Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws
Abstract
:1. Introduction
2. Modeling and Optimizing Using the RSM with Desirability Functions
3. Electroplating Process Factors Examined by Use of RSM
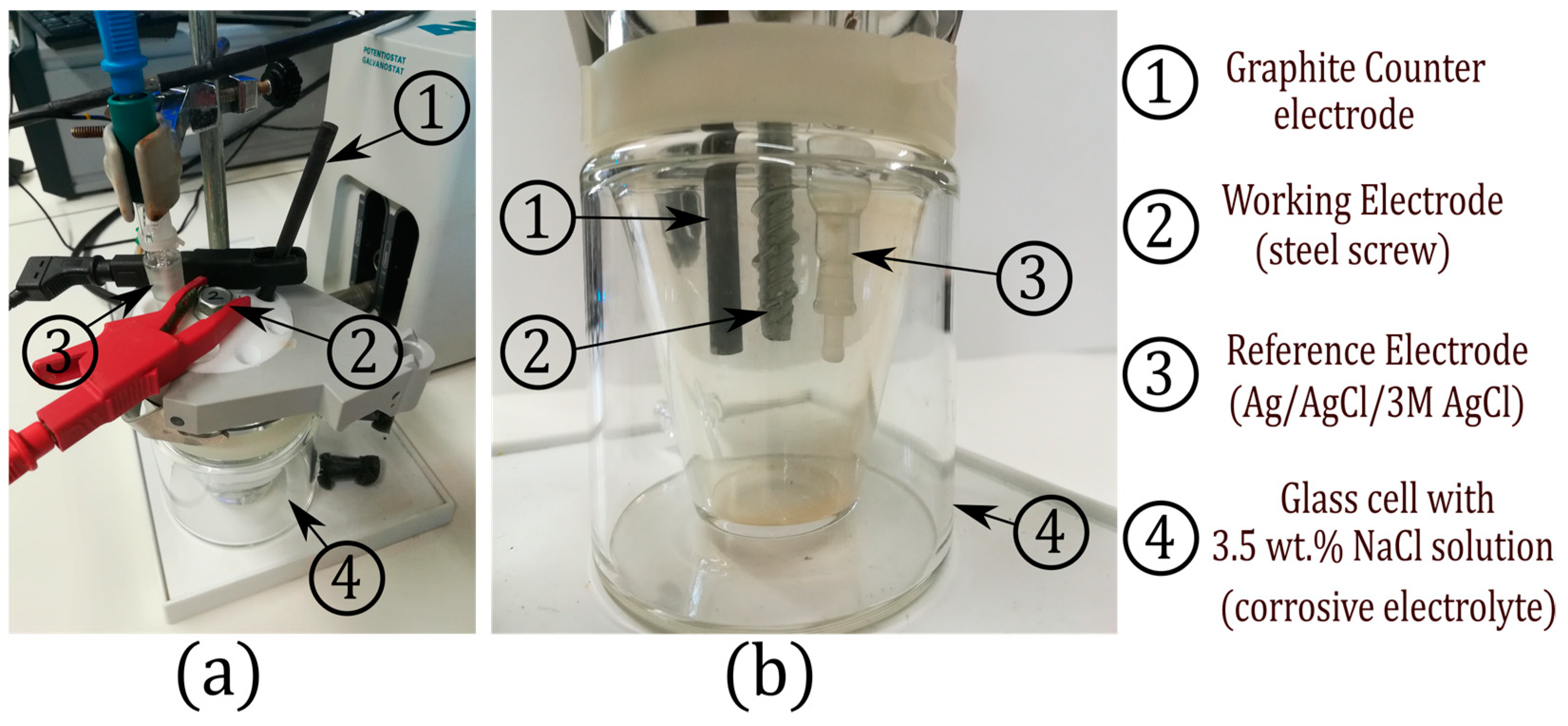
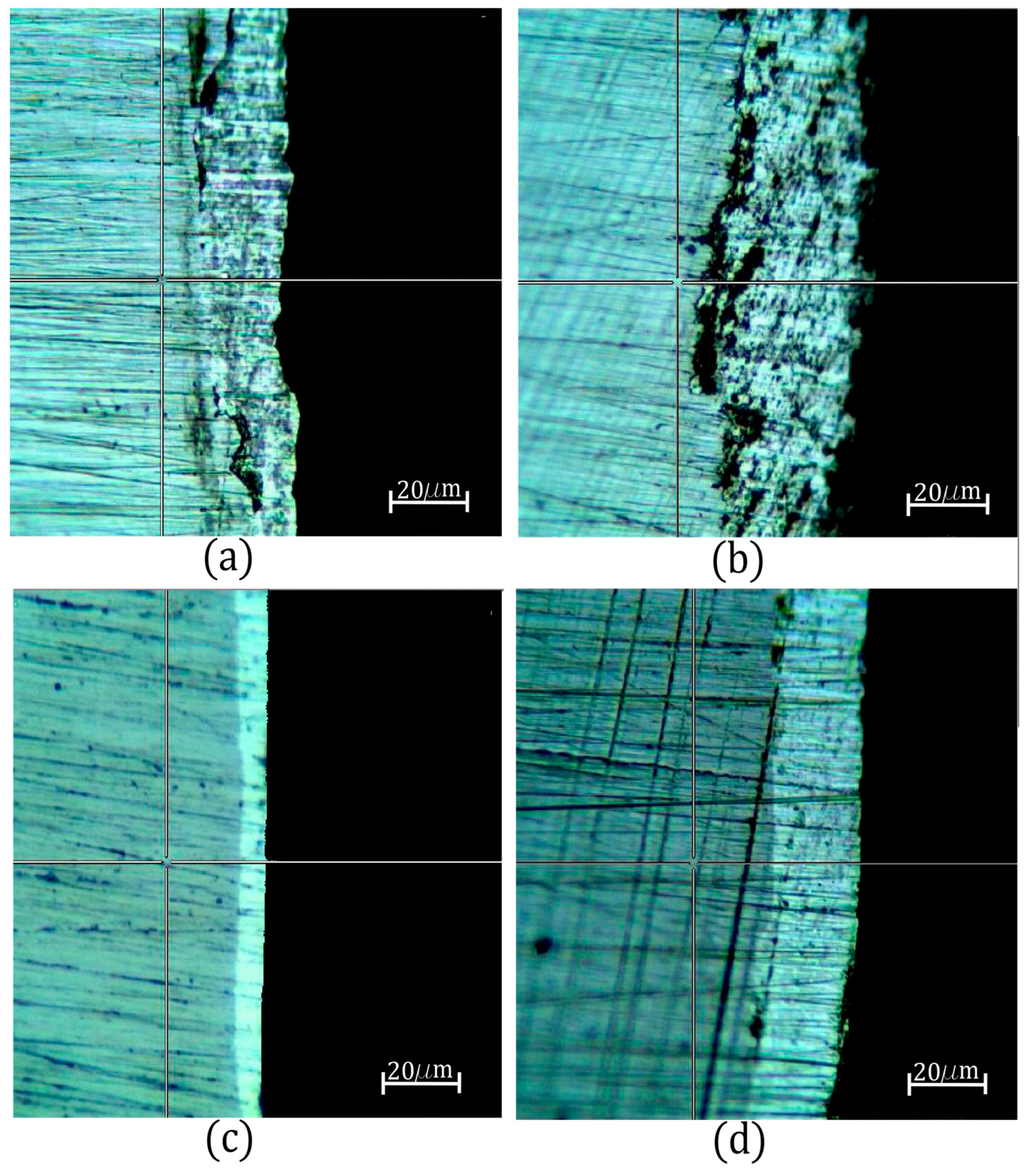
4. Experimental Setup and Results
5. Design of Experiments
6. Results and Discussion
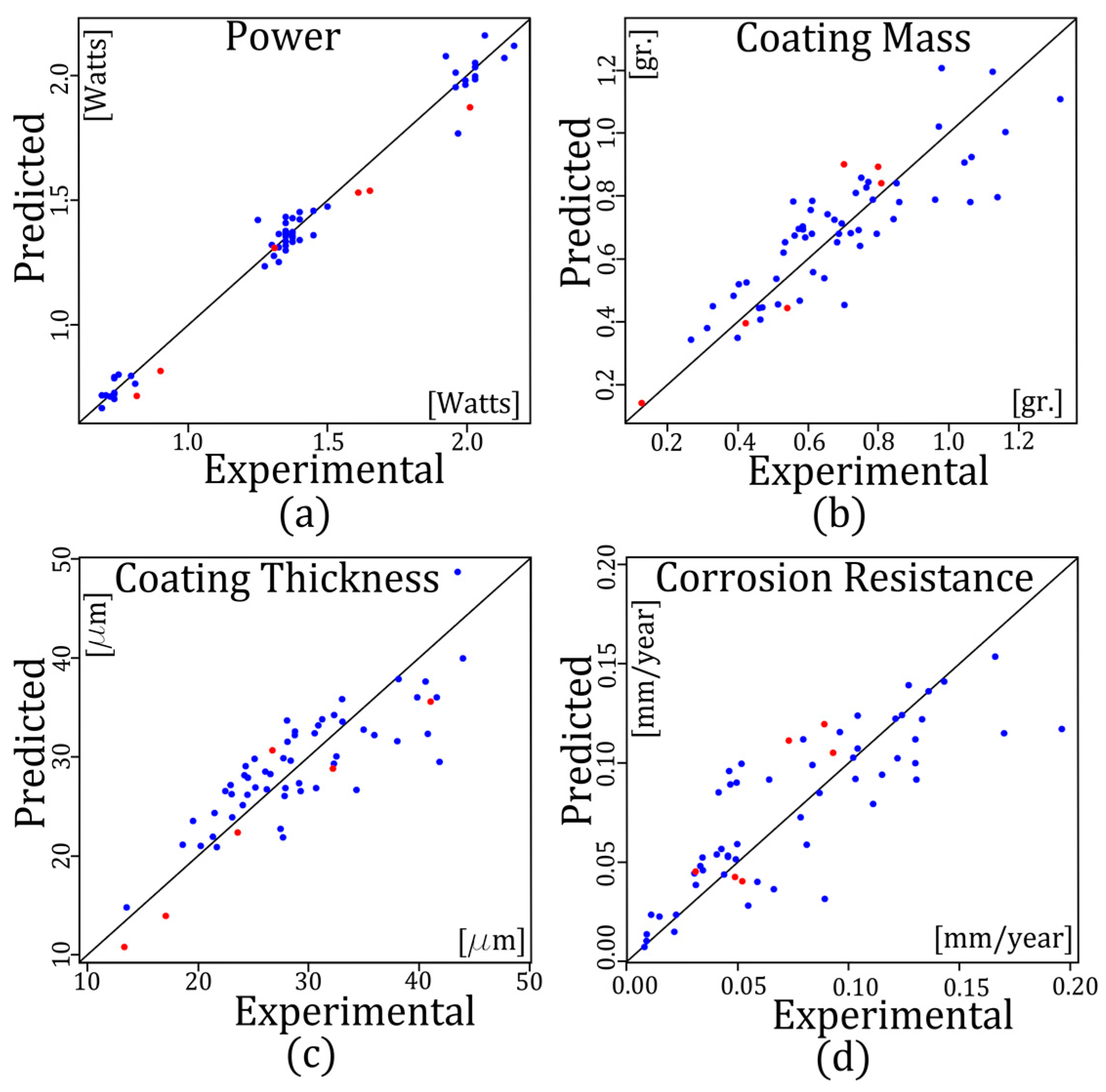
6.1. Modelling the W, Th, ΔM, and R Using RSM
6.2. Multi-Response Optimization
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yli-Pentti, A. Electroplating and electroless plating. In Comprehensive Materials Processing; Elsevier: Oxford, UK, 2014; pp. 277–306. [Google Scholar]
- Pourbaix, M. Applications of Electrochemistry in Corrosion Science and in Practice. Corros. Sci. 1974, 14, 25–82. [Google Scholar] [CrossRef]
- Schneider, S. Zinc Plating. Plat. Surf. Finish. 2007, 94, 40–41. [Google Scholar]
- Oluwole, O.O.; Oloruntoba, D.T.; Awheme, O. Effect of Zinc Plating of Low Carbon Steel on Corrosion Resistance in Cocoa Fluid Environment. Mater. Des. 2008, 29, 1266–1274. [Google Scholar] [CrossRef]
- Valentini, C.R.; Fiora, J.; Iglesias, A.M. Corrosion Behavior of Chromatized Zinc-Electroplated Mild Steel. Corrosion 2008, 64, 891–899. [Google Scholar] [CrossRef]
- Motte, C.; Maury, N.; Olivier, M.-G.; Petitjean, J.-P.; Willem, J.-F. Cerium Treatments for Temporary Protection of Electroplated Steel. Surf. Coat. Technol. 2005, 200, 2366–2375. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Imai, K.; Kimoto, M.; Hirose, Y.; Fukui, K.; Wakano, S. Performance of New Composite Zinc Electroplated Steel Sheet. SAE Tech. Pap. 1999. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Yin, R.H.; Cao, W.M.; Yuan, A.B. Electropolymerization of Aniline on Zinc-Electroplated Steel from Neutral Aqueous Medium by Single-Step Process. Acta Met. Sin. 2004, 17, 849–855. [Google Scholar]
