Effect of the Parameters of Semi-Solid Processing on the Elimination of Sharp-Edged Primary Chromium Carbides from Tool Steel
Abstract
:1. Introduction
2. Experimental Programme
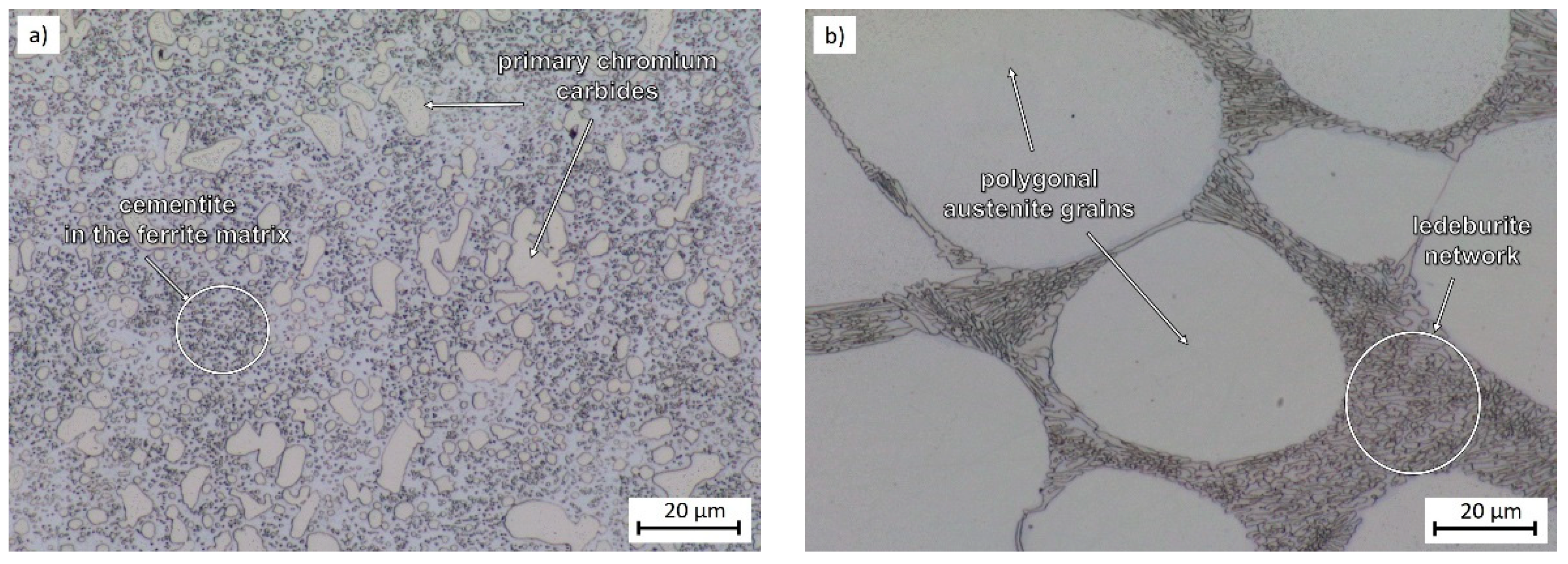
2.1. Experimental Material
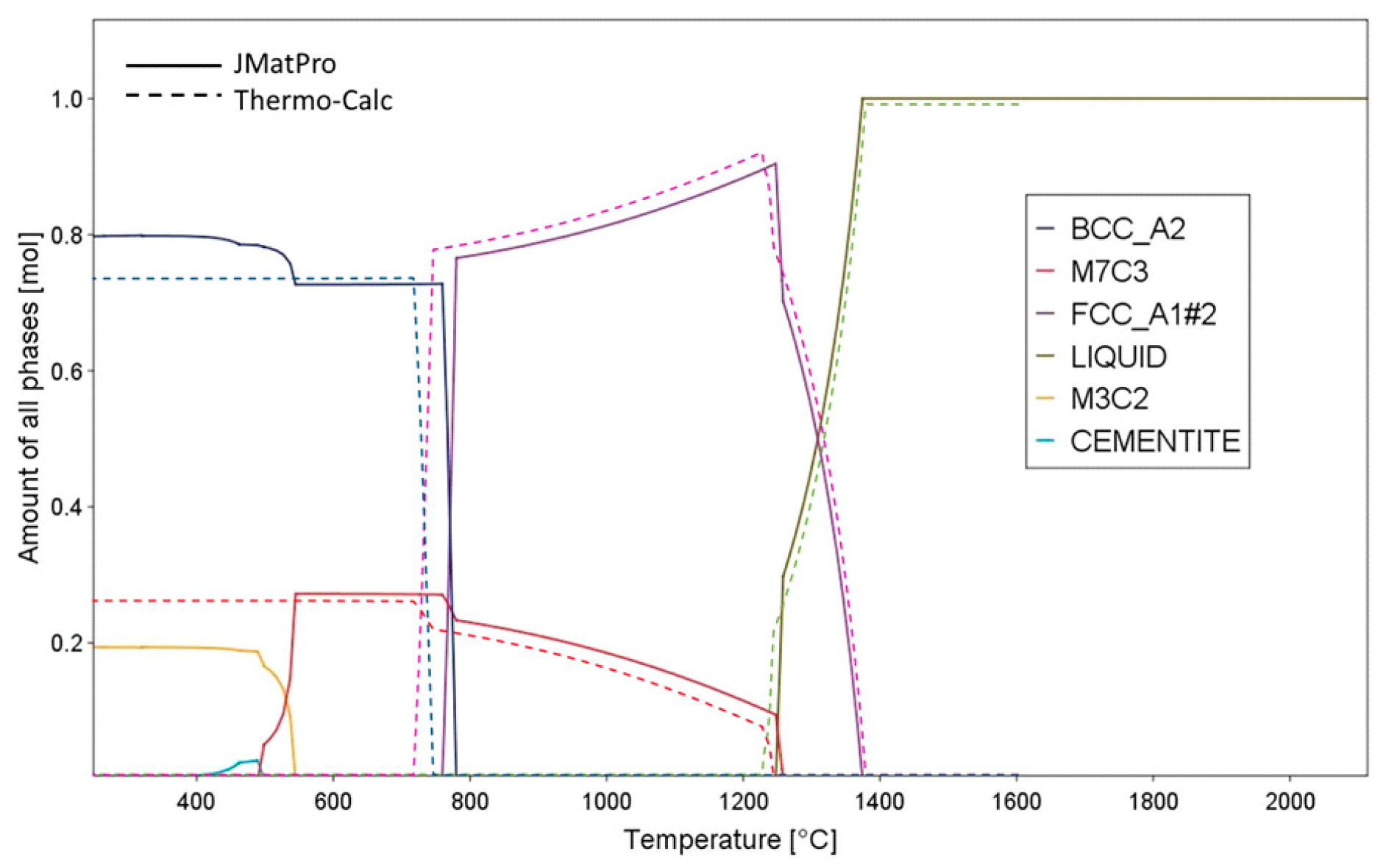
2.2. Determination of Semi-Solid Processing Parameters
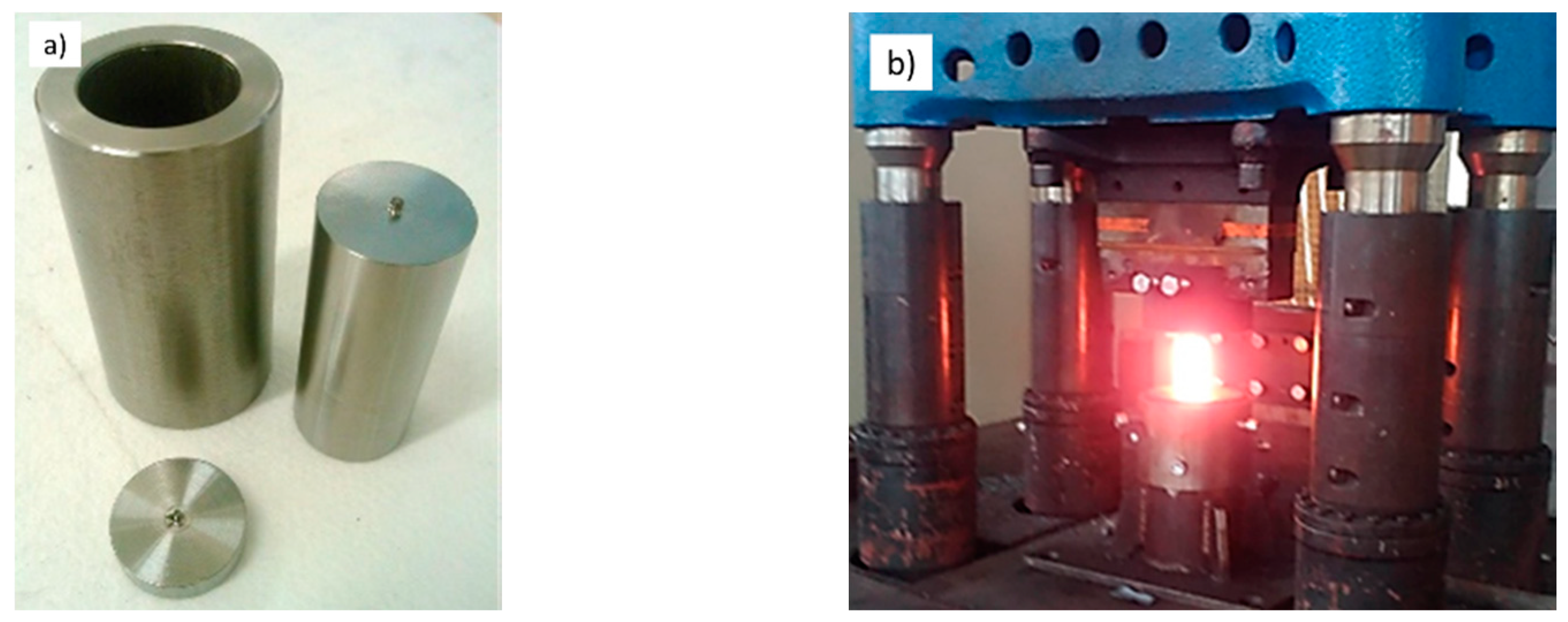
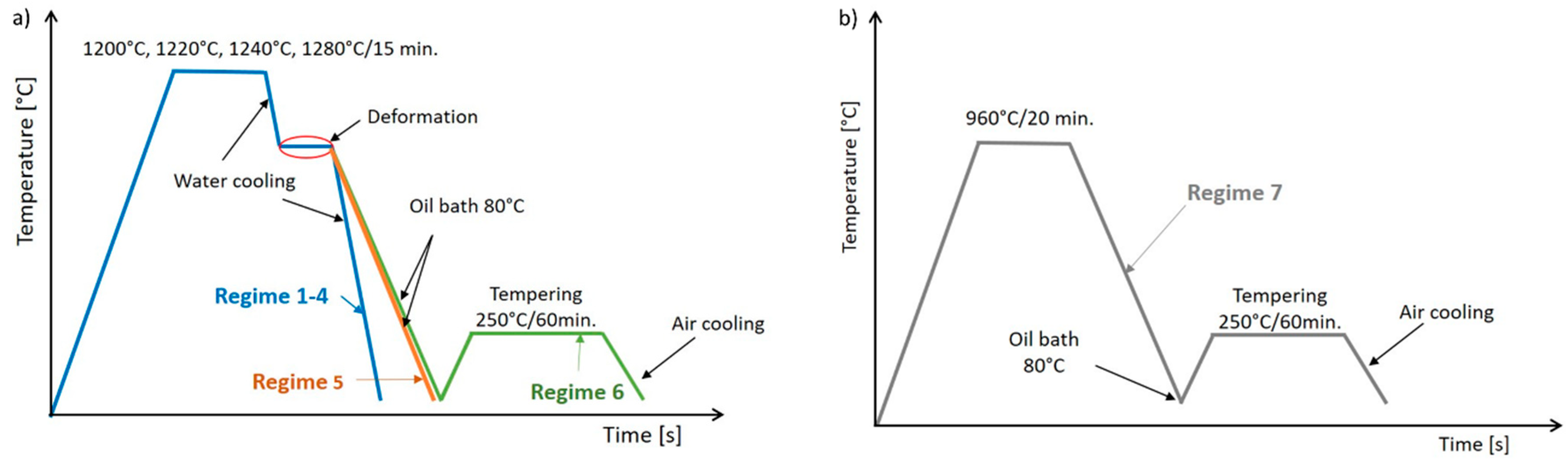
2.3. Semi-Solid Processing and Subsequent Thermomechanical Treatment
2.4. Methods of Evaluation
3. Results and Discussion
3.1. Microstructural Evolution and Process Parameters
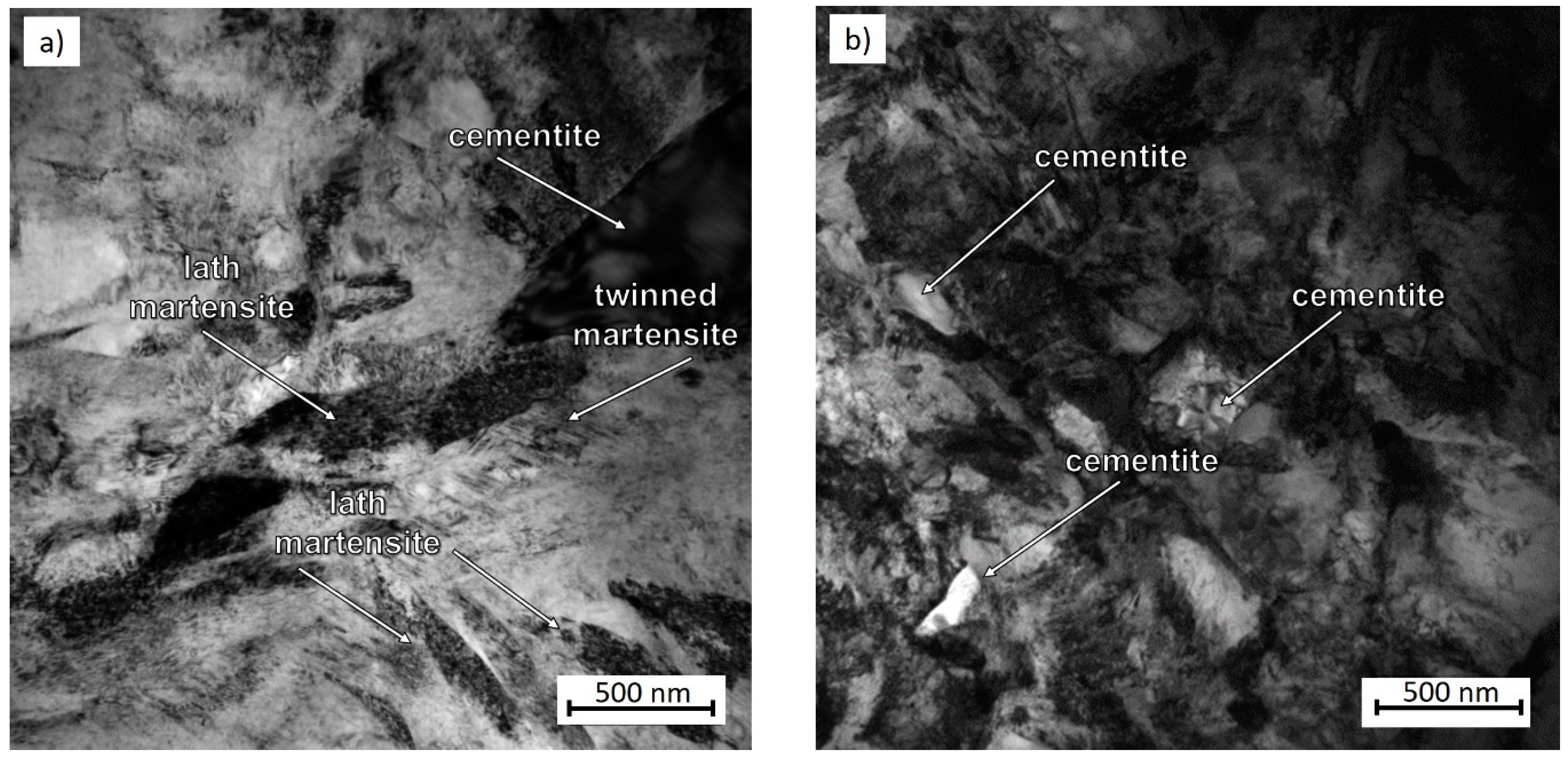
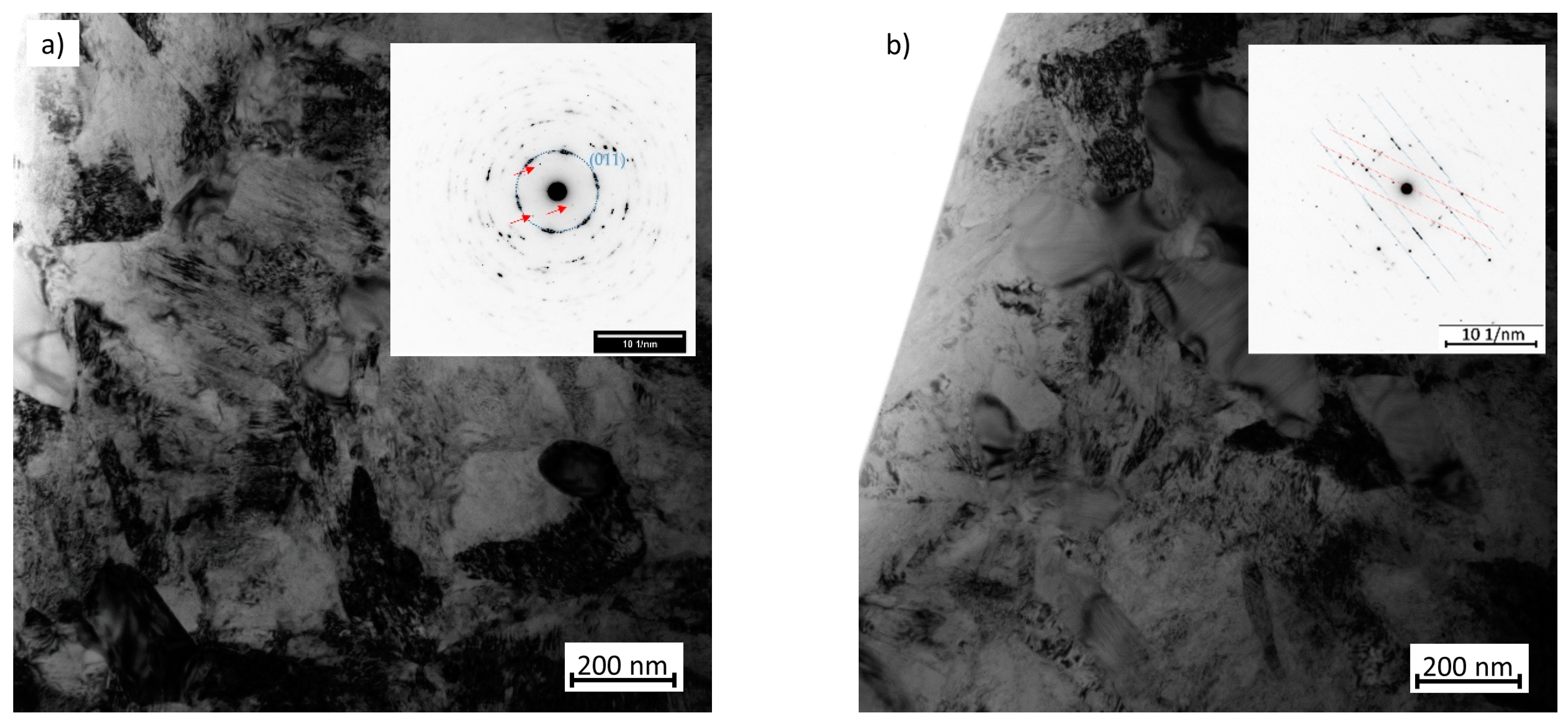
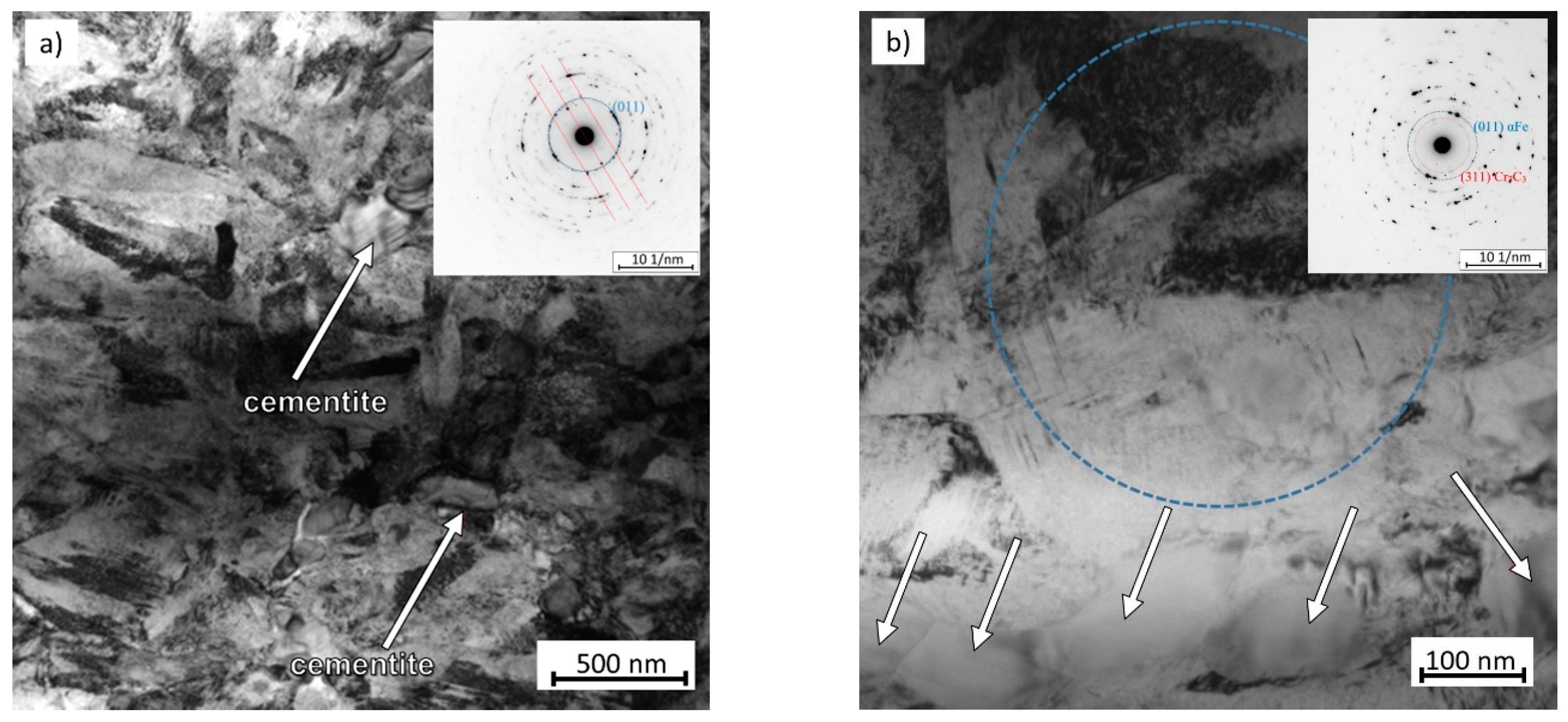
3.2. Microstructure Observation Using Transmission Electron Microscopy
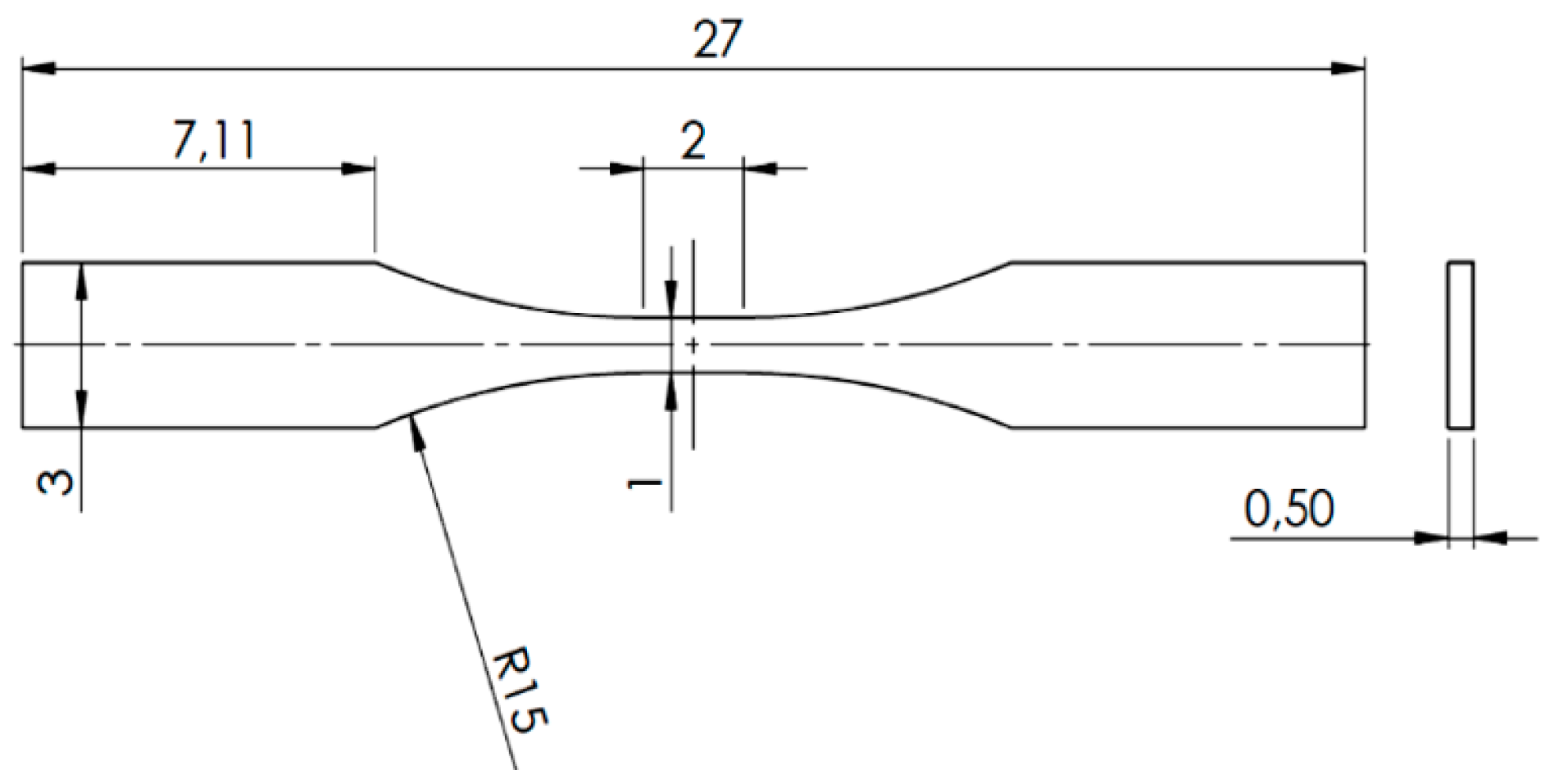
