Abstract
The thermal properties of a Pd40Ni40Si20 glassy alloy with the largest supercooled liquid region among the glassy alloys containing Si as a metalloid element are studied using static and dynamic measurement methods. The relatively wide supercooled liquid region of 45 K, defined by the temperature interval between the glass transition temperature (Tg) and the onset temperature of crystallization (Tx), and the viscosity of the supercooled liquid, varying from 2.7 × 1010 to 3.2 × 1011 Pa·s, make this glass suitable for the introduction of controlled pores by a viscous flow in the temperature range Tg~Tx. The obtained activation energy for crystallization, 272 ± 19 kJ/mol, is slightly higher than that of Tg (228 ± 11 kJ/mol), indicating the dominant contribution of the atomic transport barrier in the overall energy barrier for crystallization.
1. Introduction
Amorphous metals are significantly different from conventional metals because of their atomic structure, mechanical properties, and specific behavior in a solid state [1,2]. Reflecting their non-periodic disordered atomic arrangement, amorphous alloys have outstanding mechanical properties, such as high yield strength, a large elastic strain of about 2%, and a wide range of tensile strength (1.5–5.5 GPa) [3,4,5]. These unique properties make them extremely interesting materials, and many studies have been carried out over the last four decades. At present, these materials can be made up to a few centimeters (bulk metallic glassy alloys) [1,2,3,4,5]. The largest thickness value of the bulk glassy alloys reaches about 30 mm for the Zr-Al-Ni-Cu system [6], 50 mm for the Zr-Be-Ti-Cu-Ni system [7], and 80 mm for the Pd-Cu-Ni-P system [8]. This is expected to enable the production of bulk glassy metal foams that have high strength combined with a very low specific weight and high mechanical energy absorption capability [9]. The evolution of porosity in bulk glassy alloys is supposed to be controlled through changes in the viscosity with the strain rate and temperature in the supercooled liquid region [10,11]. The subsequent annealing in the temperature range with high viscous flowability also gives an opportunity to control the structure and morphology of the metal foam. Considering the Pd- and Ni-rich compositions, which are favourable and have high potential for the preparation of amorphous foam, in the present study, it was important to clarify the feature of the static and dynamic thermal properties of the Pd40Ni40Si20 glassy alloy. The other attractive characteristic of this glassy alloy is its low glass transition and melting temperatures and ease of preparation [12].
2. Materials and Methods
A glassy Pd40Ni40Si20 alloy ribbon with a thickness of about 40 μm and a width of 2 mm was prepared by the melt-spinning technique. The circumferential velocity of the copper wheel was 25 m/s. X-ray diffraction (Co Kα radiation) and DSC were used to prove the glassy structure of the as-spun alloy ribbon. Thermal properties were examined by differential scanning calorimetry (DSC) (Perkin Elmer DSC7) at heating rates of 10–80 K/min and the thermo-mechanical analysis (Perkin Elmer quartz thermomechanical TMS2) at a heating rate of 20 K/min [13,14]. The thermo-mechanical analysis was undertaken based on the Free Volume Model (FVM) [15].
3. Results and Discussion
The X-ray diffraction and DSC analysis revealed the glassy structure of the as-spun Pd40Ni40Si20 alloy (Figure 1).
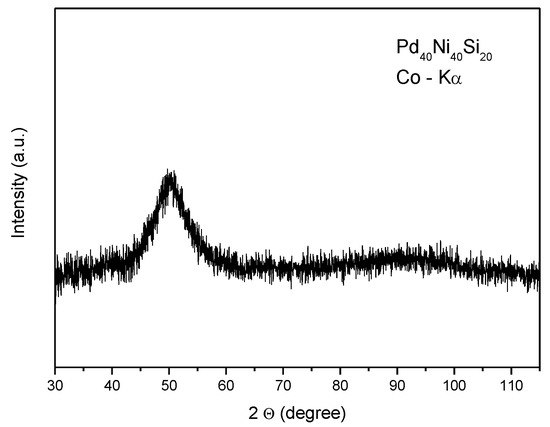
Figure 1.