- Short, N.R.; Abibsi, A.; Dennis, J.K. Corrosion resistance of electroplated zinc alloy coatings. Trans. Inst. Met. Finish. 1989, 67, 73–77. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Gudavičiute, L.; Ščit, O.; Bučinskiene, D.; Juskenas, R. Pulse plating effect on composition and corrosion properties of zinc alloy coatings. Trans. Inst. Met. Finish. 2008, 86, 103–108. [Google Scholar] [CrossRef]
- Geduld, H. Zinc Plating; Finishing Publications Ltd.: Warrington, UK, 1988. [Google Scholar]
- Wing, L.; Man, R.; Paulsen, R. A solution to reducing the cost of acid zinc plating. Met. Finish. 2009, 107, 26–30. [Google Scholar] [CrossRef]
- Laĭner, V.I. Modern Electroplating; Israel Program for Scientific Translations: Jerusalem, Israel, 1970. [Google Scholar]
- Kumar, S.; Pande, S.; Verma, P. Factor Effecting Electro-Deposition Process. Int. J. Curr. Eng. Technol. 2015, 5, 2. [Google Scholar]
- Tan, Y.J.; Lim, K.Y. Understanding and improving the uniformity of electrodeposition. Surf. Coat. Technol. 2003, 167, 255–262. [Google Scholar] [CrossRef]
- Vagaská, A.; Gombár, M.; Kmec, J.; Michal, P. Statistical analysis of the factors effect on the zinc coating thickness. Appl. Mech. Mater. 2013, 378, 184–189. [Google Scholar] [CrossRef]
- Amuda, M.O.H.; Subair, W.; Obitayo, O.W. Study of Optimum Conditions for Zinc Plating on Mild Steel. Int. J. Engine Res. Afr. 2010, 2, 31–39. [Google Scholar] [CrossRef]
