Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
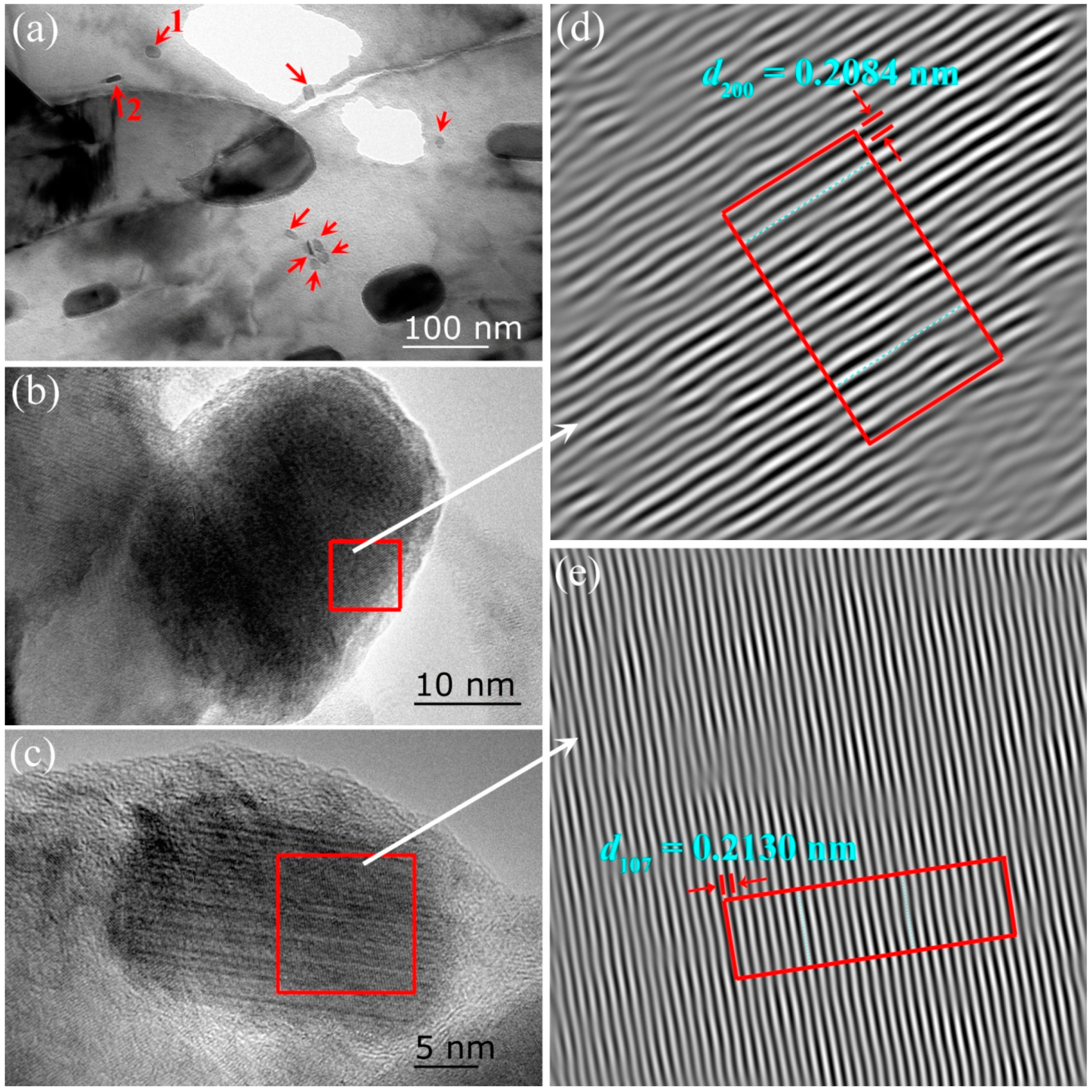
3.1. Microstructural Characterization
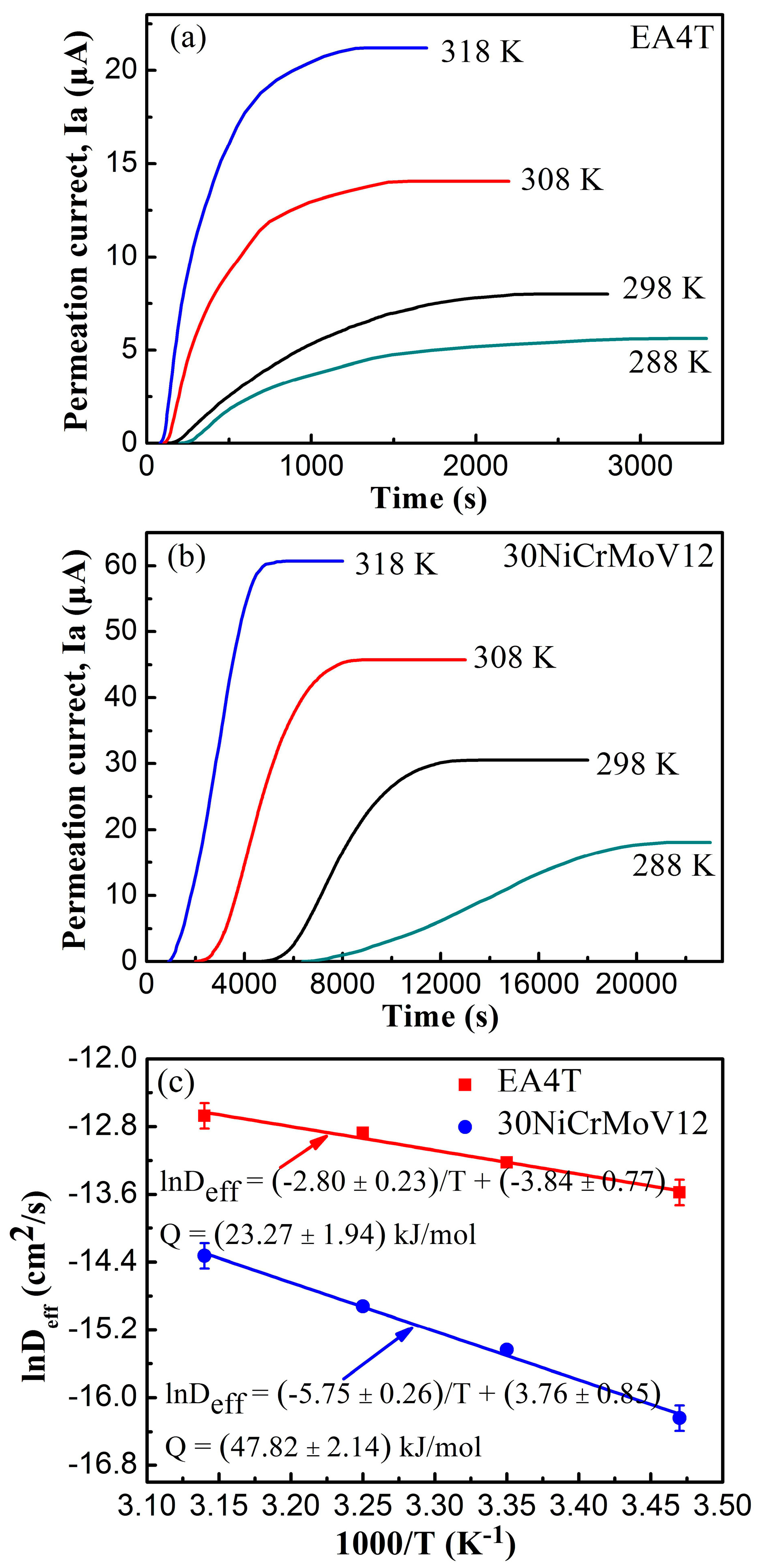
3.2. Hydrogen Permeation Behavior
3.3. Density and Nature of H-Traps
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, S.C.; Zhang, S.Q.; Xu, Z.W.; Kang, G.Z.; Cai, L.X. Cyclic plastic strain based damage tolerance for railway axles in China. Int. J. Fatigue 2016, 93, 64–70. [Google Scholar] [CrossRef]
- Huo, Y.; Bai, Q.; Lin, J.; Wang, B.; Zhou, J. A new application of unified constitutive equations for cross wedge rolling of a high-speed railway axle steel. J. Mater. Process Technol. 2015, 223, 274–281. [Google Scholar] [CrossRef]
- Beretta, S.; Conte, A.L.; Rudlin, J.; Panggabean, D. From atmospheric corrosive attack to crack propagation for A1N railway axles steel under fatigue: Damage process and detection. Eng. Fail. Anal. 2015, 47, 252–262. [Google Scholar] [CrossRef]
- Pokorny, P.; Vojtek, T.; Nahlik, L.; Hutar, P. Crack closure in near-threshold fatigue crack propagation in railway axle steel EA4T. Eng. Fract. Mech. 2017, 185, 2–19. [Google Scholar] [CrossRef]
- Yamamoto, M.; Makino, K.; Ishiduka, H. Comparison of crack growth behavior between full-scale railway axle and scaled specimen. Int. J. Fatigue 2016, 92, 159–165. [Google Scholar] [CrossRef]
- Lan, L.Y.; Kong, X.W.; Hu, Z.Y.; Qiu, C.L.; Zhao, D.W.; Du, L.X. Hydrogen permeation behavior in relation to microstructural evolution of low bainitic steel weldments. Corros. Sci. 2016, 112, 180–193. [Google Scholar] [CrossRef]
- Pereira, P.A.S.; Franco, C.S.G.; Filho, J.L.M.G.; Santos, D.S.D. Hydrogen effects on the microstructure of a 2.25Cr–1Mo–0.25V steel welded joint. Int. J. Hydrogen Energy 2015, 40, 17136–17143. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Novak, P.; Ahn, D.C.; Liu, J.B.; Sofronis, P.; Johson, D.D.; Robertson, I.M. Recent advances in the study of structural materials compatibility with hydrogen. Adv. Mater. 2010, 22, 1128–1135. [Google Scholar] [CrossRef]
