Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Solutions
2.2. Pretreatment of Substrates
2.3. Electrodeposition
2.4. Electrochemical Testing and Structural Characterization
3. Results and Discussion
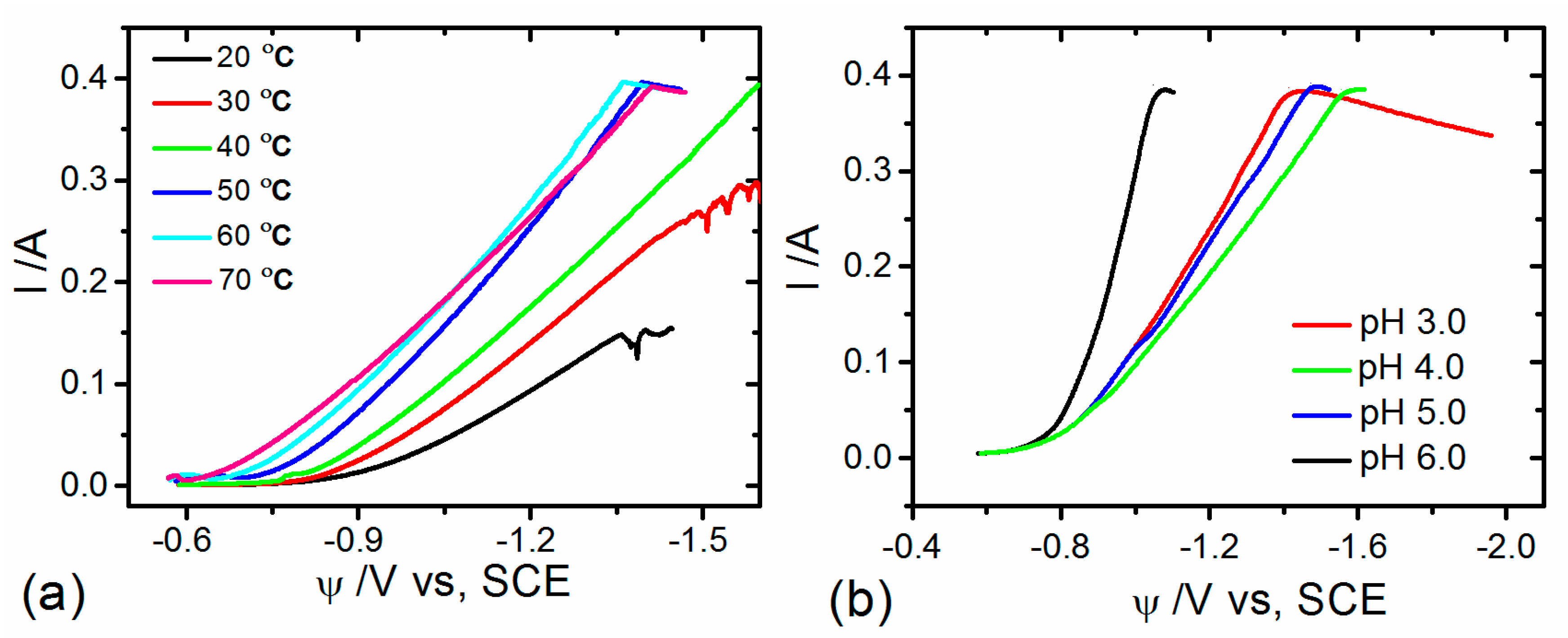
3.1. Voltametric Behavior
3.2. Effects of Current Densities on Morphology and Microstructure
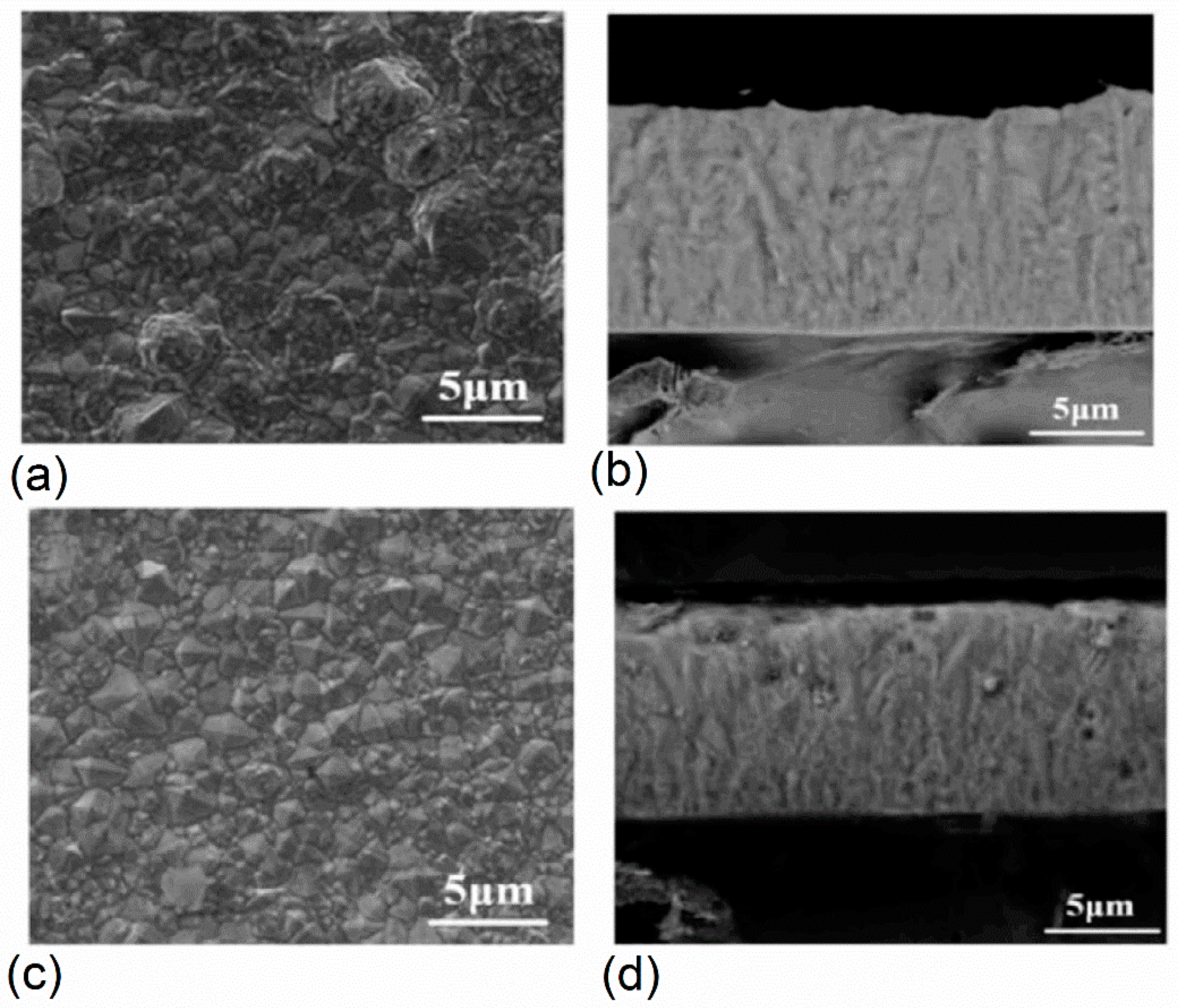
3.2.1. Morphological Characterization
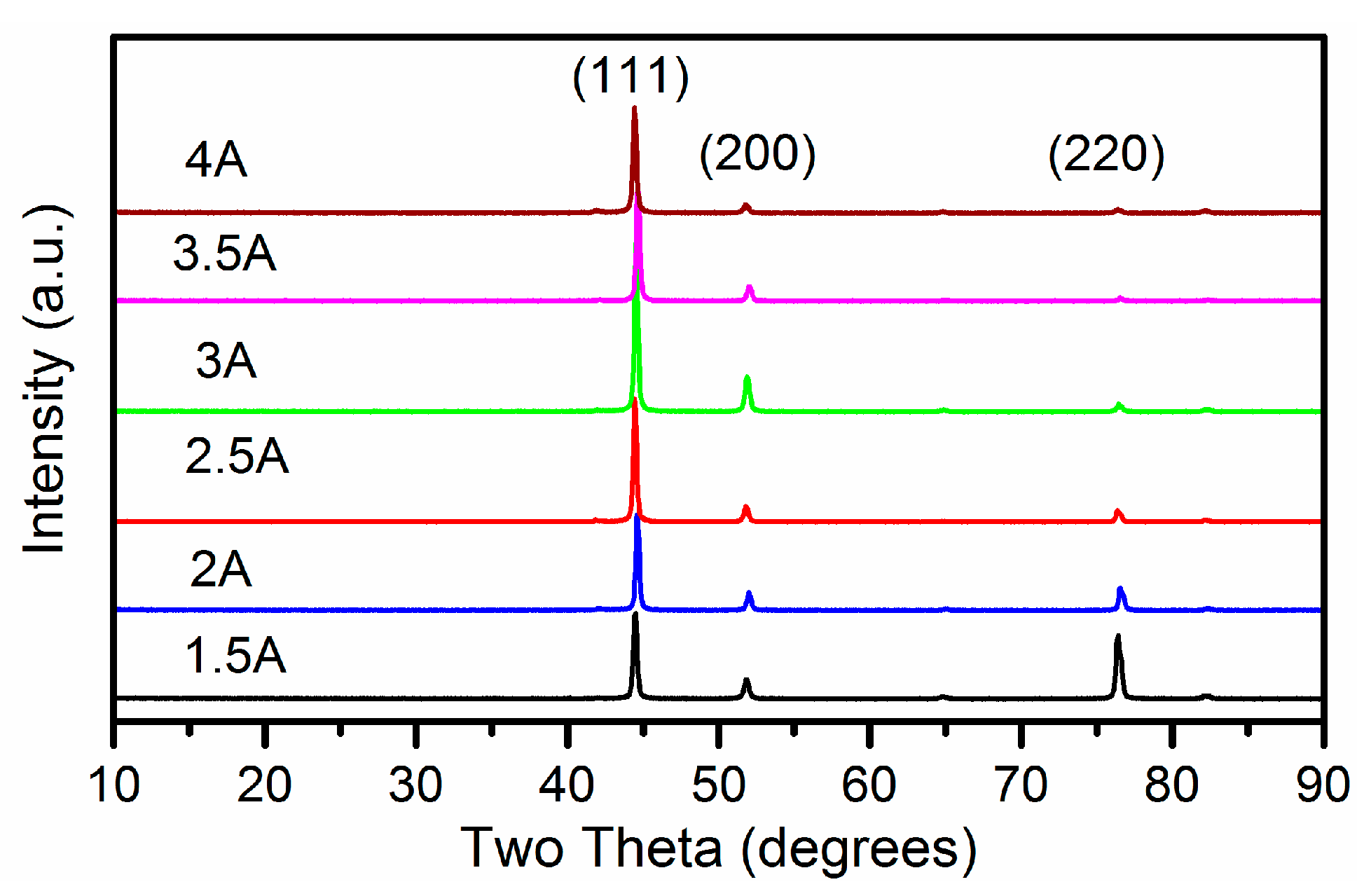
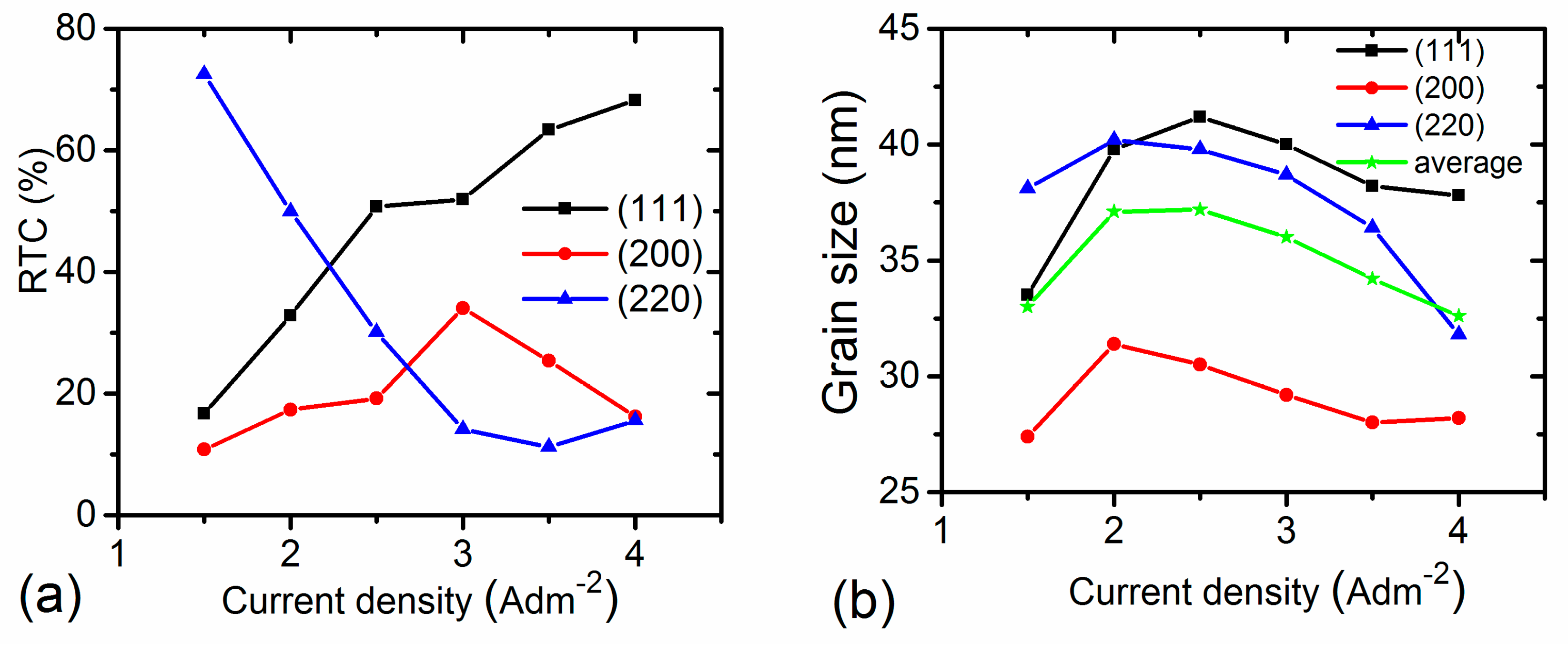
3.2.2. Structure Properties
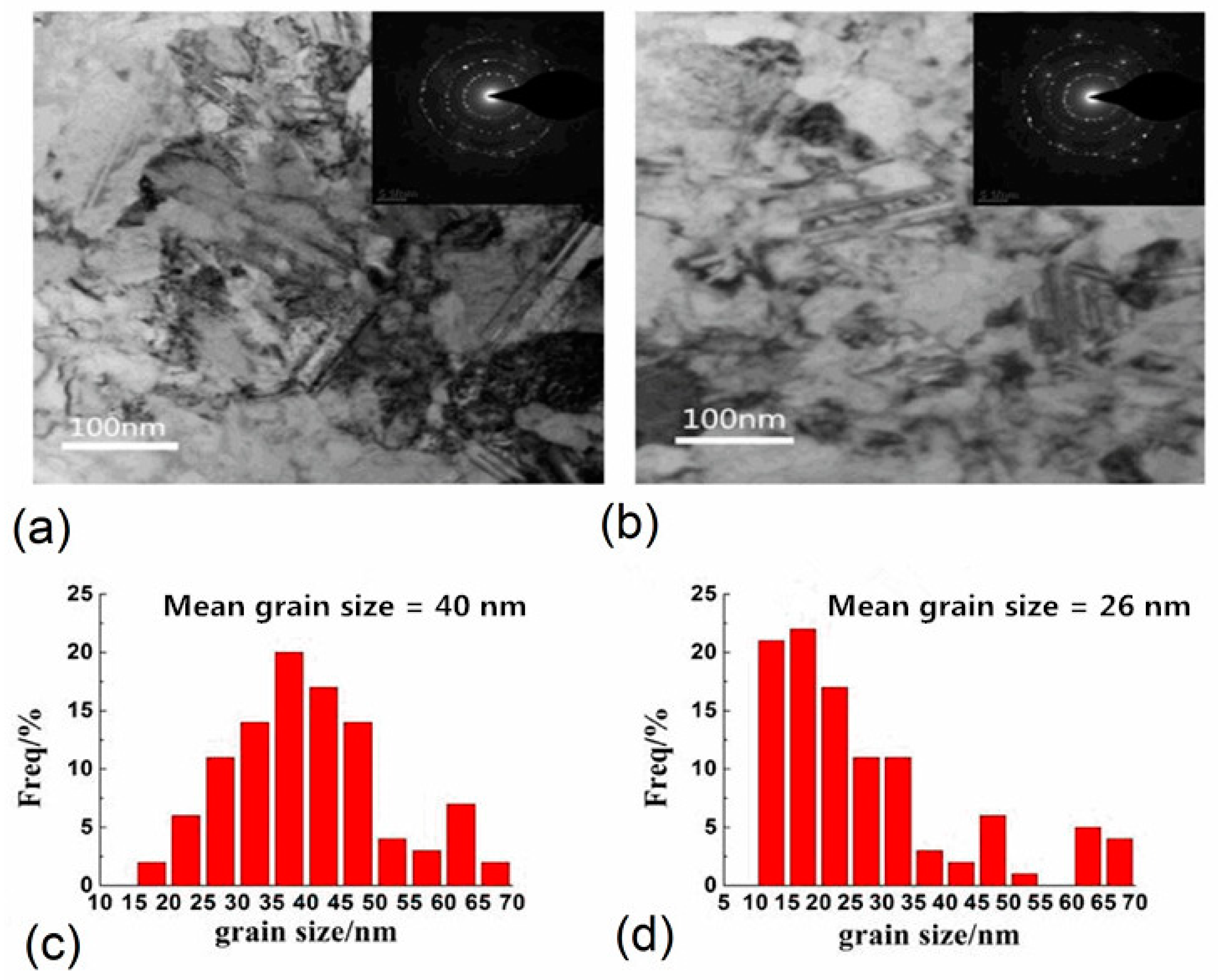
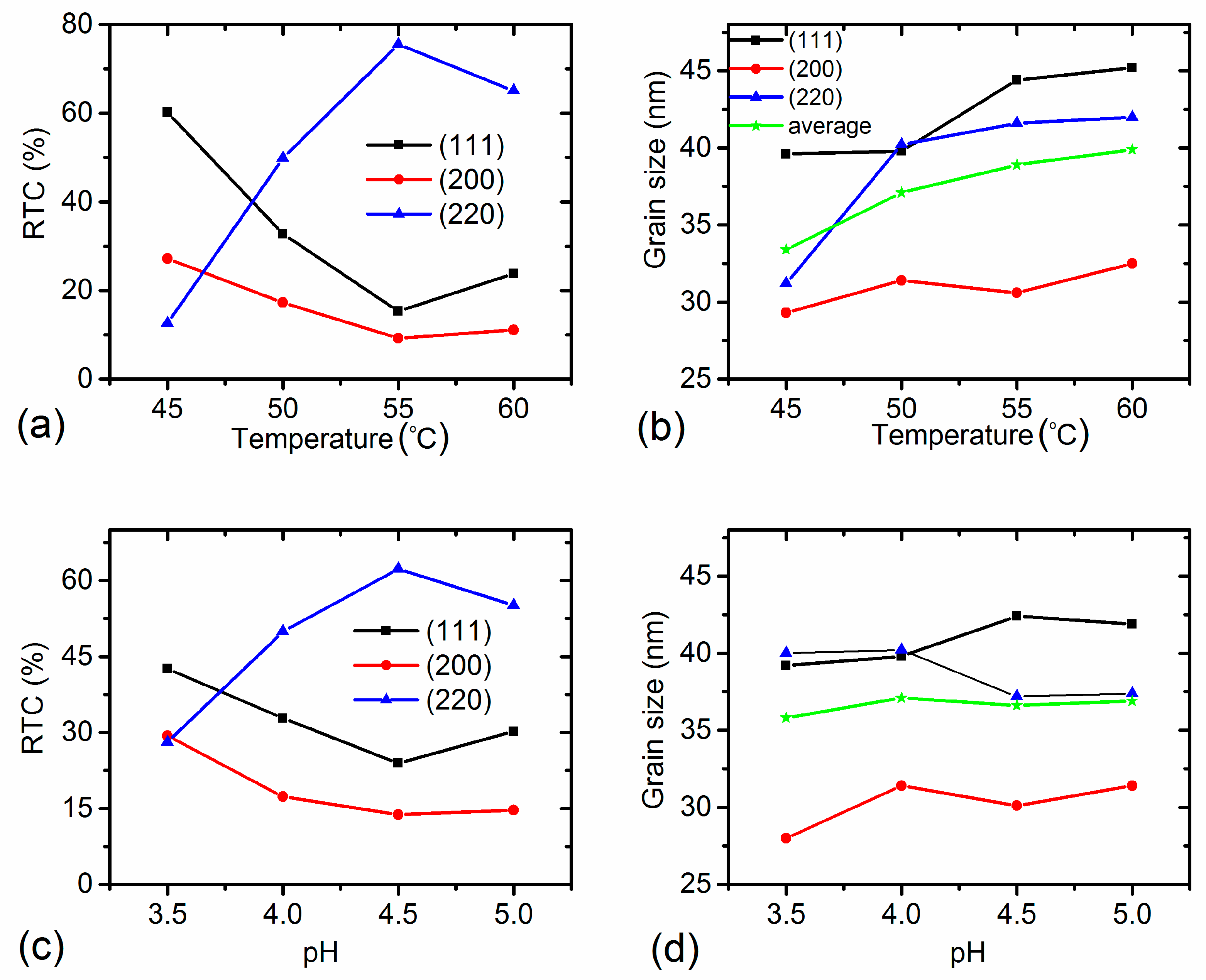
3.3. Effects of Temperature and pH Value on Morphology and Microstructure
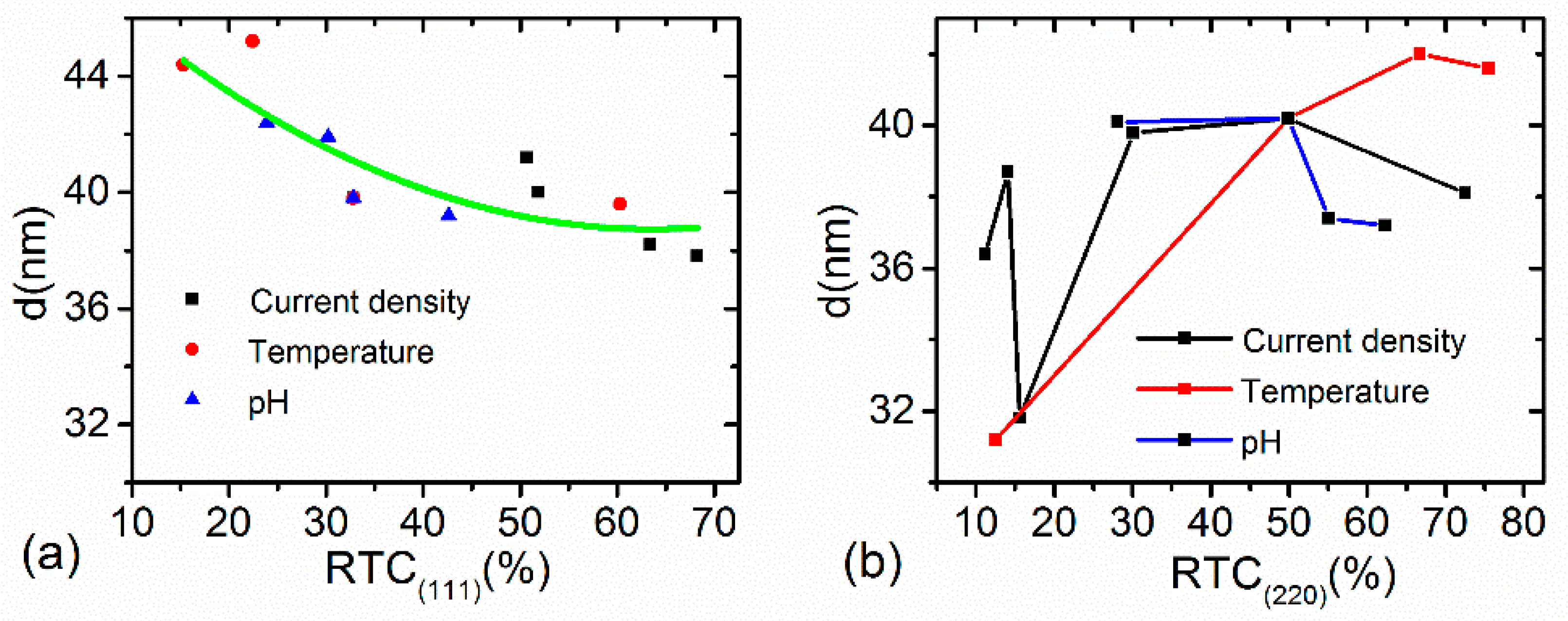
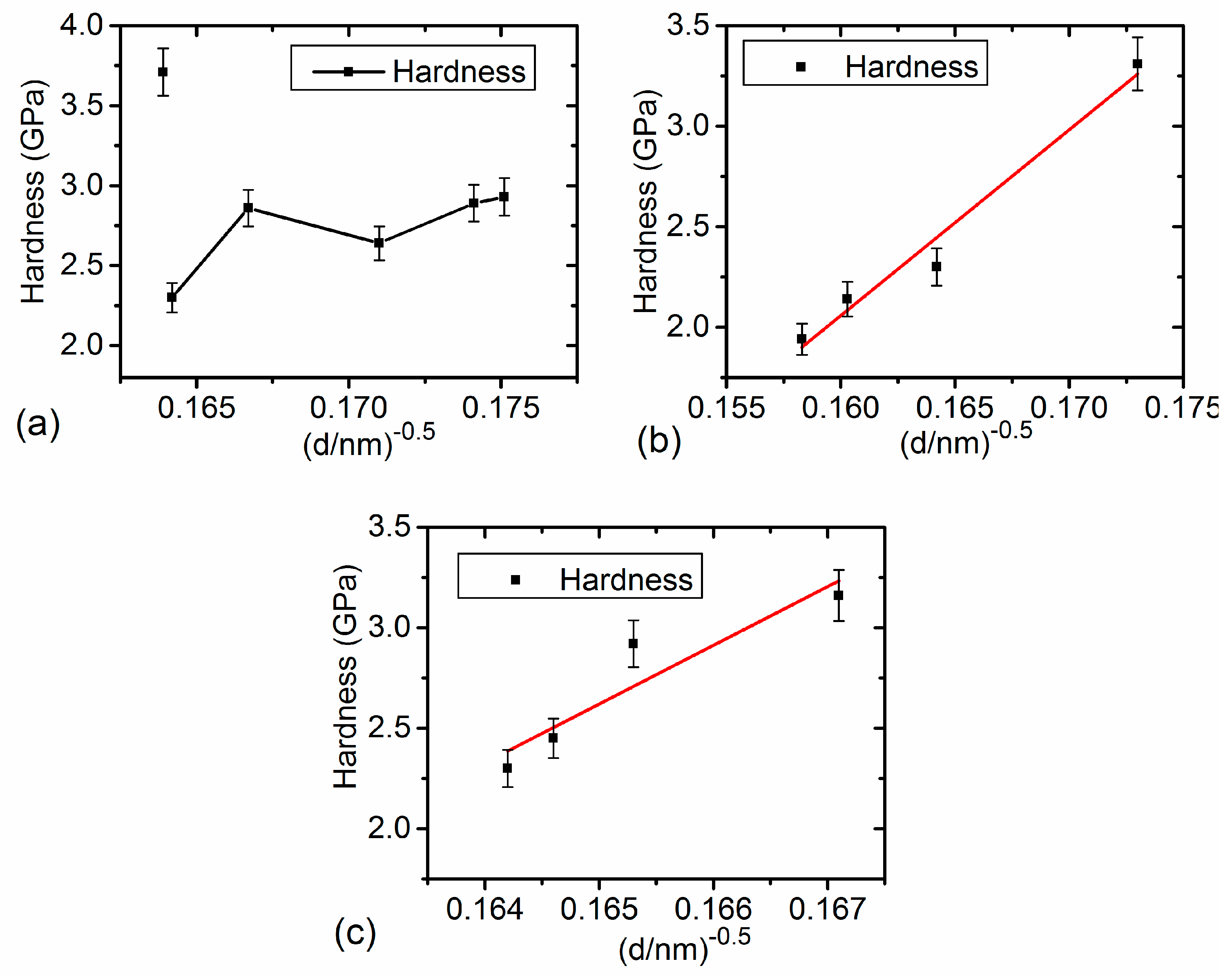
3.4. Relationship between Grain Size and Texture
4. Mechanical Properties of nc-Ni Coatings
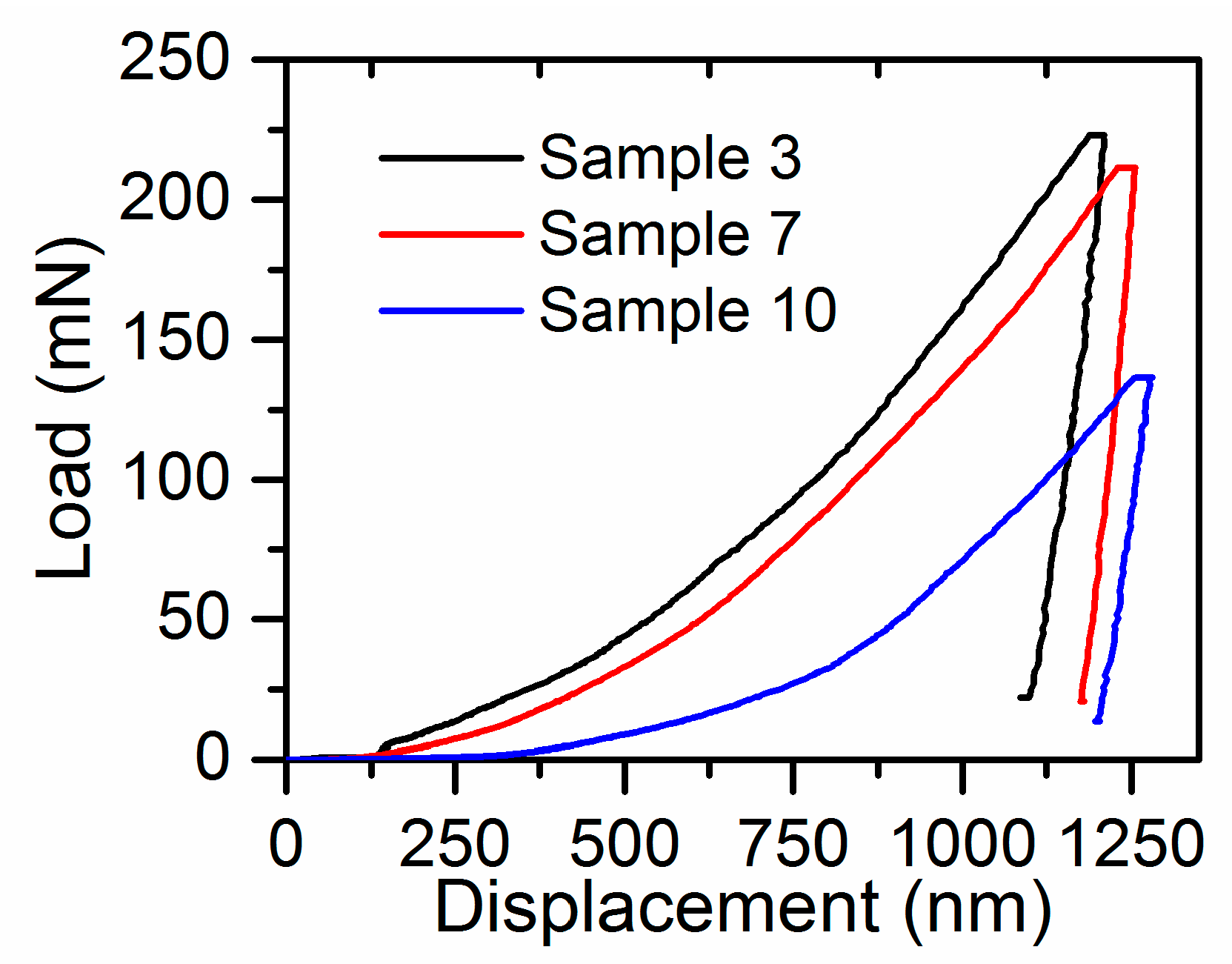
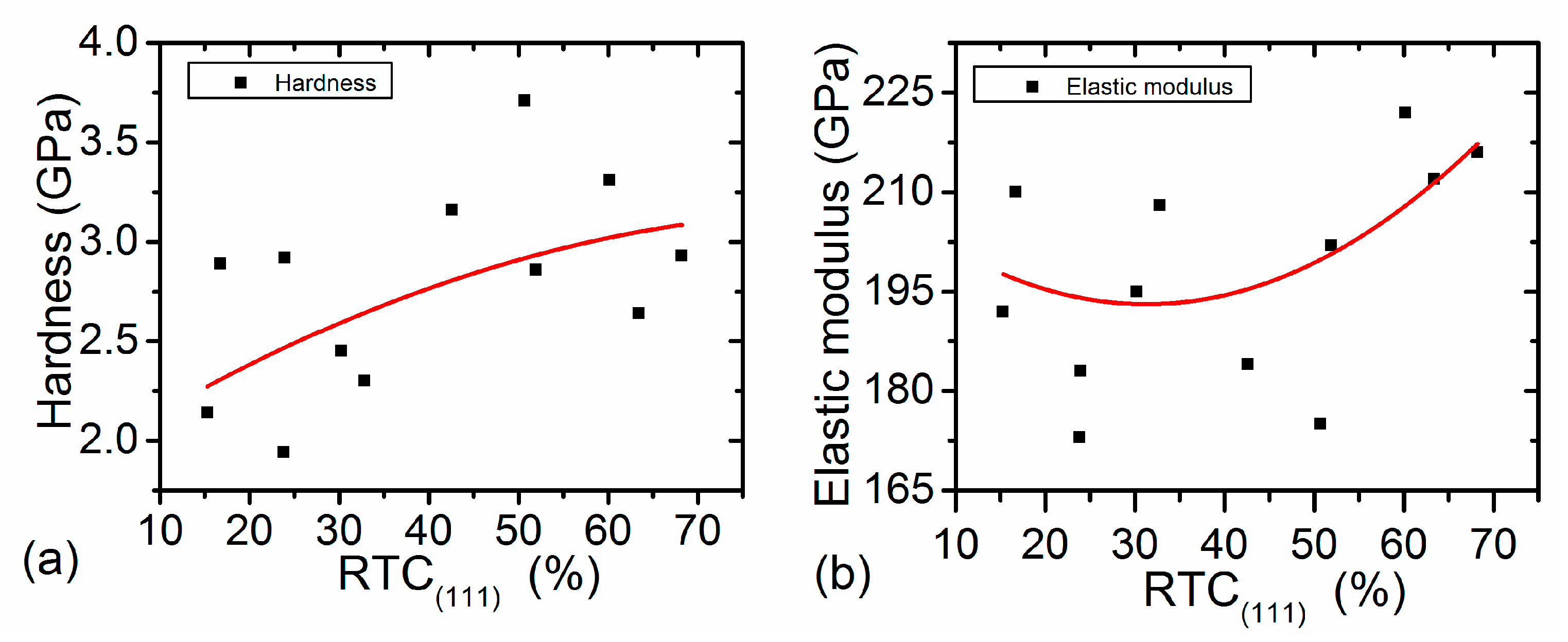
4.1. Hardness in Link with Structural Characteristics
4.2. Elastic Modulus in Link with Structural Characteristics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beltowska-Lehman, E.; Bigos, A.; Indyka, P.; Chojnacka, A.; Drewienkiewicz, A.; Zimowski, S.; Kot, M.; Szczerba, M.J. Optimisation of the electrodeposition process of Ni-W/ZrO2 nanocomposites. J. Electroanal. Chem. 2018, 813, 39–51. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Omidvar, H.; Javanbakht, M.; Mozafari, A. Textural and structural evolution of pulse electrodeposited Ni/diamond nanocomposite coatings. J. Alloy. Compd. 2017, 704, 809–817. [Google Scholar] [CrossRef]
- Kim, K. Measurement and analysis of friction and wear on electrodeposited coatings against a high carbon chrome steel ball. J. Mater. Res. 2016, 31, 1865–1872. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, C.P.; Balani, K. Synergistic role of carbonaceous reinforcements on multi length scale tribology of electrophoretically deposited nickel-boron nitride coatings. Mater Res Bull. 2018, 99, 61–72. [Google Scholar] [CrossRef]
- Ziebell, T.D.; Schuh, C.A. Residual stress in electrodeposited nanocrystalline nickel-tungsten coatings. J. Mater. Res. 2012, 27, 1271–1284. [Google Scholar] [CrossRef]
- Shen, X.; Wu, M.; Ji, D.; Xu, Q.; Cheng, D. The mechanical behavior of a layered nanostructured Ni with an alternating growth of ultrafine grains and nano-sized grains fabricated by electrodeposition. Mater. Sci. Eng. A 2018, 713, 43–51. [Google Scholar] [CrossRef]
- Bakonyi, I.; Toth-Kadar, E.; Tarnoczi, T.; Varga, L.K.; Cziraki, A.; Gerocs, I.; Fogarassy, B. Structure and properties of fine-grained electrodeposited nickel. Nanostruct. Mater. 1993, 3, 155–161. [Google Scholar] [CrossRef]