- Box, J.; Wilson, W. Central composites design. J. R. Stat. Soc. 1951, 1, 1–35. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Lostado, R.; Escribano, R.; Martínez, M.Á.; Múgica, R. Improvement in the Design of Welded Joints of EN 235JR Low Carbon Steel by Multiple Response Surface Methodology. Metals 2016, 6, 205. [Google Scholar] [CrossRef]
- Lostado, R.; García, R.E.; Martinez, R.F. Optimization of operating conditions for a double-row tapered roller bearing. Int. J. Mech. Mater. Des. 2016, 12, 353–373. [Google Scholar] [CrossRef]
- Gómez, F.S.; Lorza, R.L.; Bobadilla, M.C.; García, R.E. Improving the Process of Adjusting the Parameters of Finite Element Models of Healthy Human Intervertebral Discs by the Multi-Response Surface Method. Materials 2017, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Corral Bobadilla, M.; Lostado Lorza, R.; Escribano García, R.; Somovilla Gómez, F.; Vergara González, E.P. An Improvement in Biodiesel Production from Waste Cooking Oil by Applying Thought Multi-Response Surface Methodology Using Desirability Functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef]
- Reddy, P.B.S.; Nishina, K.; Babu, A.S. Performance improvement of a zinc plating process using Taguchi’s methodology: A case study. Met. Finish. 1998, 96, 24–34. [Google Scholar] [CrossRef]
- Kumar, A.; Clement, S.; Agrawal, V.P. Optimum selection and ranking of electroplating system process parameters: Taguchi-MADM approach. Int. J. Appl. Des. Sci. 2011, 4, 341–361. [Google Scholar] [CrossRef]
- Harrington, E.C. The desirability function. Ind. Qual. Control 1965, 21, 494–498. [Google Scholar]
- Kuhn, M. Desirability: Desirabiliy Function Optimization and Ranking. R Package Version. Available online: http://CRAN.R-project.org/package=desirability (accessed on 4 July 2018).
