L Stainless Steel for Nuclear Power" Metals 9, no. 2: 257. https://doi.org/10.3390/met9020257
APA StyleChen, X., Cheng, G., Hou, Y., & Li, J. (2019). Oxide-Inclusion Evolution in the Steelmaking Process of 304L Stainless Steel for Nuclear Power. Metals, 9(2), 257. https://doi.org/10.3390/met9020257





