Phase Transformation Behavior of a β-Solidifying γ-TiAl-Based Alloy from Different Phase Regions with Various Cooling Methods
Abstract
:1. Introduction
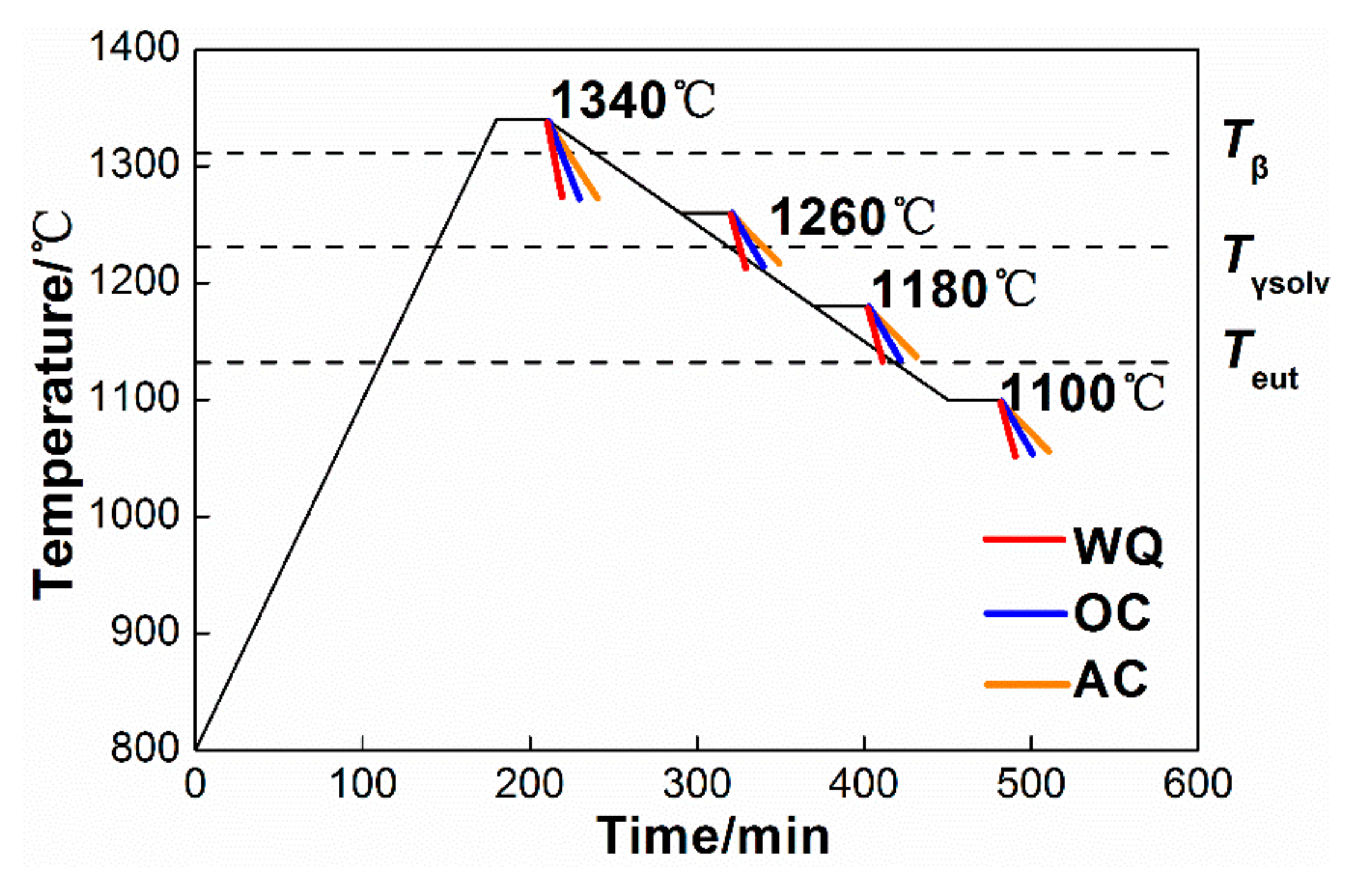
2. Experimental Methods