- Otsuka, S.; Fujita, S.; Tada, E.; Nishikata, A.; Tsuru, T. Evaluation of hydrogen absorption into steel in automobile moving environments. Corros. Sci. 2015, 98, 430–437. [Google Scholar] [CrossRef]
- Du, X.S.; Cao, W.B.; Wang, C.D.; Li, S.J.; Zhao, J.Y.; Sun, Y.F. Effect of microstructures and inclusions on hydrogen-induced cracking and blistering of A537 steel. Mater. Sci. Eng. A 2015, 642, 181–186. [Google Scholar] [CrossRef]
- Raina, A.; Deshpande, V.S.; Fleck, N.A. Analysis of thermal desorption of hydrogen in metallic alloys. Acta Mater. 2018, 144, 777–785. [Google Scholar] [CrossRef]
- Frappart, S.; Feaugas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Hydrogen solubility, diffusivity and trapping in a tempered Fe–C–Cr martensitic steel under various mechanical stress states. Mater. Sci. Eng. A 2012, 534, 384–393. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Zhang, Z.Y.; Jiao, S.H. Precipitates and hydrogen permeation in HSLA steel produced by TMCP. Corros. Eng. Sci. Technol. 2011, 46, 375–379. [Google Scholar] [CrossRef]
- Pressouyre, G.M. A classification of hydrogen traps in steel. Metall. Trans. A 1979, 10, 1571–1573. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Winzer, N.; Rott, O.; Thiessen, R.; Thomas, I.; Mraczek, K.; Hoche, T.; Wright, L.; Mrovec, M. Hydrogen diffusion and trapping in Ti-modified advanced high strength steels. Mater. Des. 2016, 92, 450–461. [Google Scholar] [CrossRef]
- Hirth, J.P.; Dienes, G.J. On the rate of equilibration of diffusional traps in solids. Acta Mater. 1982, 30, 2061–2064. [Google Scholar] [CrossRef]