- Bakonyi, I.; Toth-Kadar, E.; Pogany, L.; Cziraki, A.; Gerocs, I.; Varga-Josepovits, K.; Arnold, B.; Wetzig, K. Preparation and characterization of DC-plated nanocrystalline nickel electrodeposits. Surf. Coat. Technol. 1996, 78, 124–136. [Google Scholar] [CrossRef]
- Toth-Kadar, E.; Bakonyi, I.; Pogany, L.; Czira’ki, A. Microstructure and electrical transport properties of pulse-plated nanocrystalline nickel electrodeposits. Surf. Coat. Technol. 1997, 88, 57–65. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. Effect of electroplating parameters on microstructure of nanocrystalline nickel coatings. J. Mater. Sci. Technol. 2010, 26, 82–86. [Google Scholar] [CrossRef]
- Sarac, U.; Baykulb, M.C. Morphological and microstructural properties of two-phase Ni–Cu films electrodeposited at different electrolyte temperatures. J. Alloy. Compd. 2013, 552, 195–201. [Google Scholar] [CrossRef]
- Haciismailoglu, M.; Alper, M. Effect of electrolyte pH and Cu concentration on microstructure of electrodeposited Ni–Cu alloy films. Surf. Coat. Technol. 2011, 206, 1430–1438. [Google Scholar] [CrossRef]
- Boubatra, M.; Azizi, A.; Schmerber, G.; Dinia, A. The influence of pH electrolyte on the electrochemical deposition and properties of nickel thin films. Ionics 2012, 18, 425–432. [Google Scholar] [CrossRef]