3. Results
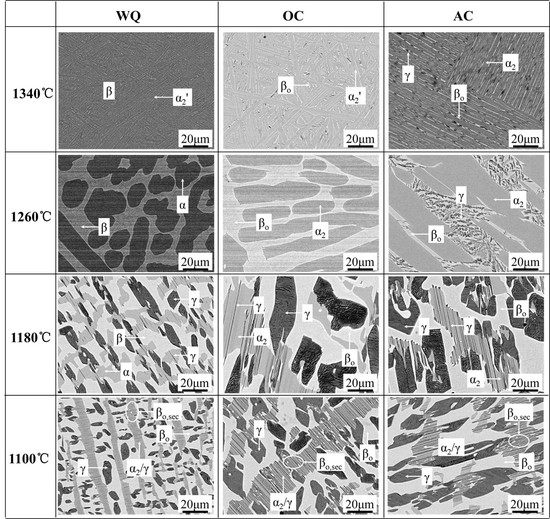
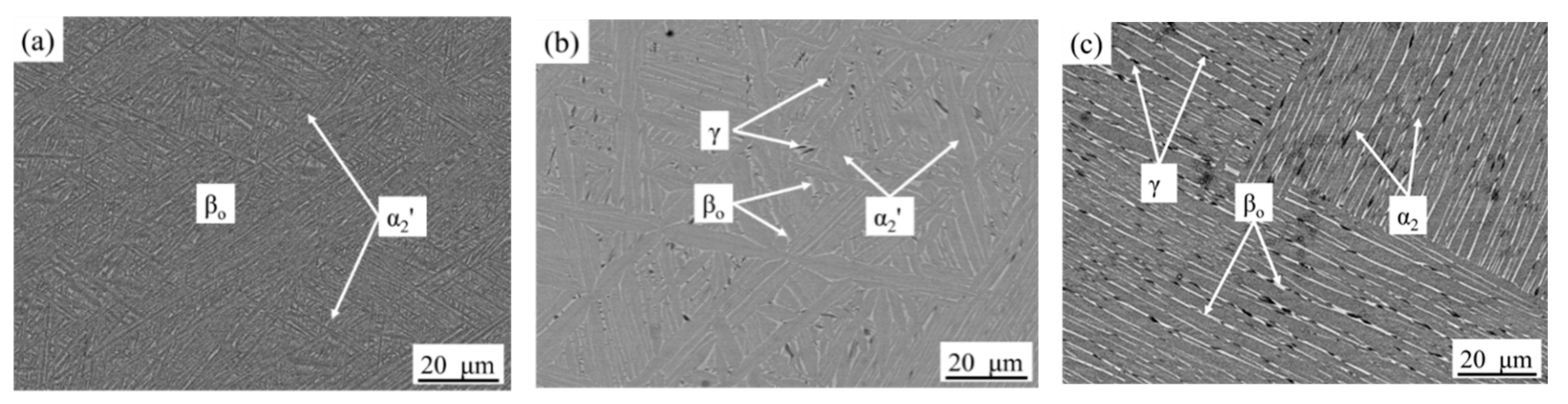
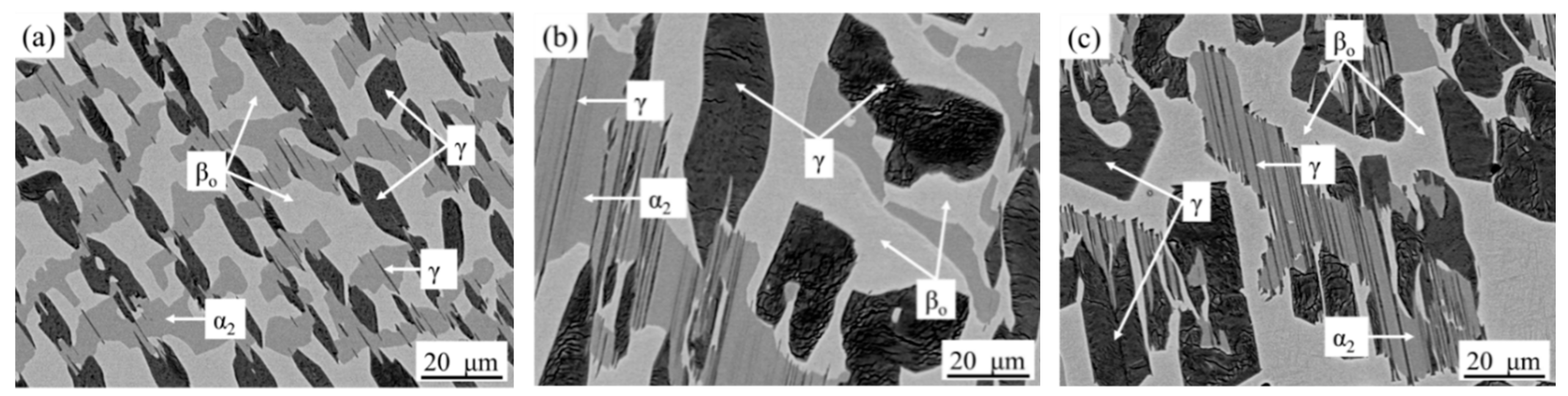
3.1. Microstructure Cooled from 1340 °C
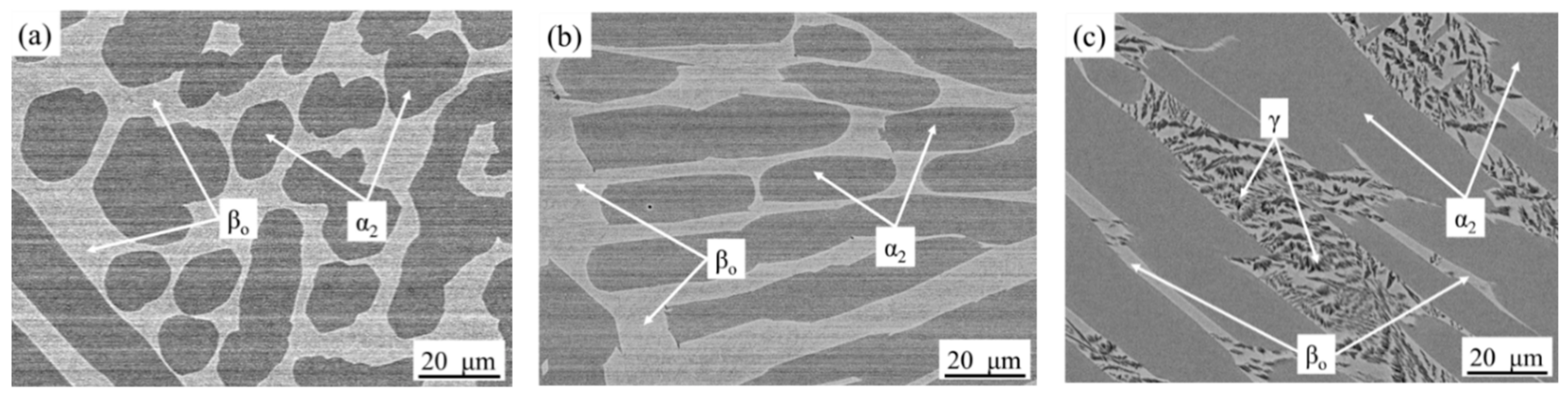