- Nagao, A.; Martin, M.L.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The effect of nanosized (Ti, Mo)C precipitates on hydrogen embrittlement of tempered lath martensitic steel. Acta Mater. 2014, 74, 244–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Zhou, T.; Liu, D.W.; Fang, X.W.; Li, H.P.; Suo, J.P. Effects of Ti and a twice quenching treatment on the microstructure and ductile brittle transition temperature of 9CrWVTiN steels. Mater. Des. 2015, 88, 675–682. [Google Scholar] [CrossRef]
- Zamberger, S.; Lang, P.; Klosch, G.; Klarner, J.; Kozeschnik, E. Long-range diffusion of H in the presence of traps in a microalloyed steel. Comp. Mater. Sci. 2016, 113, 266–274. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Liu, G. Effect of hydrogen on ductility of high strength 3Ni–Cr–Mo–V steels. Mater. Sci. Eng. A 2014, 594, 40–47. [Google Scholar] [CrossRef]
- Cui, Q.Q.; Wu, J.S.; Xie, D.H.; Wu, X.G.; Huang, Y.H.; Li, X.G. Effect of nanosized NbC precipitates on hydrogen diffusion in X80 pipeline steel. Materials 2017, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, J.C.; Margot-Marette, H.; Brass, A.M.; Aucouturier, M. Sulfide stress cracking of high strength modified Cr–Mo steels. Metall. Mater. Trans. A 1985, 16, 935–944. [Google Scholar] [CrossRef]
- Yokota, T.; Shiraga, T. Evaluation of hydrogen content trapped by Vanadium precipitates in a steel. ISIJ Int. 2003, 43, 534–538. [Google Scholar] [CrossRef]
- Asahi, H.; Hirakami, D.; Yamasaki, S. Hydrogen trapping behavior in Vanadium-added steel. ISIJ Int. 2003, 43, 527–533. [Google Scholar] [CrossRef]
- Mendibide, C.; Sourmail, T. Composition optimization of high-strength steels for sulfide stress cracking resistance improvement. Corros. Sci. 2009, 51, 2878–2884. [Google Scholar] [CrossRef]
- Parvathavarthini, N.; Saroja, S.; Dayal, R.K.; Khatak, H.S. Studies on hydrogen permeability of 2.25% Cr–1% Mo ferritic steel: Correlation with microstructure. J. Nucl. Mater. 2001, 288, 187–196. [Google Scholar] [CrossRef]