- Ghosh, P.; Petegem, S.V.; Swygenhoven, H.V.; Chokshi, A.H. An in-situ synchrotron study on microplastic flow of electrodeposited nanocrystalline nickel. Mater. Sci. Eng. A 2017, 701, 101–110. [Google Scholar] [CrossRef]
- Matsui, I.; Kanetake, M.; Mori, H.; Takigawa, Y.; Higashi, K. Relationship between grain boundary relaxation strengthening and orientation in electrodeposited bulk nanocrystalline Ni alloys. Mater. Lett 2017, 205, 211–214. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Sanaeian, M.R.; Samardak, A.S.; Sukovatitsina, E.V.; Ognev, A.V.; Chebotkevich, L.A.; Hosseini, M.G.; Abdolmaleki, M. An investigation on the effect of surface morphology and crystalline texture on corrosion behavior, structural and magnetic properties of electrodeposited nanocrystalline nickel films. Appl. Surf. Sci 2014, 292, 795–805. [Google Scholar] [CrossRef]
- Kang, J.; Zhao, W.; Zhang, G. Influence of electrodeposition parameters on the deposition rate and microhardness of nanocrystalline Ni coatings. Surf. Coat. Technol. 2009, 203, 1815–1818. [Google Scholar] [CrossRef]
- Godon, A.; Creus, J.; Cohendoz, S.; Conforto, E.; Feaugas, X.; Girault, P.; Savall, C. Effects of grain orientation on the Hall–Petch relationship in electrodeposited nickel with nanocrystalline grains. Scripta Mater. 2010, 62, 403–406. [Google Scholar] [CrossRef]