3.2. Microstructure Cooled from 1260 °C
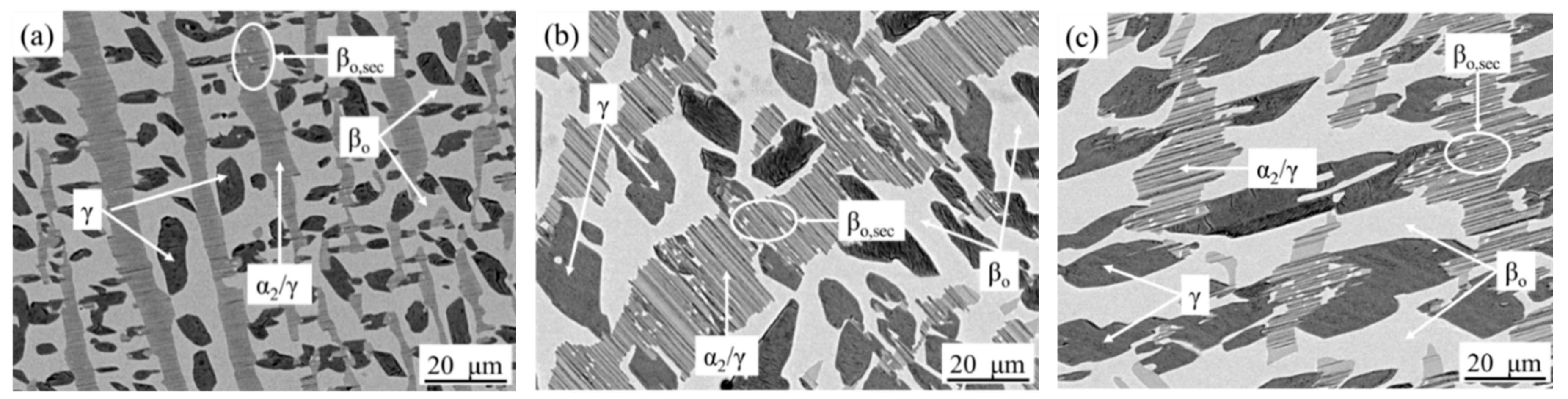
3.3. Microstructure Cooled from 1180 °C
3.4. Microstructure Cooled from 1100 °C
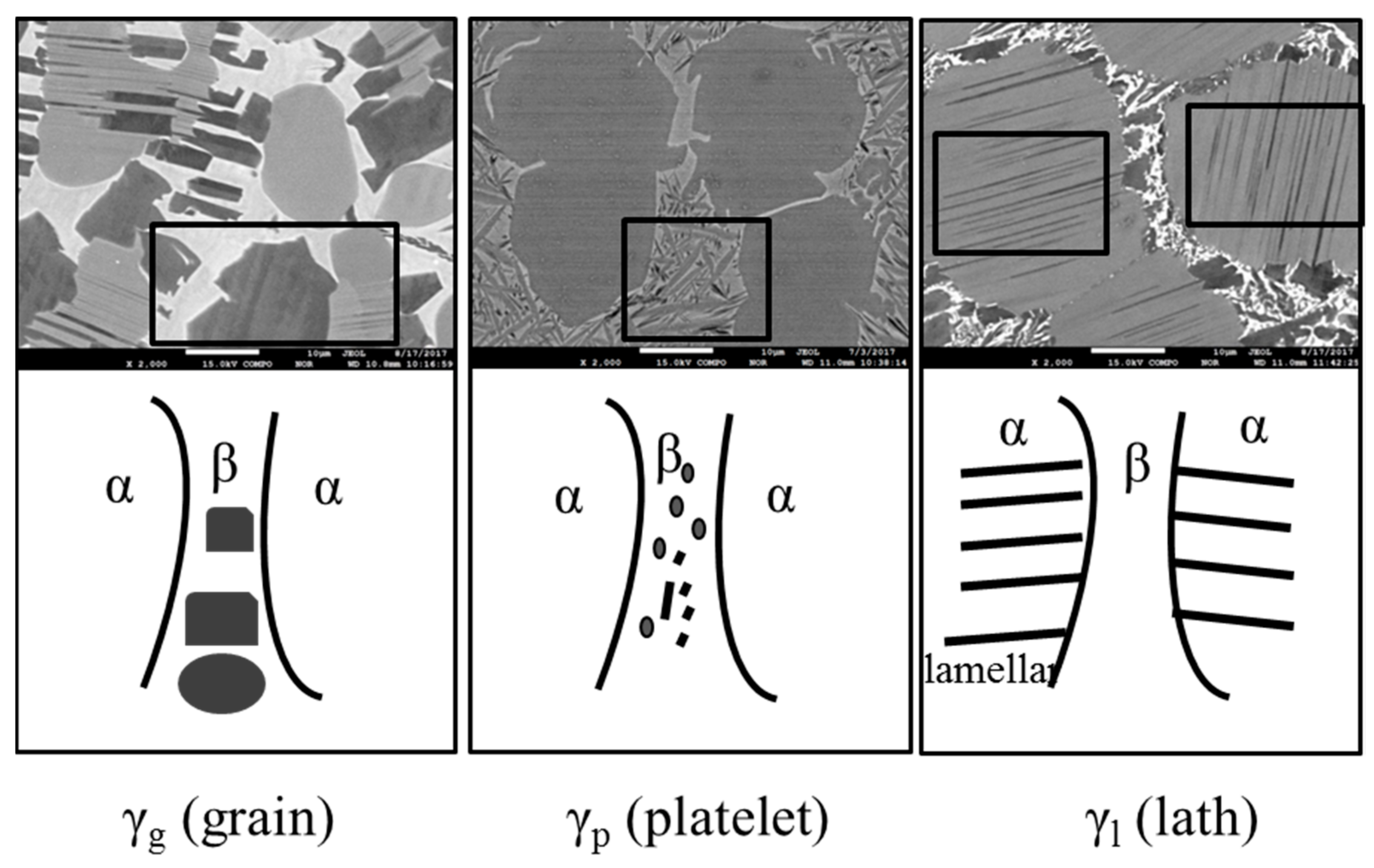
4. Discussion
5. Conclusions
- The β→α2′ takes place when this alloy is cooled at a high rate (WQ and OC) from β single phase. With the decreasing cooling rate to AC, β→α2′ is restrained and the β→γ is promoted by the formation of γ platelets.