- Li, D.; Gangloff, R.P.; Scully, J.R. Hydrogen trap states in ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. A 2004, 35, 849–864. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe–C–Ti alloys. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Evaluation of the effect of V4C3 precipitates on the hydrogen induced mechanical degrdation in Fe–C–V alloys. Mater. Sci. Eng. A 2016, 675, 299–313. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Evaluation of the role of Mo2C in hydrogen induced ductility loss in Q&T Fe–C–Mo alloys. Int. J. Hydrogen Energy 2016, 41, 14310–14329. [Google Scholar]
- Depover, T.; Verbeken, K. Hydrogen trapping and hydrogen induced mechanical degradation in lab cast Fe–C–Cr alloys. Mater. Sci. Eng. A 2016, 669, 134–149. [Google Scholar] [CrossRef]
- Herbst, S.; Besserer, H.B.; Grydin, O.; Milenin, A.; Maier, H.J.; Nurnberger, F. Holistic consideration of grain growth behavior of tempering steel 34CrNiMo6 during heating processes. J. Mater. Process Technol. 2016, 229, 61–71. [Google Scholar] [CrossRef]
- Ogawa, Y.; Matsunaga, H.; Yamaba, M.Y.; Matsuoka, H. Fatigue limit of carbon and Cr–Mo steels as a small fatigue crack threshold in high-pressure hydrogen gas. Int. J. Hydrogen Energy 2018, 43, 20133–20142. [Google Scholar] [CrossRef]
- Murakami, Y.; Nomoto, T.; Ueda, T. On the mechanism of fatigue failure in the superlong life regime (N > 107 cycles). Part I: Influence of hydrogen trapped by inclusions. Fatigue Fract. Eng. Mater. Struct. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The adsorption and diffusion on electrolytic hydrogen in palladium. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1962, 270, 90–102. [Google Scholar]
- EN ISO 17081: Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique; European Committee for Standardization: Brussels, Belgium, 2008.