- Oraon, B.; Majumdar, G.; Ghosh, B. Application of response surface method for predicting electroless nickel plating. Mater. Des. 2006, 27, 1035–1045. [Google Scholar] [CrossRef]
- Santana, R.A.C.; Campos, A.R.N.; Prasad, S.; Leite, V.D. Optimization of electrolytic bath LIGA Fe-W-B corrosionresistant. Quim. Nova 2007, 30, 360–365. [Google Scholar] [CrossRef]
- Poroch-Seritan, M.; Gutt, S.; Gutt, G.; Cretescu, I.; Cojocaru, C.; Severin, T. Design of experiments for statistical modeling and multi-response optimization of nickel electroplating process. Chem. Eng. Res. Des. 2011, 89, 136–147. [Google Scholar] [CrossRef]
- Poroch-Seritan, M.; Bulai, P.; Severin, T.L.; Gutt, G. Modelling and optimization study on hardness of Ni-Fe alloythin films through electroplating process. Appl. Mech. Mater. 2014, 657, 286–290. [Google Scholar] [CrossRef]
- Poroch-Seritan, M.; Cretescu, I.; Cojocaru, C.; Amariei, S.; Suciu, C. Experimental design for modelling and multi-response optimization of Fe-Ni electroplating process. Chem. Eng. Res. Des. 2015, 96, 138–149. [Google Scholar] [CrossRef]
- Catia, version v5 R18; Dassault Systèmes: Woodlands Hills, CA, USA, 2007.
- ASTM B499-09. Standard Specification for Measurement of Coating Thicknesses by the Magnetic Method: Nonmagnetic Coatings on Magnetic Basis Metals; ASTM International: Washington, DC, USA, 2014. [Google Scholar]
- ASTM E3-95. Standard Specification for Preparation of Metallographic Specimens; ASTM International: Washington, DC, USA, 2017. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Manickam, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium Insertion into Manganese Dioxide Electrode in MnO2/Zn Aqueous Battery: Part I. A Preliminary Study. J. Power Sources 2004, 130, 254–259. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium Insertion into Manganese Dioxide Electrode in MnO2/Zn Aqueous Battery Part II. Comparison of the Behavior of EMD and Battery Grade MnO2 in Zn|MnO2| Aqueous LiOH Electrolyte. J. Power Sources 2004, 138, 319–322. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium Insertion into Manganese Dioxide Electrode in MnO2/Zn Aqueous Battery: Part III. Electrochemical Behavior of γ-MnO2 in Aqueous Lithium Hydroxide Electrolyte. J. Power Sources 2006, 153, 165–169. [Google Scholar] [CrossRef]
- Radwan, A.B.; Mohamed, A.M.A.; Abdullah, A.M.; Al-Maadeed, M.A. Corrosion Protection of Electrospun PVDF-ZnO Superhydrophobic Coating. Surf. Coat. Technol. 2016, 289, 136–143. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Yu, L.; Jiang, T. Hydrophobic polystyrene/electro-Spun Polyaniline Coatings for Corrosion Protection. Synth. Met. 2017, 234, 166–174. [Google Scholar] [CrossRef]
- Autolab Software: Advanced Electrochemical Software, AUTOLAB NOVA; Metrohm Autolab: Herisau, Switzerland, 2016.
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning Superhydrophobic Nanofibrous Poly(Vinylidene Fluoride)/stearic Acid Coatings with Excellent Corrosion Resistance. Thin Solid Films 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Zhang, Z.; Yu, L. Superhydrophobic polyaniline/polystyrene micro/nanostructures as Anticorrosion Coatings. React. Funct. Polym. 2017, 119, 95–104. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2008. [Google Scholar]
- Box, G.E.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- R Development Core Team. R Language and Environment for Statistical Computing; R Foundation for Statistical Com-Putting: Vienna, Austria, 2011; ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 4 July 2018).