- Pathak, S.; Guinard, M.; Vernooij, M.G.C.; Cousin, B.; Wang, Z.; Michler, J.; Philippe, L. Influence of lower current densities on the residual stress and structure of thick nickel electrodeposits. Surf. Coat. Technol. 2011, 205, 3651–3657. [Google Scholar] [CrossRef]
- Baskaran, I.; Narayanan, T.S.N.S.; Stephen, A. Pulsed electrodeposition of nanocrystalline Cu–Ni alloy films and evaluation of their characteristic properties. Mater. Lett. 2006, 60, 1990–1995. [Google Scholar] [CrossRef]
- Savall, C.; Godon, A.; Creus, J.; Feaugas, X. Influence of deposition parameters on microstructure and contamination of electrodeposited nickel coatings from additive-free sulphamate bath. Surf. Coat. Technol. 2012, 206, 4394–4402. [Google Scholar] [CrossRef]
- Bolelli, G.; Candeli, A.; Lusvargh, L.; Manfredini, T.; Denoirjean, A.; Valette, S.; Ravaux, A.; Meillot, E. “Hybrid” plasma spraying of NiCrAlY+Al2O3+h-BN composite coatings for sliding wear applications. Wear 2017, 68, 378–379. [Google Scholar]
- Bund, A.; Thiemig, D. Influence of bath composition and pH on the electrocodeposition of alumina nanoparticles and nickel. Surf. Coat. Technol. 2007, 201, 7092–7099. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Pellicer, E.; Varea, A.; Pané, S.; Sivaraman, K.M.; Nelson, B.J.; Suriñach, S.; Baró, M.D.; Sort, J. A comparison between fine-grained and nanocrystalline electrodeposited Cu–Ni films. Insights on mechanical and corrosion performance. Surf. Coat. Technol. 2011, 205, 5285–5293. [Google Scholar] [CrossRef]