3.3. Micro-Tensile Test (M-TT)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoyoung, K.; Jun-Yun, K.; Dongmin, S.; Tae-Ho, L.; Kyung-Mox, C. Evolution of carbides in cold-work tool steels. Mater. Charact. 2015, 107, 376–385. [Google Scholar] [CrossRef]
- Wang, J.; Guo, W.; Sun, H.; Li, H.; Gou, H.; Zhang, J. Plastic deformation behaviours and hardening mechanism of M7C3 carbid. Mater. Sci. Eng. A 2016, 662, 88–94. [Google Scholar] [CrossRef]
- Casellas, D.; Caro, J.; Molas, S.; Prado, J.M.; Valls, I. Fracture toughness of carbides in tool steels evaluated by nanoindentation. Acta Mater. 2007, 55, 4277–4286. [Google Scholar] [CrossRef]
- Bombac, D.; Fazarinc, M.; Saha Podder, A.; Kugler, G. Study of Carbide Evolution During Thermo-mechanical Processing of AISI D2 Tool Steel. J. Mater. Eng. Perform. 2013, 22, 742–747. [Google Scholar] [CrossRef]
- Song, T.; Kang, Y.; Zhao, A. Semi-solid rolling process of steel strips. J. Mater. Process. Technol. 2008, 198, 291–299. [Google Scholar] [CrossRef]
- Rassili, A.; Atkinson, H.V. A review of steel thixoforming. Trans. Nonferrous Met. Soc. China 2010, 20, 1048–1054. [Google Scholar] [CrossRef]
- Jirková, H.; Aišman, D.; Mašek, B. Unconventional structure of X210Cr12 steel obtained by thixoforming. J. Alloy. Compd. 2010, 504, S500–S503. [Google Scholar] [CrossRef]
- Puttgen, W.; Hallstedt, B.; Bleck, W.; Uggowitzer, P.J. On the microstructure formation in chromium steels rapidly cooled from the semi-solid state. Acta Mater. 2007, 55, 1033–1042. [Google Scholar] [CrossRef]
- Balan, T.; Becker, E.; Langlois, L.; Bigot, R. A new route for semi-solid steel forging. CIRP Ann. 2017, 66, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Kopp, R.; Kallweit, J.; Möller, T.; Seidl, I. Forming and joining of commercial steel grades in the semi-solid state. J. Mater. Process. Technol. 2002, 130–131, 562–568. [Google Scholar] [CrossRef]
- Uhlenhaut, D.I.; Kradolfer, J.; Puttgen, W.; Loffler, J.F.; Uggowitzer, P.J. Structure and properties of a hypoeutectic chromium steel processed in the semi-solid state. Acta Mater. 2006, 54, 2727–2734. [Google Scholar] [CrossRef]
- Aišman, D.; Jirková, H.; Kučerová, L.; Mašek, B. Metastable structure of austenite base obtained by rapid solidification in a semi-solid state. J. Alloy. Compd. 2011, 509, S312–S315. [Google Scholar] [CrossRef]
- Aišman, D.; Jirková, H.; Mašek, B. The influence of deformation and cooling parameters after transition through semi-solid state on structure development of ledeburite steel. J. Alloy. Compd. 2012, 536, S204–S207. [Google Scholar] [CrossRef]
- Rogal, Ł.; Dutkiewicz, J. Deformation behavior of high strength X210CrW12 steel after semi-solid processing. Mater. Sci. Eng. A 2014, 603, 93–97. [Google Scholar] [CrossRef]
- Mašek, B.; Aišman, D.; Jirková, H.; Wurster, S. Micro-compression test of thixoformed austenite. Solid State Phenom. 2013, 192, 215–218. [Google Scholar] [CrossRef]
- ČSN 41 9436. Ocel 19436 chromová (Tool Steel 19 436); Český Normalizační Institut: Praha, Czech Republic, 1994.
- Bučovice, JKZ. X210Cr12. Available online: http://www.jkz.cz/cs/produkty/nastrojove-oceli/pro-prace-za-studena/w-nr-12080/ (accessed on 20 June 2017).
- JMatPro, Release 9.0, Sente Software Ltd.: Guildford, UK, 2016.
- Thermo-Calc, 2017a, Thermo-Calc Software AB: Pittsburgh, PA, USA, 2018.