- Jiang, B.; Wu, M.; Zhang, M.; Liu, Y.Z. Microstructural characterization, strengthening and toughening mechanisms of a quenched and tempered steel: Effect of heat treatment parameters. Mater. Sci. Eng. A 2017, 707, 306–314. [Google Scholar] [CrossRef]
- Yen, S.K.; Huang, I.B. Critical hydrogen concentration for hydrogen induced blistering on AISI 430 stainless steel. Mater. Chem. Phys. 2003, 80, 662–666. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.L.; Zhang, M.X.; Zhou, Q.J.; Atrens, A. The influence of hydrogen on the mechanical and fracture properties of some martensitic advanced high strength steels studied using the linearly increasing stress test. Corros. Sci. 2015, 99, 98–117. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: An experimental proof of the HELP mechanism. Int. J. Hydrogen Energy 2018, 43, 3050–3061. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Kwon, Y.J.; Mun, D.J.; Yoo, J.Y.; Lee, C.S. Role of Mo/V carbides in hydrogen embrittlement of tempered martensitic steel. Corros. Rev. 2015, 33, 433–441. [Google Scholar] [CrossRef]
- Araujo, D.F.; Vilar, E.O.; Palma Carrasco, J. A critical review of mathematical models used to determine the density of hydrogen trapping sites in steels and alloys. Int. J. Hydrogen Energy 2014, 39, 12194–12200. [Google Scholar] [CrossRef]