- The room-temperature microstructure is βo+α2 when this alloy is cooled by WQ and OC from (β + α) the dual-phase. However, under AC, the β→γ takes place and γ platelets form.
- The α2→γ occurs when this alloy is cooled from the (β + α + γ) temperature, slightly higher than Teut (1132 °C), by WQ, OC and AC, forming incomplete lamellae (α2/γ) structures in the α2 phase. In this situation, plenty of globular γ grains rather than γ platelets are nucleated from the β phase, and the size of γ grains increased intensively with the decreasing cooling rate.
- When the alloy cooled from 1100 °C (<Teut), the α2/γ→βo,sec occurs and complete lamellae are generated in the α2 phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.; Joris, O.P.; Sankaran, A.; Weekes, H.E.; Bull, D.J.; Prior, T.J.; Dye, D.; Errandonea, D.; Proctor, J.E. On the high-pressure phase stability and elastic properties of β-titanium alloys. J. Phys. Condens. Matter 2017, 29, 155401. [Google Scholar] [CrossRef] [PubMed]
- Errandonea, D.; Meng, Y.; Somayazulu, M.; Häusermann, D. Pressure-induced α→ω transition in titanium metal: A systematic study of the effects of uniaxial stress. Physica B 2005, 355, 116–125. [Google Scholar] [CrossRef]
- Halevy, I.; Zamir, G.; Winterrose, M.; Sanjit, G.; Grandini, C.R.; Moreno-Gobbi, A. Crystallographic structure of Ti-6Al-4V, Ti-HP and Ti-CP under high-pressure. J. Phys. Conf. Ser. 2010, 215, 1683–1688. [Google Scholar] [CrossRef]
- MacLeod, S.G.; Tegner, B.E.; Cynn, H.; Evans, W.J.; Proctor, J.E.; McMahon, M.I.; Ackland, G.J. Experimental and theoretical study of Ti-6Al-4V to 220 GPa. Phys. Rev. B 2012, 85, 224202–224207. [Google Scholar] [CrossRef]
- Appel, F.; Wagner, R. Microstructure and deformation of two-phase γ-titanium aluminides. Mater. Sci. Eng. R Rep. 1998, 22, 187–268. [Google Scholar] [CrossRef]
- Clemens, H.; Kestler, H. Processing and applications of intermetallic γ-TiAl based alloys. Adv. Eng. Mater. 2000, 2, 551–570. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Lin, J.; Chang, H.; Li, F. Phase transformation behavior and kinetics of high Nb–TiAl alloy during continuous cooling. J. Alloys Compd. 2016, 668, 22–26. [Google Scholar] [CrossRef]
- Imayev, V.; Oleneva, T.; Imayev, R.; Christ, H.J.; Fecht, H.J. Microstructure and mechanical properties of low and heavy alloyed γ-TiAl + α2-Ti3Al based alloys subjected to different treatments. Intermetallics 2012, 26, 91–97. [Google Scholar] [CrossRef]
- Naka, S.; Thomas, M.; Sanchez, C.; Khan, T. Development of third generation castable gamma titanium aluminides: Role of solidification paths. In Structural Intermetallics; Nathal, M.V., Darolia, R., Liu, C.T., Martin, P.L., Miracle, D.B., Wagner, R., Yamaguchi, M., Eds.; TMS: Warrendale, PA, USA, 1997; pp. 313–322. [Google Scholar]
- Kim, Y.W.; Kim, S.L. Advances in gammalloy materials–processes–application technology: Successes, dilemmas, and future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef]
- Yang, J.R.; Xiao, B.; Han, P.; Kou, H.C.; Li, J.S. Microstructure evolution in the mushy zone of a β-solidifying TiAl alloy under different cooling processes. Adv. Eng. Mater. 2016, 18, 1667–1673. [Google Scholar] [CrossRef]
- Kim, Y.W. Intermetallic alloys based on gamma titanium aluminide. JOM 1989, 41, 24–30. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Mayer, S.; Erdely, P.; Fischer, F.D.; Holec, D.; Kastenhuber, M.; Klein, T.; Clemens, H. Intermetallic β-solidifying γ-TiAl based alloys-from fundamental research to application. Adv. Eng. Mater. 2017, 19, 1600735. [Google Scholar] [CrossRef]