XRD diffraction pattern of as-quenched Pd40Ni40Si20 alloy.
Figure 2a shows the typical elongation vs. temperature curves of the glassy Pd40Ni40Si20 alloy ribbon under four different loads. The overall strain of the glassy ribbon attained at temperature T under an applied tensile stress σ, under a continuous heating condition, is given by
where l0 and l(T) are the initial length and the current length of the specimen at temperature T, respectively, and is the elastic strain of the ribbon at the Young’s modulus of the material, E(T). It is worth adding that represents the possible anelastic contribution to the overall strain, and takes into account the contribution of any relaxation effects to the overall strain, represents the contribution of the thermal expansion to the overall strain, while takes into account the viscous flow contribution to the overall strain. It is shown that the subtraction of the strains obtained at different loads gives [5]
where is caused by the effective stress Δσ1,2 = σ1 – σ2. Applying the Newtonian relation for viscous flow and taking into account the shear stress τ = σ/3, one obtains
where is the difference of the strain rates and , caused by the applied shear stresses τ1 and τ2, while Δτ1,2 = τ1 – τ2.
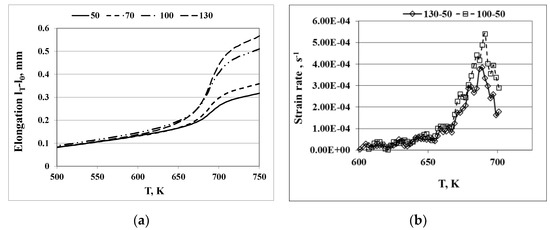
Figure 2.
Experimental elongation [l(T) − l0] vs. the temperature curve of the Pd40Ni40Si20 glassy alloy at a heating rate of 20 K/min with 4 different loads: 50 g (11.9 MPa), 70 g (16.7 MPa), 100 g (23.8 MPa), and 130 g (31.0 MPa) (a) and the temperature dependences of the strain rates (b).
Thus, the experimental elongation vs. temperature results (Figure 2a) was used to determine the temperature dependence of the viscosity around the glass transition temperature Tg. The typical temperature dependences of the strain rates , caused by shear stress differences for Pd40Ni40Si20, are shown in Figure 2b. The curves are obtained by numerical differentiation of the temperature dependence of the degree of deformation (l(T) − l0). The strain rate increases smoothly up to approximately 660 K and then rapidly near Tg. Well-outlined maxima are observed in the range of the onset temperature of crystallization.
Figure 3a shows the temperature dependence of the viscosity (points) calculated from the experimental data for the deformation velocity. The line is a smoothed curve (fits in least squares) across all experimental data. Two linear sections of the curve are observed. The steeper part of the viscosity temperature dependence represents a quasi-equilibrium structural state of the supercooled liquid of the alloy. The one in the lower temperature region is the non-equilibrium viscosity of the vitrified alloy. Figure 3b reveals the measured (points) and calculated (curvilinear) temperature dependencies of the viscosity for the glassy alloy according to the Free Volume Model at a heating rate of 20 K/min. A detailed description of the theory and experimental method used are presented in our previous studies [13,14]. Thus, the glass transition temperature Tg determined by the thermo-mechanical analysis is 611 K, and theviscosities are in the range of 2.7 × 1010 to 3.2 × 1011 Pa·s are obtained in the temperature range of the supercooled liquid. Similar viscosity values of the supercooled liquid were reported for a glassy alloy with a composition of Pd40Ni40P20 [16] and for the Zr65Cu18Ni7Al10 bulk metallic glass [17].
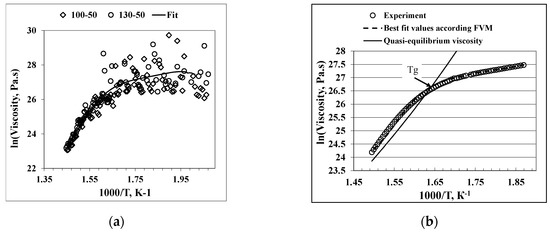
Figure 3.