- Godon, A.; Creus, J.; Feaugas, X.; Conforto, E.; Pichon, L.; Armand, C.; Savall, C. Characterization of electrodeposited nickel coatings from sulphamate electrolyte without additive. Mater. Charact. 2011, 62, 164–173. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Vering, G.; Talian, I.; Smith, R.M.; Arlinghaus, H.F. Influence of pH on the electrolytic deposition of Ni–Co films. Thin Solid Films 2008, 516, 3045–3050. [Google Scholar] [CrossRef]
- Moharana, M.; Mallik, A. Nickel electrocrystallization in different electrolytes: An in-process and post synthesis analysis. Electrochimica. Acta. 2013, 98, 1–10. [Google Scholar] [CrossRef]
- Xue, Z.; Lei, W.; Wang, Y.; Qian, H.; Li, Q. Effect of pulse duty cycle on mechanical properties and microstructure of nickel-graphene composite coating produced by pulse electrodepositionunder supercritical carbon dioxide. Surf. Coat. Technol. 2017, 325, 417–428. [Google Scholar] [CrossRef]
- Sribalaji, M.; Asiq Rahman, O.S.; Laha, T.; Keshri, A.K. Nanoindentation and nanoscratch behavior of electroless deposited nickel-phosphorous coating. Mater. Chem. Physics 2016, 177, 220–228. [Google Scholar] [CrossRef]