- Dong, C.F.; Li, X.G.; Liu, Z.Y.; Zhang, Y.R. Hydrogen induced cracking and healing behavior of X70 steel. J. Alloy Compd. 2009, 484, 966–972. [Google Scholar] [CrossRef]
- Ramunni, V.P.; de Paiva, T.; de Miranda, P.E.V. Interaction of hydrogen with the microstructure of low-carbon steel. Mater. Sci. Eng. A 2006, 435–436, 504–514. [Google Scholar] [CrossRef]
- Haq, A.J.; Muzaka, K.; Dunne, D.P.; Calka, A.; Pereloma, E.V. Effect of microstructure and composition on hydrogen permeation in X70 pipeline steels. Int. J. Hydrogen Energy 2013, 38, 2544–2556. [Google Scholar] [CrossRef]
- Symons, D.M.; Young, G.A.; Scully, J.R. The effect of strain on the trapping of hydrogen at grain-boundary carbides in Ni–Cr–Fe alloys. Metall. Mater. Trans. A 2001, 32, 369–377. [Google Scholar] [CrossRef]
- Van den Eeckhout, E.; Depover, T.; Verbeken, K. The effect of microstructural characteristics on the hydrogen permeation transient in qenched and tempered martensitic alloys. Metals 2018, 8, 779. [Google Scholar] [CrossRef]
- Chu, W.Y. Hydrogen Embrittlement and Stress Corrosion Cracking; Science Press: Beijing, China, 2013. [Google Scholar]
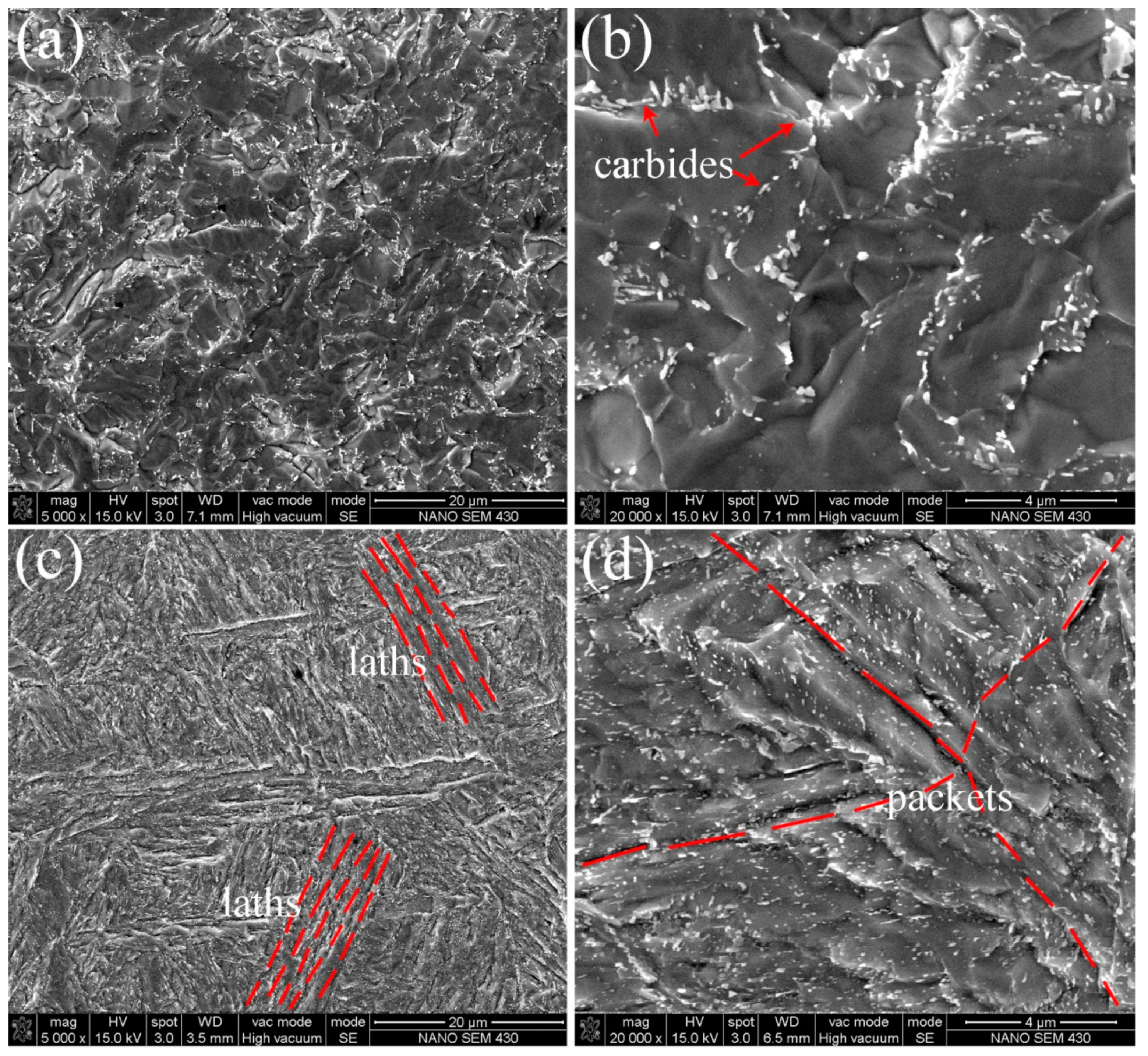
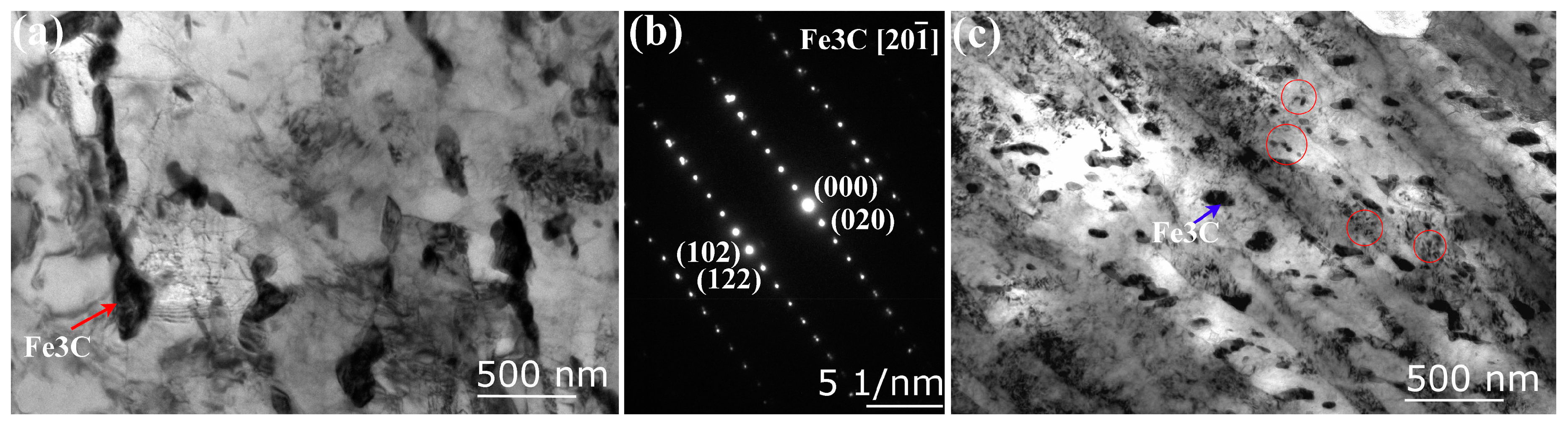
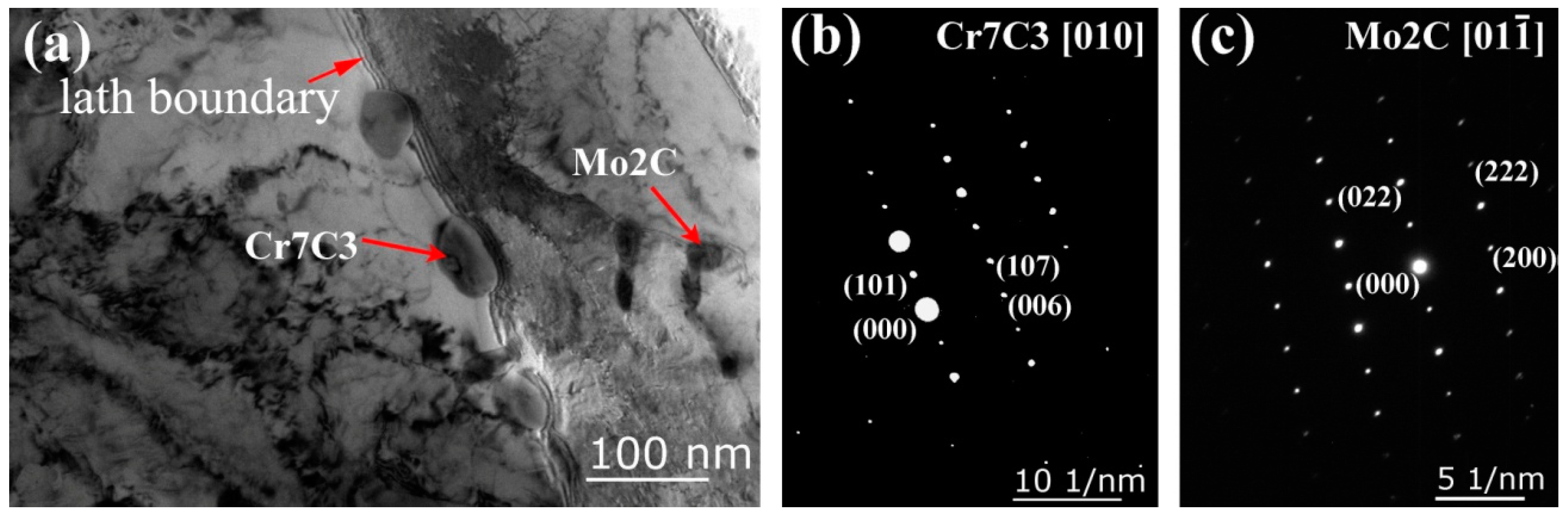
| Sample | C | Si | Mn | Cr | Ni | Mo | V | S | P |
|---|---|---|---|---|---|---|---|---|---|
| EA4T | 0.28 | 0.31 | 0.72 | 0.95 | 0.21 | 0.12 | - | 0.006 | 0.008 |