Viscosity temperature dependence of the Pd40Ni40Si20 glassy alloy at a heating rate of 20 K/min, calculated from load differences (a) and the viscosity temperature dependence of the Pd40Ni40Si20 glass: points—experimental values; broken line—best fit values, according to FVM; and the steep solid line—quasi-equilibrium viscosity (b). The cross point of the non-equilibrium and quasi- equilibrium viscosity determines the glass transition temperature Tg.
To confirm the glassy character of the as-spun alloy, in addition to XRD (Figure 1), DSC measurements with different heating rates were also carried out. Figure 4a presents the DSC curves for the as-spun Pd40Ni40Si20 ribbon, measured with heating rates in the range of 10–80 K/min. Both, Tg and Tx can clearly be detected over the whole heating rate range. It is necessary to note that the Tg obtained by DSC is slightly higher than that determined from the viscosity measurement (611 K) due to the dynamic character in the thermomechanical measurement. The Tg value obtained from the thermomechanical experiments corresponds to the beginning of the transition from glass to supercooled liquid measured by DSC at a lower heating rate of 10 K/min.
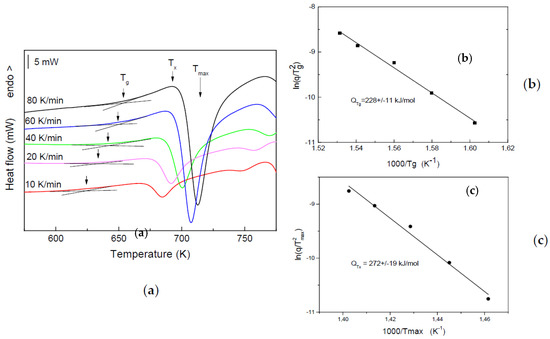
Figure 4.
DSC scans of the as-cast Pd40Ni40Si20 ribbon, measured with heating rates in the range 10–80 K/min (a); Kissinger plot of glass transition temperature Tg (b) and Kissinger plot of the temperature of crystallization Tmax (temperature of the maximum of the first crystallization peak) (c).
The change of Tg with the heating rate was used to determine the activation energy of the glass transition, applying the Kissinger equation [18] (Figure 4b). The value of the activation energy 228 ± 11 kJ/mol is close to that obtained for the glassy Pd40Ni40P16Si4 alloy [19]. The activation energy of the glass transition can be considered as an energy barrier for mass transport, which is usually evaluated by the temperature dependence of the viscosity. Indeed, both activation energies (of the viscous flow and glass transition) reflect the transition from a frozen glassy state to a supercooled liquid, i.e., cooperative atomic mobility unlocking [19]. The obtained activation energy for the crystallization of the Pd40Ni40Si20 glassy alloy, 272 ± 19 kJ/mol (Figure 4c) is slightly higher than that of Tg, indicating the dominant contribution of the atomic transport barrier compared to the nucleation barrier in the overall energy barrier for crystallization. The last conclusion is expected for a solid state crystallization process like the one studied. This relatively large temperature interval of the supercooled liquid (Tx − Tg > 40 K) is proof of the alloy’s high glass forming ability and suggests an opportunity for the development of porous glassy by appropriate thermomechanical treatment in the supercooled liquid.
4. Conclusions
A glassy Pd40Ni40Si20 alloy was obtained by melt spinning. The thermal behaviour of the as-spun glassy alloy ribbon was studied by DSC and thermomechanical analysis, and the glass transition temperature Tg and the onset temperature of crystallization Tx were determined under static and dynamic heating conditions. The activation energies for glass transition and crystallization obtained by DSC are 228 ± 11 kJ/mol and 272 ± 19 kJ/mol, respectively, suggesting that these transitions occur through a mass transport mechanism rather than a nucleation-controlled process. It was established that the supercooled liquid region between Tg and Tx for the present glassy alloy is large enough (> 40 K) and the viscosity is in a low enough range (2.7 × 1010–3.2 × 1011 Pa·s) to allow the development of a porous glass by appropriate thermomechanical treatment of the supercooled liquid.