- Müller, T.; Grimwood, J.; Bachmaier, A.; Pippan, R. Electrodeposition of Fe-C alloys from citrate baths: structure, mechanical properties, and thermal stability. Metals 2018, 8, 363. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xu, T.; Xue, Q. A comparative study on the tribological behavior of nanocrystalline nickel and cobalt coatings correlated with grain size and phase structure. Mater. Chem. Phys. 2006, 99, 96–103. [Google Scholar] [CrossRef]
- Mishra, A.C.; Thakur, A.K.; Srinivas, V. Effect of deposition parameters on microstructure of electrodeposited nickel thin films. J. Mater. Sci. 2009, 44, 3520–3527. [Google Scholar] [CrossRef]
- Torrents, A.; Yang, H.; Mohamed, A.F. Effect of annealing on hardness and the modulus of elasticity in bulk nanocrystalline nickel. Metall. Mater. Trans. A 2010, 41, 621–630. [Google Scholar] [CrossRef]
- Milstein, F.; Chantasiriwan, S. Theoretical study of the response of 12 cubic metals to uniaxial loading. Phys. Rev. B 1998, 58, 6006–6018. [Google Scholar] [CrossRef]
| Sample | Varied Parameters | |
|---|---|---|
| Current density (A dm−2) (T = 50 °C, pH 4) | 1 | 1.5 |
| 2 | 2 | |
| 3 | 2.5 | |
| 4 | 3 | |
| 5 | 3.5 | |
| 6 | 4 | |
| Temperature (°C) (pH 4, I = 2 A·dm−2) | 7 | 45 |
| 8 | 50 | |
| 9 | 55 | |
| 10 | 60 | |
| pH (I = 2 A·dm−2, T = 50 °C) | 11 | 3.5 |
| 12 | 4 | |
| 13 | 4.5 | |
| 14 | 5 |