- Rorgal, Ł.; Dutkiewicz, J.; Szklarz, Z.; Zimowski, S.; Kot, M.; Zimowski, S. Mechanical properties and corrosion resistance of steel X210CrW12 after semi-solid processing and heat treatment. Mater. Charact. 2014, 88, 100–110. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 6892-1:2016: Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Konopík, P.; Džugan, J. Determination of Tensile Properties of Low Carbon Steel and Alloyed Steel 34CrNiMo6 by Small Punch Test and Micro-Tensile Test. In Proceedings of the 2nd International conference—Determination of Mechanical Properties of Materials by Small Punch and Other Miniature Techniques, Ostrava, Czech Republic, 2–4 October 2012; Matocha, K., Hurst, R., Sun, W., Eds.; Ocelot: Ostrava-Vítkovice, Czech Republic, 2012; pp. 319–328. [Google Scholar]
- Rund, M.; Procházka, R.; Konopík, P.; Džugan, J.; Folgar, H. Investigation of Sample-size Influence on Tensile Test Results at Different Strain Rate. Procedia Eng. 2015, 114, 410–415. [Google Scholar] [CrossRef]
- Prochazka, R.; Dzugan, J.; Konopik, P.; Rund, M. Investigation of high-strength stainless steel using small specimen test techniques—Tensile and fatigue properties. In Proceedings of the 7th International Conference on Mechanics and Materials in Design (M2D), Albufeira, Portugal, 11–15 June 2017; Gomes, J.F.S., Meguid, S.A., Eds.; INEGI/FEUP: Porto, Portugal, 2017; pp. 343–354. [Google Scholar]