- Leitner, T.; Schloffer, M.; Mayer, S.; Eßlinger, J.; Clemens, H.; Pippan, R. Fracture and R-curve behavior of an intermetallic β-stabilized TiAl alloy with different nearly lamellar microstructures. Intermetallics 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Tetsui, T.; Shindo, K.; Kobayashi, S.; Takeyama, M. A newly developed hot worked TiAl alloy for blades and structural components. Scr. Mater. 2002, 47, 399–403. [Google Scholar] [CrossRef]
- Tetsui, T.; Shindo, K.; Kaji, S.; Kobayashi, S.; Takeyama, M. Fabrication of TiAl components by means of hot forging and machining. Intermetallics 2005, 13, 971–978. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Harada, H. Achieving high strength and low cost for hot-forged TiAl based alloy containing β phase. Mater. Sci. Eng. A 2012, 552, 345–352. [Google Scholar] [CrossRef]
- Tetsui, T.; Harada, H. The influence of oxygen concentration and phase composition on the manufacturability and high-temperature strength of Ti-42Al-5Mn (at%) forged alloy. J. Mater. Process. Technol. 2013, 213, 752–758. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Xing, W.; Shu, L.; Ma, Y.C.; Liu, K. Phase transformation behavior in an as-cast Ti-42Al-5Mn alloy after subsequent quenching at different temperatures. J. Alloys Compd. 2018, in press. [Google Scholar]
- Xu, H.; Li, X.; Xing, W.; Shu, L.; Ma, Y.; Liu, K. Phase transformation behavior of a Mn containing β-solidifying γ-TiAl alloy during continuous cooling. Intermetallics 2018, 99, 51–58. [Google Scholar] [CrossRef]
- Hu, D.; Huang, A.J.; Gregoire, A.; Li, X.; Wu, X.; Loretto, M. Determining continuous cooling phase transformation behavior in Ti-46Al-8Nb using Jominy end quenching. Mater. Sci. Forum 2005, 29, 172–175. [Google Scholar]
- Klein, T. Impact of Mo on the ωo phase in β-solidifying Ti Al alloys: An experimental and computational approach. Intermetallics 2017, 85, 26–33. [Google Scholar] [CrossRef]
- Yang, H.W.; Lin, C. Phase transformation and microstructural evolution in Ti-44Al-4Nb-4Zr alloy during heat treatment. Metall. Mater. Trans. A 2006, 37, 3191–3196. [Google Scholar] [CrossRef]
- Mayer, S.; Petersmann, M.; Fischer, F.D.; Clemens, H.; Waitz, T.; Antretter, T. Experimental and theoretical evidence of displacive martensite in an intermetallic Mo-containing gamma-TiAl based alloy. Acta Mater. 2016, 115, 242–249. [Google Scholar] [CrossRef]
- Takeyama, M.; Kobayashi, S. Physical metallurgy for wrought gamma titanium aluminides microstructure control through phase transformations. Intermetallics 2005, 13, 993–999. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, H. Martensite in a TiAl alloy quenched from beta phase field. Intermetallics 2015, 56, 87–95. [Google Scholar] [CrossRef]
- Schloffer, M.; Rashkova, B.; Schöberl, T.; Schwaighofer, E.; Zhang, Z.; Clemens, H.; Mayer, S. Evolution of the ωo phase in a β-stabilized multi-phase TiAl alloy and its effect on hardness. Acta Mater. 2014, 64, 241–252. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Clemens, H.; Mayer, S.; Lindemann, J.; Klose, J.; Smarsly, W.; Güther, V. Microstructural design and mechanical properties of a cast and heat-treated intermetallic multi-phase γ-TiAl based alloy. Intermetallics 2014, 44, 128–140. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Xing, W.; Shu, L.; Tang, H.; Chen, B.; Ma, Y.; Liu, K. Microstructural evolution and mechanical properties of a forged beta solidified γ-TiAl alloy in different heat treatments. J. Alloys Compd. 2018, inpress. [Google Scholar]