| j (A·dm2) | H (GPa) | E (GPa) | T (°C) | H (GPa) | E (GPa) | pH | H (GPa) | E (GPa) |
|---|---|---|---|---|---|---|---|---|
| 1.5 | 2.89 ± 0.3 | 210.0 ± 4.2 | 45 | 3.31 ± 0.3 | 222.8 ± 4.1 | 3.5 | 3.16 ± 0.3 | 184.4 ± 3.8 |
| 2.0 | 2.30 ± 0.3 | 208.0 ± 3.4 | 50 | 2.30 ± 0.3 | 208.0 ± 5.3 | 4.0 | 2.30 ± 0.3 | 208.0 ± 4.3 |
| 2.5 | 3.71 ± 0.3 | 175.0 ± 5.1 | 55 | 2.14 ± 0.3 | 191.7 ± 4.3 | 4.5 | 2.92 ± 0.3 | 183.8 ± 4.1 |
| 3.0 | 2.86 ± 0.3 | 202.4 ± 3.6 | 60 | 1.94 ± 0.3 | 172.7 ± 3.9 | 5.0 | 2.45 ± 0.3 | 195.2 ± 3.9 |
| 3.5 | 2.64 ± 0.3 | 212.0 ± 3.8 | ||||||
| 4.0 | 2.93 ± 0.3 | 216.0 ± 5.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Ren, Y.-Y.; Zhang, Y.-H.; Wang, S.; Li, L. Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings. Metals 2019, 9, 188. https://doi.org/10.3390/met9020188
Feng L, Ren Y-Y, Zhang Y-H, Wang S, Li L. Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings. Metals. 2019; 9(2):188. https://doi.org/10.3390/met9020188
Chicago/Turabian StyleFeng, Lu, Yong-Yue Ren, Yan-Heng Zhang, Shibin Wang, and Linan Li. 2019. "Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings" Metals 9, no. 2: 188. https://doi.org/10.3390/met9020188
APA StyleFeng, L., Ren, Y.-Y., Zhang, Y.-H., Wang, S., & Li, L. (2019). Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings. Metals, 9(2), 188. https://doi.org/10.3390/met9020188