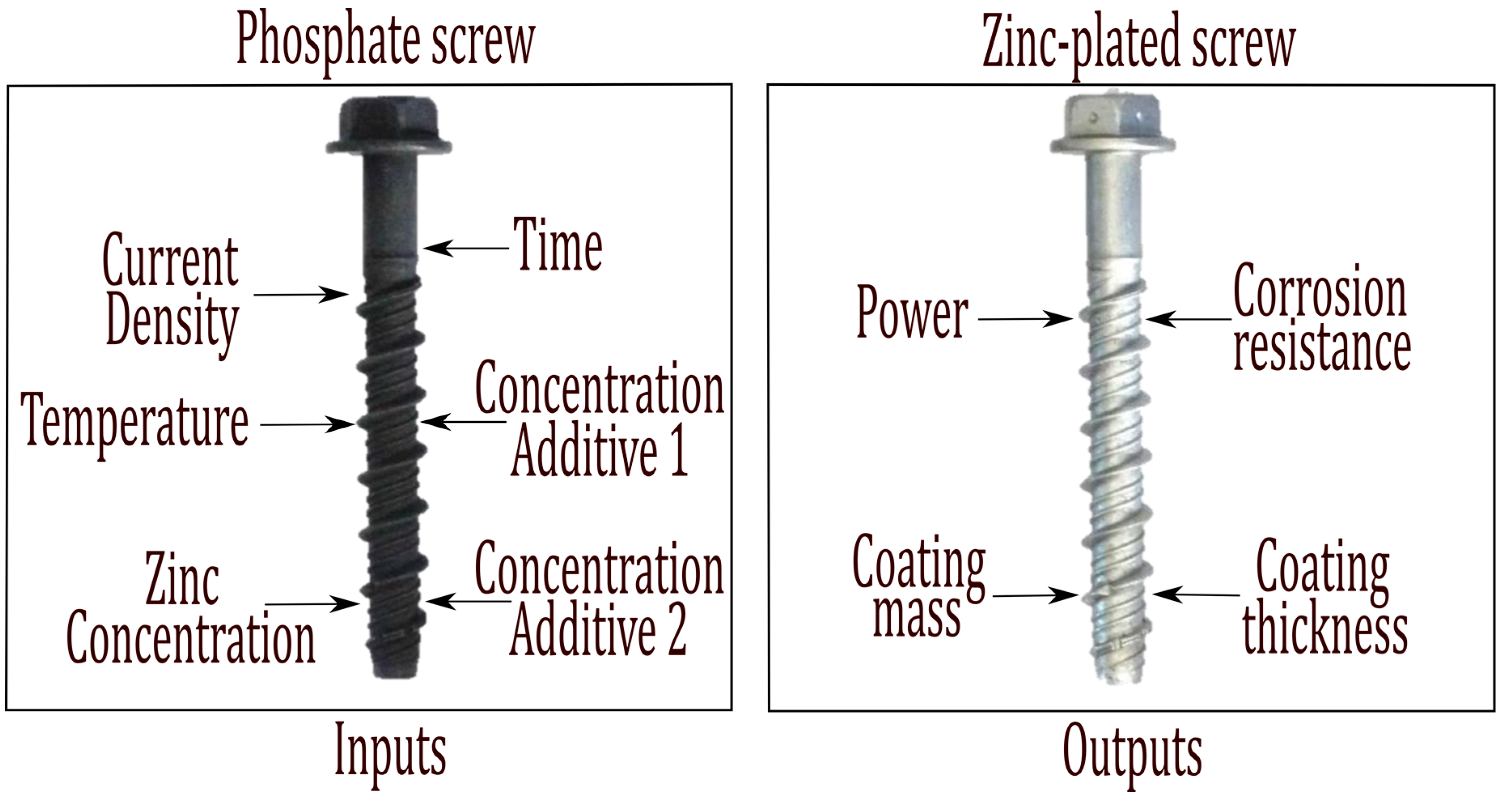

| Input | Notation | Magnitude | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Current Density | ρ | amps/dm² | 0.30 | 0.50 | 0.70 |
| Temperature | T | °C | 20.00 | 30.00 | 40.00 |
| Zinc Concentration | C | g/L | 8.00 | 11.00 | 14.00 |
| Deposition Time | t | min | 45.00 | 67.50 | 90 |
| Concentration Additive 1 | CA2 | mL/L | 25.00 | 27.00 | 30.00 |
| Concentration Additive 2 | CA1 | mL/L | 1.00 | 2.00 | 3.00 |
| Exp.No. | Inputs | Outputs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | T | C | t | CA1 | CA2 | W | Th | ΔM | R | |
| (amps/dm2) | (°C) | (g/L) | (min) | (mL/L) | (mL/L) | (Watts) | (μm) | (gr) | (mm/year) | |
| 1 | 0.5 | 20.0 | 14.0 | 135 | 60.0 | 25.0 | 1.31 | 0.68 | 21.34 | 0.034 |
| 2 | 0.5 | 40.0 | 14.0 | 135 | 60.0 | 25.0 | 1.97 | 1.13 | 43.49 | 0.021 |
| 3 | 0.5 | 30.0 | 14.0 | 135 | 90.0 | 27.0 | 1.35 | 1.32 | 43.96 | 0.104 |
| 4 | 0.7 | 30.0 | 14.0 | 135 | 60.0 | 27.0 | 1.93 | 0.97 | 35.96 | 0.015 |
| 5 | 0.5 | 30.0 | 14.0 | 135 | 45.0 | 27.0 | 1.33 | 0.61 | 27.73 | 0.143 |
| 6 | 0.3 | 30.0 | 14.0 | 135 | 60.0 | 27.0 | 0.71 | 0.56 | 18.60 | 0.166 |
| 7 | 0.7 | 30.0 | 14.0 | 135 | 60.0 | 27.0 | 1.96 | 0.75 | 21.70 | 0.111 |
| 8 | 0.5 | 30.0 | 14.0 | 135 | 45.0 | 27.0 | 1.35 | 0.78 | 25.19 | 0.041 |
| 9 | 0.3 | 30.0 | 14.0 | 135 | 60.0 | 27.0 | 0.74 | 0.53 | 27.84 | 0.011 |
| 10 | 0.5 | 30.0 | 14.0 | 135 | 90.0 | 27.0 | 1.38 | 0.96 | 30.68 | 0.046 |
| 11 | 0.5 | 40.0 | 14.0 | 135 | 60.0 | 30.0 | 1.38 | 0.75 | 24.05 | 0.13 |
| 12 | 0.5 | 20.0 | 14.0 | 135 | 60.0 | 30.0 | 1.35 | 0.46 | 13.54 | 0.044 |
| 13 | 0.5 | 20.0 | 8.0 | 135 | 60.0 | 25.0 | 1.35 | 0.66 | 21.49 | 0.049 |
| 14 | 0.5 | 40.0 | 8.0 | 135 | 60.0 | 25.0 | 1.28 | 0.98 | 33.04 | 0.081 |
| 15 | 0.7 | 30.0 | 8.0 | 135 | 60.0 | 27.0 | 2.03 | 0.86 | 27.91 | 0.078 |
| 16 | 0.5 | 30.0 | 8.0 | 135 | 45.0 | 27.0 | 1.35 | 0.59 | 26.10 | 0.096 |
| 17 | 0.3 | 30.0 | 8.0 | 135 | 60.0 | 27.0 | 0.74 | 0.74 | 24.50 | 0.05 |
| 18 | 0.5 | 30.0 | 8.0 | 135 | 90.0 | 27.0 | 1.40 | 1.06 | 40.79 | 0.046 |
| 19 | 0.3 | 30.0 | 8.0 | 135 | 60.0 | 27.0 | 0.74 | 0.56 | 28.75 | 0.009 |
| 20 | 0.7 | 30.0 | 8.0 | 135 | 60.0 | 27.0 | 2.07 | 0.69 | 22.94 | 0.009 |
| 21 | 0.5 | 30.0 | 8.0 | 135 | 45.0 | 27.0 | 1.33 | 0.51 | 20.25 | 0.03 |
| 22 | 0.5 | 30.0 | 8.0 | 135 | 90.0 | 27.0 | 1.38 | 0.84 | 32.51 | 0.066 |
| 23 | 0.5 | 20.0 | 8.0 | 135 | 60.0 | 30.0 | 1.40 | 0.46 | 27.69 | 0.087 |
| 24 | 0.5 | 40.0 | 8.0 | 135 | 60.0 | 30.0 | 1.35 | 0.74 | 29.14 | 0.089 |
| 25 | 0.5 | 20.0 | 11.0 | 135 | 60.0 | 25.0 | 1.35 | 0.77 | 33.06 | 0.031 |
| 26 | 0.5 | 40.0 | 11.0 | 135 | 60.0 | 25.0 | 1.25 | 1.05 | 40.59 | 0.034 |
| 27 | 0.3 | 30.0 | 11.0 | 135 | 90.0 | 25.0 | 0.74 | 1.16 | 38.04 | 0.022 |
| 28 | 0.3 | 30.0 | 11.0 | 135 | 45.0 | 25.0 | 0.74 | 0.43 | 26.55 | 0.133 |
| 29 | 0.7 | 30.0 | 11.0 | 135 | 45.0 | 25.0 | 2.00 | 0.77 | 28.09 | 0.05 |
| 30 | 0.7 | 20.0 | 11.0 | 135 | 60.0 | 25.0 | 2.03 | 0.61 | 24.30 | 0.059 |
| 31 | 0.5 | 30.0 | 11.0 | 135 | 90.0 | 25.0 | 1.30 | 1.14 | 41.60 | 0.17 |
| 32 | 0.5 | 40.0 | 11.0 | 135 | 60.0 | 25.0 | 1.35 | 0.85 | 38.15 | 0.104 |
| 33 | 0.7 | 20.0 | 11.0 | 135 | 90.0 | 27.0 | 2.14 | 0.80 | 41.85 | 0.033 |
| - | - | - | - | - | - | - | - | - | - | - |
| 44 | 0.7 | 40.0 | 11.0 | 135 | 90.0 | 27.0 | 2.00 | 1.07 | 39.83 | 0.046 |
| 48 | 0.7 | 30.0 | 11.0 | 135 | 90.0 | 30.0 | 0.69 | 0.72 | 34.99 | 0.136 |
| 49 | 0.5 | 40.0 | 11.0 | 135 | 60.0 | 30.0 | 2.03 | 0.57 | 23.04 | 0.055 |