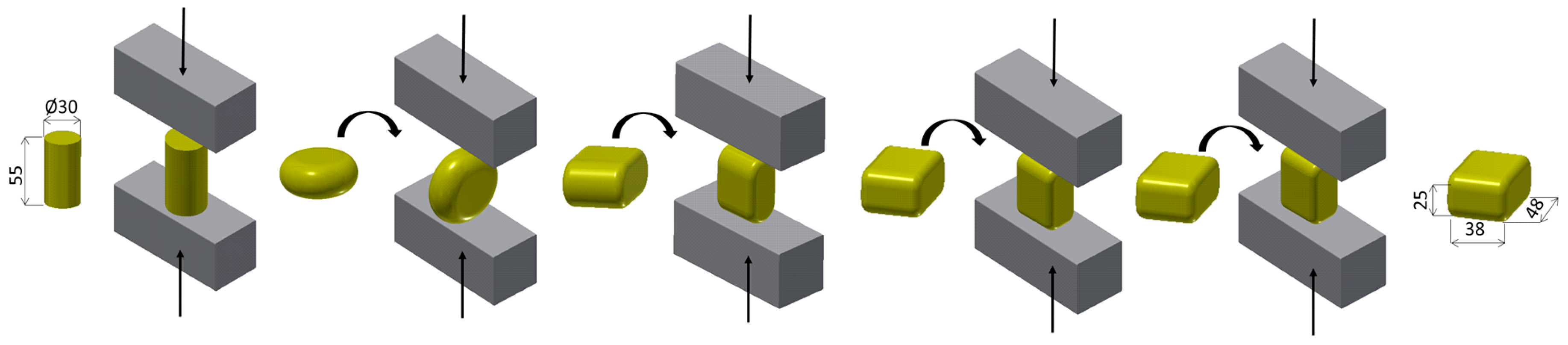
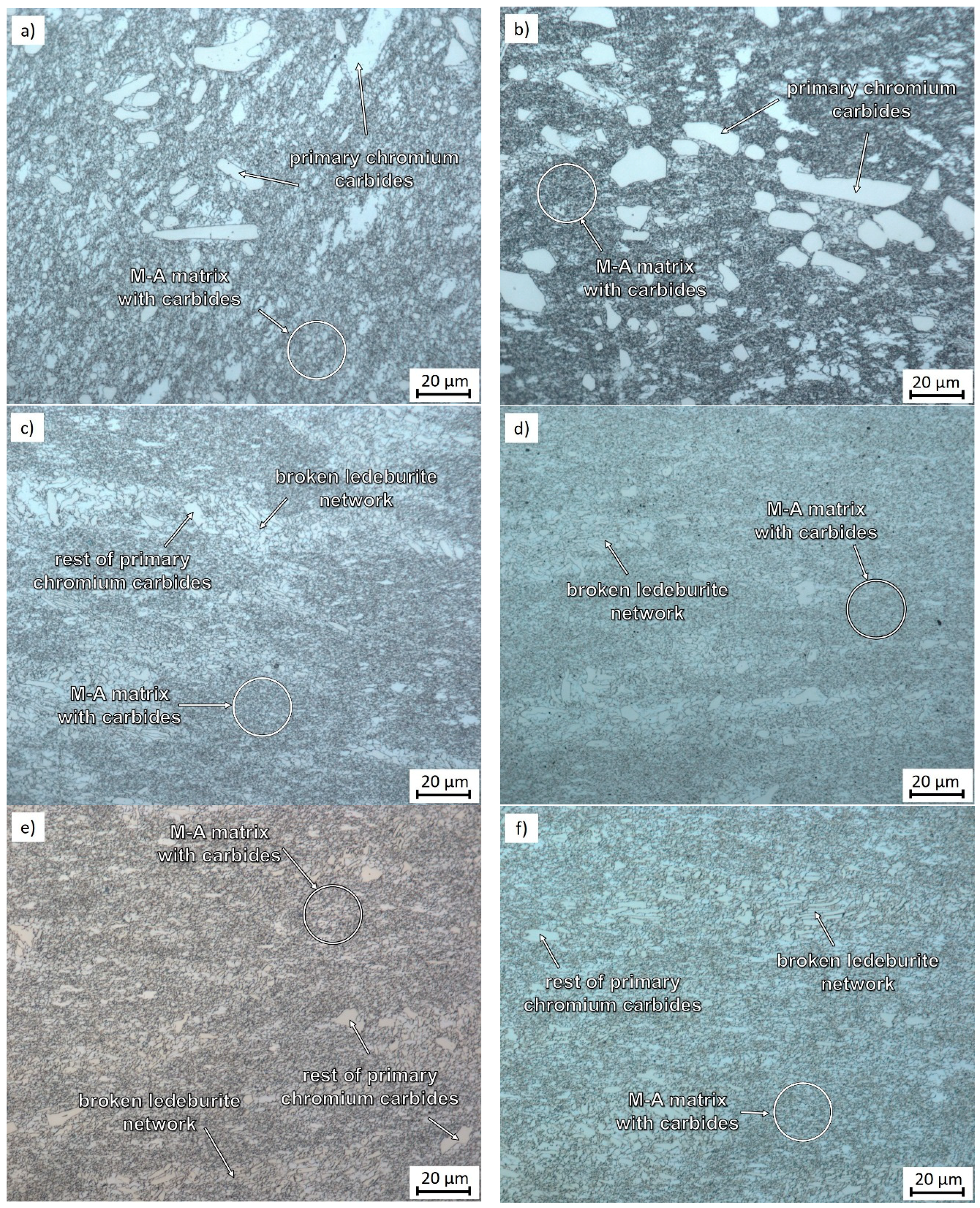
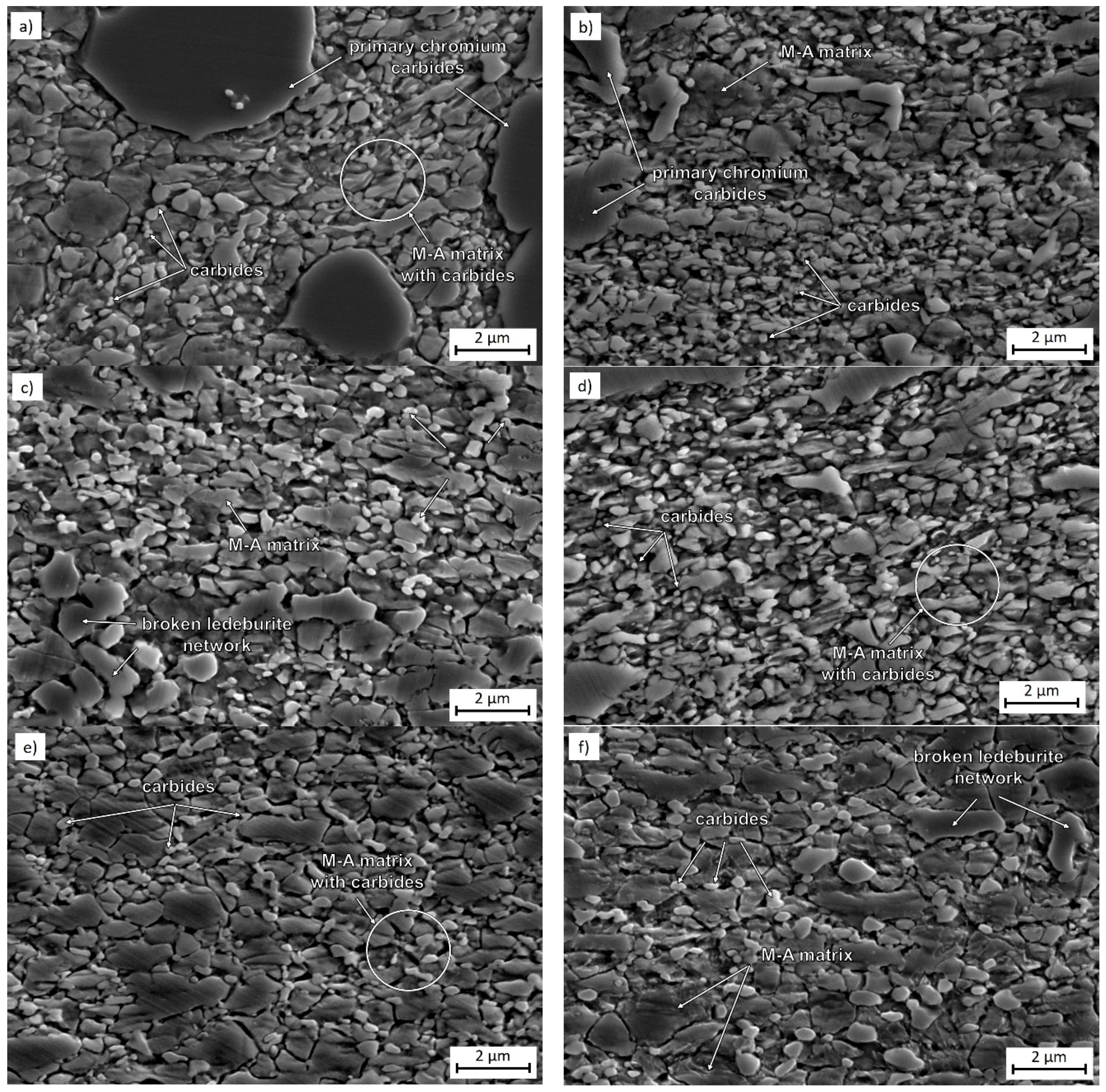
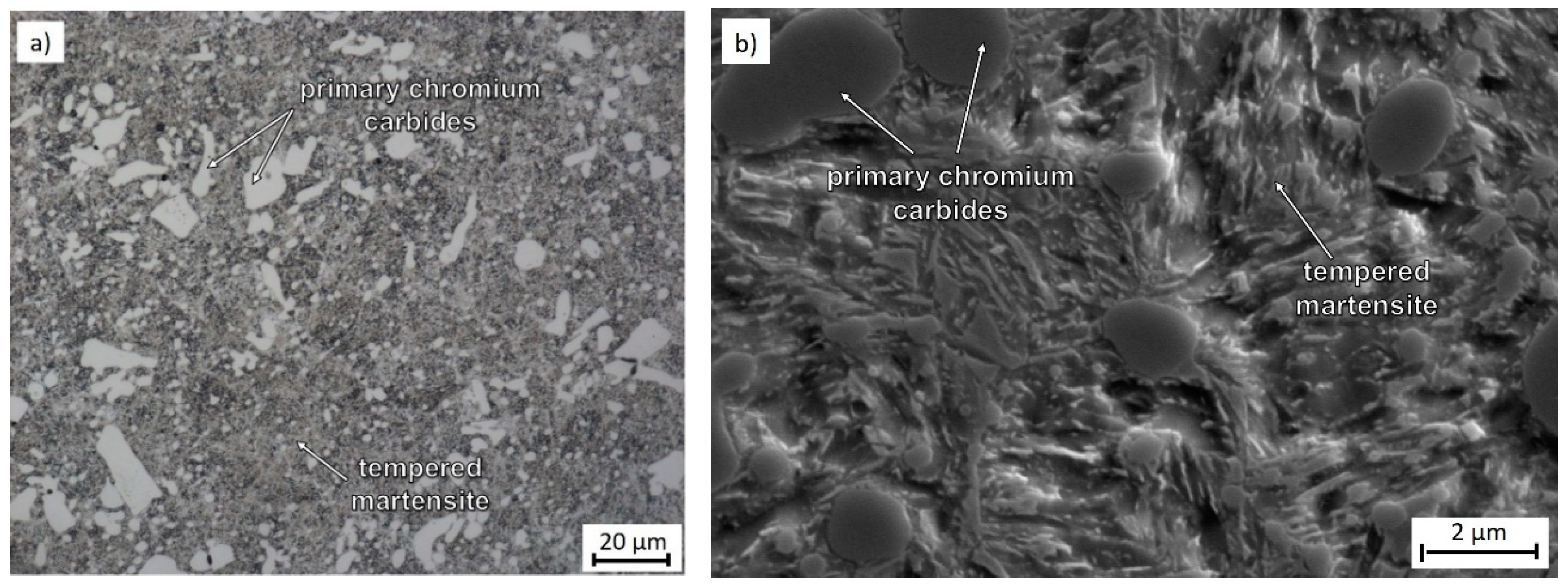


| C | Cr | Mn | Si | Ni | P | S | HV10 (–) | BCC-Fe | Cr7C3 | Fe3C |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.8 | 11 | 0.2 | 0.2 | 0.5 max | 0.03 max | 0.01 max | 220 | 70 | 6 | 24 |
| JMatPro | Thermo-Calc | DSC | |||
|---|---|---|---|---|---|
| Tsolidus (°C) | Tliquidus (°C) | Tsolidus (°C) | Tliquidus (°C) | Tsolidus (°C) | Tliquidus (°C) |
| 1225 | 1373 | 1245 | 1376 | 1237 | 1416 |
| Regime | Soaking Temp. (°C) | Time at Temp. (min) | Forging Temp. (°C) | Time at Temp. (min) | Number of Def. Steps | Cooling Medium | Tempering Temp. (°C)/Time (min) | HV10 (–) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1200 | 15 | 1080 | 1.5 | 4 | Water | - | 848 |
| 2 | 1220 | 15 | 1080 | 1.5 | 4 | Water | - | 803 |
| 3 | 1240 | 15 | 1080 | 1.5 | 5 | Water | - | 864 |
| 4 | 1280 | 15 | 1080 | 1.5 | 5 | Water | - | 866 |
| 5 | 1240 | 15 | 1080 | 1.5 | 5 | Oil 80 °C | - | 778 |
| 6 | 1240 | 15 | 1080 | 1.5 | 5 | Oil 80 °C | 250/60 | 677 |
| 7 | 960 | 20 | - | - | - | Oil 80 °C | 250/60 | 535 |
| Regime No. | Process | FCC-Fe (vol. %) | BCC-Fe (vol. %) | Cr7C3 (vol. %) | Fe3C (vol. %) |
|---|---|---|---|---|---|
| 1 | 1200 °C/1080 °C forging/water | 13 | 56 | 17 | 14 |
| 2 | 1220 °C/1080 °C forging/water | 15 | 49 | 16 | 20 |