Author Contributions
T.S., L.D., and S.G. conceived and designed the experiments; T.S. and G.S. performed the experiments; S.G., T.S., A.I., and L.D. analyzed the data; T.S., S.G., and A.I. wrote and edited the paper.
Funding
This research was funded by the Bulgarian Scientific Research Fund, grant number DN 07/17.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic glasses on the threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk amorphous metal—An emerging engineering material. JOM 2002, 54, 40–43. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Inoue, A.; Zhang, T. Fabrication of bulk glassy Zr55Al10Ni5Cu30 alloy of 30 mm in diameter by a suction casting method. Mater. Trans. JIM 1996, 37, 185–187. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk glass-forming metallic alloys: Science and technology. MRS Bull. 1999, 24, 42–56. [Google Scholar] [CrossRef]
- Nishiyama, N.; Takenaka, K.; Miura, H.; Saidoh, N.; Zeng, Y.; Inoue, A. The world’s biggest glassy alloy ever made. Intermetallics 2012, 30, 19–24. [Google Scholar] [CrossRef]
- Wada, T.; Inoue, A. Formation of Porous Pd-based Bulk Glassy Alloys by a High Hydrogen Pressure Melting-Water Quenching Method and Their Mechanical Properties. Mater. Trans. 2004, 45, 2761–2765. [Google Scholar] [CrossRef]
- Wada, T.; Inoue, A.; Greer, A.L. Enhancement of room-temperature plasticity in a bulk metallic glass by finely dispersed porosity. Appl. Phys. Lett. 2005, 86, 251907. [Google Scholar] [CrossRef]
- Nishiyama, N.; Inoue, A. Glass Transition Behavior and Viscous Flow Working of Pd40Cu30Ni10P20 Amorphous Alloy. Mater. Trans. JIM 1999, 40, 64–71. [Google Scholar] [CrossRef]
- Inoue, A.; Masumoto, Y.; Yano, N.; Kawashima, A.; Hashimoto, K.; Masumoto, T. Production of Ni-Pd-Si and Ni-Pd-P amorphous wires and their mechanical and corrosion properties. J. Mat. Sci. 1985, 20, 97–104. [Google Scholar] [CrossRef]
- Gyurov, S.; Czeppe, T.; Drenchev, L.; Stefanov, G.; Russew, K. Thermo-mechanical study of rapidly solidified NiNbZrTiAl amorphous metallic alloys. Mater. Sci. Eng. A 2017, 684, 222–228. [Google Scholar] [CrossRef]
- Stojanova, L.; Russew, K.; Fazakas, E.; Varga, L.K. Thermo-mechanical study of rapidly solidified amorphous alloys Al85Ni5Co2RE8. J. Alloys Compd. 2012, 540, 192–197. [Google Scholar] [CrossRef]
- Russew, K.; Stojanova, L. Glassy Metals; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kawamura, Y.; Inoue, A. Newtonian viscosity of supercooled liquid in a Pd40Ni40P20Pd40Ni40P20 metallic glass. Appl. Phys. Lett. 2000, 77, 1114. [Google Scholar] [CrossRef]
- Zhang, C.; Qiao, J.C.; Pelletier, J.M.; Yao, Y. Arrhenius activation of Zr65Cu18Ni7Al10 bulk metallic glass in the supercooled liquid region. Intermetallics 2017, 86, 88–93. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.A.; Caron, A.; Chen, P.C.; Lin, Y.C.; Louzguine-Luzgin, D.V.; Yao, K.F.; Esashi, M.; Inoue, A. Glass-forming ability and thermoplastic formability of a Pd40Ni40Si4P16 glassy alloy. J. Mater. Sci. 2011, 46, 2091–2096. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).