| 50 | 0.3 | 30.0 | 11.0 | 135 | 45.0 | 30.0 | 1.45 | 0.39 | 25.11 | 0.13 |
| 51 | 0.5 | 20.0 | 11.0 | 135 | 60.0 | 30.0 | 1.96 | 0.70 | 31.24 | 0.102 |
| 52 | 0.3 | 30.0 | 11.0 | 135 | 90.0 | 30.0 | 1.33 | 0.47 | 29.29 | 0.124 |
| 53 | 0.7 | 30.0 | 11.0 | 135 | 45.0 | 30.0 | 0.69 | 0.27 | 19.55 | 0.121 |
| 54 | 0.5 | 40.0 | 11.0 | 135 | 60.0 | 30.0 | 1.50 | 0.51 | 24.19 | 0.008 |
| Var. | Df | Sum of Sq. | Mean Square | F-Value | p-Value | Sig. Code |
|---|---|---|---|---|---|---|
| ρ | 1 | 9.9975 | 9.9975 | 2367.3553 | <2.2 × 10−16 | *** |
| T | 1 | 0.0018 | 0.0018 | 0.4307 | 0.5152293 | |
| T2 | 1 | 0.0006 | 0.0006 | 0.1409 | 0.7092685 | |
| ρ × C | 1 | 0.0022 | 0.0022 | 0.5319 | 0.4698578 | |
| T × C | 1 | 0.0648 | 0.0648 | 15.3523 | 0.0003227 | *** |
| C × t | 1 | 0.0012 | 0.0012 | 0.2741 | 0.6033136 | |
| CA1 | 1 | 0.0012 | 0.0012 | 0.2728 | 0.6042199 | |
| C × CA1 | 1 | 0.0756 | 0.0756 | 17.9063 | 0.0001232 | *** |
| t × CA1 | 1 | 0.1337 | 0.1337 | 31.6694 | 1.36 × 10−6 | *** |
| ρ × CA2 | 1 | 0.0001 | 0.0001 | 0.028 | 0.8679105 | |
| T × CA2 | 1 | 0.041 | 0.041 | 9.7123 | 0.0032967 | ** |
| Residuals | 42 | 0.1774 | 0.0042 | |||
| R2 | 0.983 |
| Var. | Df | Sum of Sq. | Mean Square | F-Value | p-Value | Sig. Code |
|---|---|---|---|---|---|---|
| ρ2 | 1 | 0.05302 | 0.05302 | 2.6297 | 0.1125484 | |
| C | 1 | 0.06219 | 0.06219 | 3.0849 | 0.0864911 | . |
| T × C | 1 | 0.27127 | 0.27127 | 13.4557 | 0.0006964 | *** |
| ρ × t | 1 | 0.00304 | 0.00304 | 0.1508 | 0.6997883 | |
| T × t | 1 | 0.21622 | 0.21622 | 10.7249 | 0.0021536 | ** |
| C × t | 1 | 0.01746 | 0.01746 | 0.8663 | 0.3574404 | |
| t2 | 1 | 0.20946 | 0.20946 | 10.3898 | 0.0024865 | ** |
| T × CA1 | 1 | 0.69365 | 0.69365 | 34.4063 | 6.717 × 10−7 | *** |
| CA2 | 1 | 0.20869 | 0.20869 | 10.3513 | 0.0025282 | ** |
| T × CA2 | 1 | 0.03151 | 0.03151 | 1.5632 | 0.2182895 | |
| CA1 × CA2 | 1 | 0.0427 | 0.0427 | 2.118 | 0.1531931 | |
| CA22 | 1 | 0.30681 | 0.30681 | 15.2186 | 0.0003485 | *** |
| Residuals | 41 | 0.82658 | 0.02016 | |||
| R2 | 0.924 |
| Var. | Df | Sum of Sq. | Mean Square | F-Value | p-Value | Sig. Code |
|---|---|---|---|---|---|---|
| ρ2 | 1 | 1.54 | 1.54 | 0.0739 | 0.7871258 | |
| C | 1 | 3.81 | 3.81 | 0.1831 | 0.6711062 | |
| T × C | 1 | 290.19 | 290.19 | 13.9526 | 0.0005988 | *** |
| C² | 1 | 62.95 | 62.95 | 3.0268 | 0.0897857 | . |
| T × t | 1 | 1.69 | 1.69 | 0.0813 | 0.7770587 | |
| C × t | 1 | 138.92 | 138.92 | 6.6793 | 0.0136118 | * |
| t2 | 1 | 146.32 | 146.32 | 7.0355 | 0.0114962 | * |
| ρ × CA1 | 1 | 91.75 | 91.75 | 4.4117 | 0.0422102 | * |
| CA2 | 1 | 430.74 | 430.74 | 20.7106 | 0.00005107 | *** |
| ρ × CA2 | 1 | 4.19 | 4.19 | 0.2013 | 0.6561237 | |
| T × CA2 | 1 | 157.55 | 157.55 | 7.575 | 0.0089353 | ** |
| C × CA2 | 1 | 90.58 | 90.58 | 4.355 | 0.0434848 | * |
| CA1 × CA2 | 1 | 206.85 | 206.85 | 9.9457 | 0.0030991 | ** |
| CA22 | 1 | 70.48 | 70.48 | 3.3887 | 0.0732603 | . |
| Residuals | 39 | 811.13 | 20.8 | |||
| R2 | 0.893 |
| Var. | Df | Sum of Sq. | Mean Square | F-Value | p-Value | Sig. Code |
|---|---|---|---|---|---|---|
| T2 | 1 | 0.001987 | 0.0019869 | 2.0993 | 0.1557926 | |
| T | 1 | 0.003524 | 0.0035243 | 3.7236 | 0.0613421 | . |
| ρ × C | 1 | 0.001202 | 0.0012019 | 1.2699 | 0.2670446 | |
| C2 | 1 | 0.001382 | 0.0013825 | 1.4607 | 0.2344929 | |
| t | 1 | 0.000116 | 0.0001159 | 0.1225 | 0.7283625 | |
| ρ × t | 1 | 0.008211 | 0.0082107 | 8.6752 | 0.0055502 | ** |
| C × t | 1 | 0.01551 | 0.0155102 | 16.3875 | 0.000253 | *** |
| t2 | 1 | 0.003981 | 0.003981 | 4.2062 | 0.0474079 | * |