- Klein, T.; Usategui, L.; Rashkova, B.; Nό, M.L.; San Juan, J.; Clemens, H.; Mayer, S. Mechanical behavior and related microstructural aspects of a nano-lamellar Ti Al alloy at elevated temperatures. Acta Mater. 2017, 128, 440–450. [Google Scholar] [CrossRef]
- Bolz, S.; Oehring, M.; Lindemann, J.; Pyczak, F.; Paul, J.; Stark, A.; Lippmann, T.; Schrüfer, S.; Roth-Fagaraseanu, D.; Schreyer, A.; et al. Microstructure and mechanical properties of a forged β-solidifying γ-Ti Al alloy in different heat treatment conditions. Intermetallics 2015, 58, 71–83. [Google Scholar] [CrossRef]
- Prasad, U.; Chaturvedi, M.C. Influence of alloying elements on the kinetics of massive transformation in gamma titanium aluminides. Metall. Mater. Trans. A 2003, 34, 2053–2066. [Google Scholar] [CrossRef]
- Ramanujan, R.V. Phase transformations in gamma based titanium aluminides. Int. Mater. Rev. 2000, 45, 217–240. [Google Scholar] [CrossRef]
- Cheng, T.T.; Loretto, M.H. The decomposition of the beta phase in Ti-44Al-8Nb and Ti-44Al-4Nb-4Zr-0.2Si alloys. Acta Mater. 1998, 46, 4801–4819. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; Chemical Industry Press: Beijing, China, 2003; p. 110. [Google Scholar]
- Holec, D.; Legut, D.; Isaeva, L.; Souvatzis, P.; Clemens, H.; Mayer, S. Interplay between effect of Mo and chemical disorder on the stability of β/βo-TiAl phase. Intermetallics 2015, 61, 85–90. [Google Scholar] [CrossRef]

| Temperature/°C | Phase Region [21] | Phase Transformation | ||
|---|---|---|---|---|
| WQ | OC | AC | ||
| 1340 | β | β→α′2 | β→α′2 | β→α, β→γ |
| 1260 | β + α | / | / | β→α, β→γ |
| 1180 | β + α + γ | α→α + γ | α→α + γ | α→α + γ |
| 1100 | β + α + γ | α→α2/γ,α/γ→βo,sec | α→α2/γ,α/γ→βo,sec | α→α2/γ, α/γ→βo,sec |
| Temperature/°C | 1340 | 1260 | 1180 | 1100 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooling method | WQ | OC | AC | WQ | OC | AC | WQ | OC | AC | WQ | OC | AC |
| γg | × | × | × | × | × | × | √ | √ | √ | √ | √ | √ |
| γp | × | × | √ | × | × | √ | × | × | × | × | × | × |
| γl | × | × | × | × | × | × | √ | √ | √ | √ | √ | √ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, H.; Xing, W.; Chen, B.; Ma, Y.; Liu, K. Phase Transformation Behavior of a β-Solidifying γ-TiAl-Based Alloy from Different Phase Regions with Various Cooling Methods. Metals 2018, 8, 731. https://doi.org/10.3390/met8090731
Li X, Xu H, Xing W, Chen B, Ma Y, Liu K. Phase Transformation Behavior of a β-Solidifying γ-TiAl-Based Alloy from Different Phase Regions with Various Cooling Methods. Metals. 2018; 8(9):731. https://doi.org/10.3390/met8090731
Chicago/Turabian StyleLi, Xiaobing, Hao Xu, Weiwei Xing, Bo Chen, Yingche Ma, and Kui Liu. 2018. "Phase Transformation Behavior of a β-Solidifying γ-TiAl-Based Alloy from Different Phase Regions with Various Cooling Methods" Metals 8, no. 9: 731. https://doi.org/10.3390/met8090731
APA StyleLi, X., Xu, H., Xing, W., Chen, B., Ma, Y., & Liu, K. (2018). Phase Transformation Behavior of a β-Solidifying γ-TiAl-Based Alloy from Different Phase Regions with Various Cooling Methods. Metals, 8(9), 731. https://doi.org/10.3390/met8090731