| 3 | 1240 °C/1080 °C forging/water | 17 | 48 | 18 | 17 |
| 4 | 1280 °C/1080 °C forging/water | 8 | 51 | 20 | 21 |
| 5 | 1240 °C/1080 °C forging/oil | 21 | 59 | 11 | 9 |
| 6 | 1240 °C/1080 °C forging/oil–250 °C/60 min | 24 | 48 | 17 | 6 |
| 7 | 960 °C/oil–250 °C/60 min | 6 | 65 | 14 | 15 |
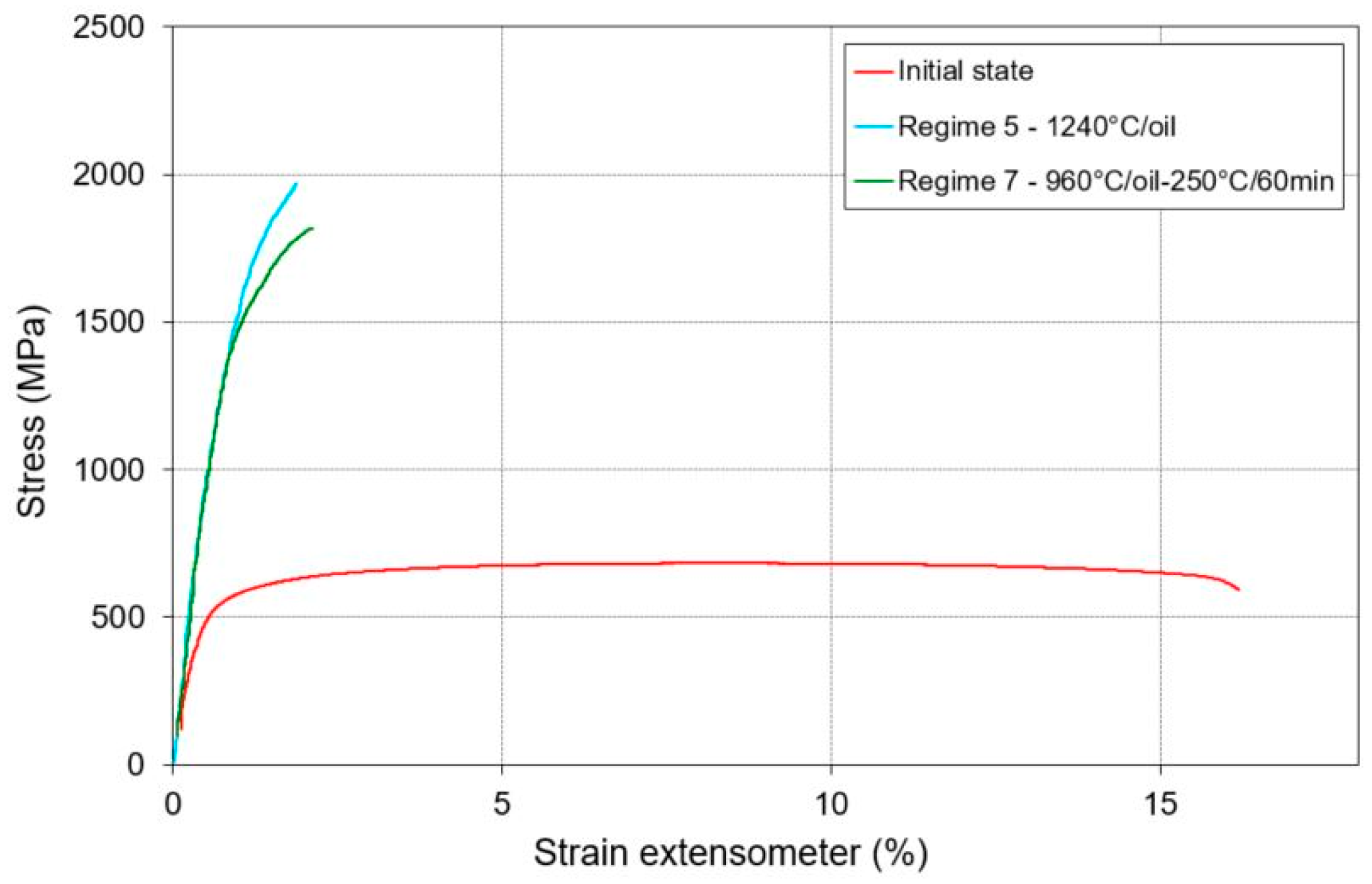
| Regime | Process | Rp0.2 (OYS) (%) | Rm (UTS) (%) | A5 (%) | Z (%) |
|---|---|---|---|---|---|
| – | Initial condition | 541 | 676 | 16.3 | 24.8 |
| 5 | 1240 °C/oil | 1466 | 1985 | 1.0 | 5.8 |
| 7 | 960 °C/oil–250 °C/60 min | 1413 | 1810 | 1.3 | 4.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirková, H.; Rubešová, K.; Konopík, P.; Opatová, K. Effect of the Parameters of Semi-Solid Processing on the Elimination of Sharp-Edged Primary Chromium Carbides from Tool Steel. Metals 2018, 8, 713. https://doi.org/10.3390/met8090713
Jirková H, Rubešová K, Konopík P, Opatová K. Effect of the Parameters of Semi-Solid Processing on the Elimination of Sharp-Edged Primary Chromium Carbides from Tool Steel. Metals. 2018; 8(9):713. https://doi.org/10.3390/met8090713
Chicago/Turabian StyleJirková, Hana, Kateřina Rubešová, Pavel Konopík, and Kateřina Opatová. 2018. "Effect of the Parameters of Semi-Solid Processing on the Elimination of Sharp-Edged Primary Chromium Carbides from Tool Steel" Metals 8, no. 9: 713. https://doi.org/10.3390/met8090713