| ρ × CA1 | 1 | 0.000019 | 0.0000192 | 0.0202 | 0.8876521 | |
| T × CA1 | 1 | 0.003611 | 0.0036113 | 3.8156 | 0.058371 | . |
| t × CA1 | 1 | 0.001303 | 0.0013035 | 1.3772 | 0.2480777 | |
| T × CA2 | 1 | 0.013708 | 0.0137082 | 14.4836 | 0.0005142 | *** |
| C × CA2 | 1 | 0.0006 | 0.0006004 | 0.6344 | 0.4308356 | |
| t × CA2 | 1 | 0.001398 | 0.0013982 | 1.4773 | 0.2318955 | |
| CA1 × CA2 | 1 | 0.000483 | 0.0004834 | 0.5108 | 0.4792938 | |
| CA22 | 1 | 0.023774 | 0.0237743 | 25.1191 | 0.0000136 | *** |
| Residuals | 37 | 0.035019 | 0.0009465 | |||
| R2 | 0.887 |
| Var. | Train | Train |
|---|---|---|
| MAE | RMSE | |
| W | 0.0277 | 0.0387 |
| ΔM | 0.0971 | 0.1176 |
| Th | 0.1048 | 0.1273 |
| R | 0.0989 | 0.1354 |
| Exp.No. | Inputs | Outputs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | T | C | t | CA1 | CA2 | W | Th | ΔM | R | |
| (amps/dm2) | (°C) | (g/L) | (min) | (mL/L) | (mL/L) | (Watts) | (μm) | (gr) | (mm/year) | |
| 1 | 0.47 | 24.0 | 9.5 | 60.0 | 25.0 | 1.0 | 1.31 | 0.81 | 32.20 | 0.052 |
| 2 | 0.66 | 29.4 | 9.5 | 90.0 | 30.0 | 3.0 | 2.01 | 0.54 | 17.08 | 0.049 |
| 3 | 0.56 | 24.7 | 11.5 | 60.0 | 25.0 | 1.0 | 1.65 | 0.70 | 41.04 | 0.031 |
| 4 | 0.34 | 26.9 | 11.5 | 90.0 | 30.0 | 3.0 | 0.90 | 0.13 | 23.59 | 0.073 |
| 5 | 0.59 | 24.4 | 15.0 | 60.0 | 25.0 | 1.0 | 1.61 | 0.80 | 26.74 | 0.093 |
| 6 | 0.32 | 31.7 | 15.0 | 30.0 | 30.0 | 3.0 | 0.82 | 0.42 | 13.34 | 0.089 |
| Var. | Test | Test |
|---|---|---|
| MAE | RMSE | |
| W | 0.0590 | 0.0657 |
| ΔM | 0.0732 | 0.0945 |
| Th | 0.1081 | 0.1158 |
| R | 0.1000 | 0.1169 |
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.3 | 1.00 |
| T (°C) | min | 20.0 | 40.0 | 20.0 | 0.99 |
| C (g/L) | inRange | 8.0 | 14.0 | 13.9 | 1.00 |
| t (min) | inRange | 45 | 90 | 45 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 28.5 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 2.8 | 1.00 |
| W (Watts) | min | 0.69 | 2.17 | 0.75 | 0.95 |
| ΔM (gr) | min | 0.26 | 1.31 | 0.26 | 1.00 |
| Th (μm) | inRange | 13.53 | 43.96 | 13.76 | 1.00 |
| R (mm/year) | inRange | 0.008 | 0.190 | 0.009 | 1.00 |
| Overall Desirability | 0.98 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.5 | 1.00 |
| T (°C) | inRange | 20.0 | 40.0 | 24.6 | 1.00 |
| C (g/L) | inRange | 8.0 | 14.0 | 13.9 | 1.00 |
| t (min) | min | 45 | 90 | 45 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 26.9 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 1.1 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.10 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.32 | 0.55 | 1.00 |
| Th (μm) | inRange | 13.53 | 43.96 | 23.87 | 1.00 |
| R (mm/year) | inRange | 0.008 | 0.190 | 0.090 | 1.00 |
| Overall Desirability | 1.00 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.6 | 1.00 |
| T (°C) | inRange | 20.0 | 40.0 | 32.4 | 1.00 |
| C (g/L) | inRange | 8.0 | 14.0 | 14.0 | 1.00 |
| t (min) | inRange | 45 | 90 | 45 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 28.7 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 2.5 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.41 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.31 | 0.52 | 1.00 |
| Th (μm) | inRange | 13.53 | 43.96 | 21.35 | 1.00 |
| R (mm/year) | max | 0.008 | 0.190 | 0.210 | 1.00 |
| Overall Desirability | 1.00 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.7 | 1.00 |
| T (°C) | inRange | 20.0 | 40.0 | 38.4 | 1.00 |
| C (g/L) | inRange | 8.0 | 14.0 | 12.2 | 1.00 |