| 30CrNiMoV12 | 0.29 | 0.33 | 0.68 | 1.12 | 3.15 | 0.58 | 0.13 | 0.005 | 0.009 |
| Sample | Parameter | Temperature (K) | |||
|---|---|---|---|---|---|
| 288 | 298 | 308 | 318 | ||
| EA4T | L (mm) | 0.85 | 1.01 | 0.85 | 0.85 |
| tL (s) | 948 | 940 | 470 | 385 | |
| Deff (cm2 s−1) | 1.27 × 10−6 | 1.81 × 10−6 | 2.56 × 10−6 | 3.13 × 10−6 | |
| Q (kJ mol−1) | 23.27 ± 1.94 | ||||
| 30NiCrMoV12 | L (mm) | 0.80 | 1.00 | 1.00 | 0.99 |
| tL (s) | 14,600 | 8405 | 5045 | 3180 | |
| Deff (cm2 s−1) | 0.88 × 10−7 | 1.98 × 10−7 | 3.30 × 10−7 | 6.00 × 10−7 | |
| Q (kJ mol−1) | 47.82 ± 2.14 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, T.; Liu, Y.; Zhang, Q.; Liu, D.; Li, Y. Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels. Metals 2019, 9, 164. https://doi.org/10.3390/met9020164
Si T, Liu Y, Zhang Q, Liu D, Li Y. Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels. Metals. 2019; 9(2):164. https://doi.org/10.3390/met9020164
Chicago/Turabian StyleSi, Tingzhi, Yunpeng Liu, Qingan Zhang, Dongming Liu, and Yongtao Li. 2019. "Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels" Metals 9, no. 2: 164. https://doi.org/10.3390/met9020164
APA StyleSi, T., Liu, Y., Zhang, Q., Liu, D., & Li, Y. (2019). Effect of Microstructure on Hydrogen Permeation in EA4T and 30CrNiMoV12 Railway Axle Steels. Metals, 9(2), 164. https://doi.org/10.3390/met9020164