| t (min) | inRange | 45 | 90 | 45 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 26.5 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 1.5 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.88 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.31 | 1.11 | 1.00 |
| Th (μm) | max | 13.53 | 43.96 | 45.37 | 1.00 |
| R (mm/year) | inRange | 0.008 | 0.190 | 0.120 | 1.00 |
| Overall Desirability | 1.00 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.7 | 1.00 |
| T (°C) | min | 20.0 | 40.0 | 26.7 | 0.66 |
| C (g/L) | min | 8.0 | 14.0 | 12.1 | 0.52 |
| t (min) | min | 45 | 90 | 45 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 25.0 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 2.0 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.87 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.31 | 0.26 | 1.00 |
| Th (μm) | inRange | 13.53 | 43.96 | 22.97 | 1.00 |
| R (mm/year) | max | 0.008 | 0.196 | 0.160 | 0.84 |
| Overall Desirability | 0.73 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.6 | 1.00 |
| T (°C) | min | 20.0 | 40.0 | 23.3 | 0.81 |
| C (g/L) | min | 8.0 | 14.0 | 10.4 | 0.83 |
| t (min) | min | 45 | 90 | 45 | 0.80 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 29.5 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 1.3 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.98 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.31 | 0.65 | 1.00 |
| Th (μm) | max | 13.53 | 43.96 | 36.43 | 0.75 |
| R (mm/year) | inRange | 0.008 | 0.190 | 0.008 | 1.00 |
| Overall Desirability | 0.79 | ||||
| Variables | Goal | Min. | Max. | Results | Desirability |
|---|---|---|---|---|---|
| ρ (amps/dm2) | inRange | 0.3 | 0.7 | 0.5 | 1.00 |
| T (°C) | min | 20.0 | 40.0 | 20.0 | 0.99 |
| C (g/L) | min | 8.0 | 14.0 | 9.6 | 0.99 |
| t (min) | inRange | 45 | 90 | 89.71 | 1.00 |
| CA1 (mL/L) | inRange | 25.0 | 30.0 | 30.0 | 1.00 |
| CA2 (mL/L) | inRange | 1.0 | 3.0 | 1.2 | 1.00 |
| W (Watts) | inRange | 0.69 | 2.17 | 1.36 | 1.00 |
| ΔM (gr) | inRange | 0.26 | 1.31 | 0.56 | 1.00 |
| Th (μm) | max | 13.53 | 43.96 | 26.22 | 0.41 |
| R (mm/year) | max | 0.008 | 0.190 | 0.150 | 0.75 |
| Overall Desirability | 0.74 | ||||
| Criterion | Optimal Values Obtained | |||||
|---|---|---|---|---|---|---|
| W (Watts) | ΔM (gr) | Th (μm) | R (mm/year) | MAE | RMSE | |
| 1st Criterion | 0.78 | 0.28 | 13.62 | 0.024 | 0.049 | 0.073 |
| 2nd Criterion | 1.14 | 0.48 | 23.72 | 0.187 | 0.090 | 0.099 |
| 3rd Criterion | 1.43 | 0.61 | 21.41 | 0.299 | 0.066 | 0.071 |
| 4th Criterion | 1.91 | 1.24 | 45.33 | 0.155 | 0.061 | 0.074 |
| 5th Criterion | 1.91 | 0.29 | 23.15 | 0.220 | 0.077 | 0.098 |
| 6th Criterion | 1.96 | 0.60 | 36.58 | 0.022 | 0.058 | 0.079 |
| 7th Criterion | 1.37 | 0.63 | 26.37 | 0.129 | 0.062 | 0.083 |
| MAE | 0.027 | 0.065 | 0.125 | 0.047 | ||
| RMSE | 0.029 | 0.075 | 0.134 | 0.057 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorza, R.L.; Calvo, M.Á.M.; Labari, C.B.; Fuente, P.J.R. Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals 2018, 8, 711. https://doi.org/10.3390/met8090711
Lorza RL, Calvo MÁM, Labari CB, Fuente PJR. Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals. 2018; 8(9):711. https://doi.org/10.3390/met8090711
Chicago/Turabian StyleLorza, Ruben Lostado, María Ángeles Martínez Calvo, Carlos Berlanga Labari, and Pedro J. Rivero Fuente. 2018. "Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws" Metals 8, no. 9: 711. https://doi.org/10.3390/met8090711
APA StyleLorza, R. L., Calvo, M. Á. M., Labari, C. B., & Fuente, P. J. R. (2018). Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals, 8(9), 711. https://doi.org/10.3390/met8090711








