Effect of CaCl2 on Microstructure of Calciothermic Reduction Products of Ti2O3 to Prepare Porous Titanium
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Experimental Procedures
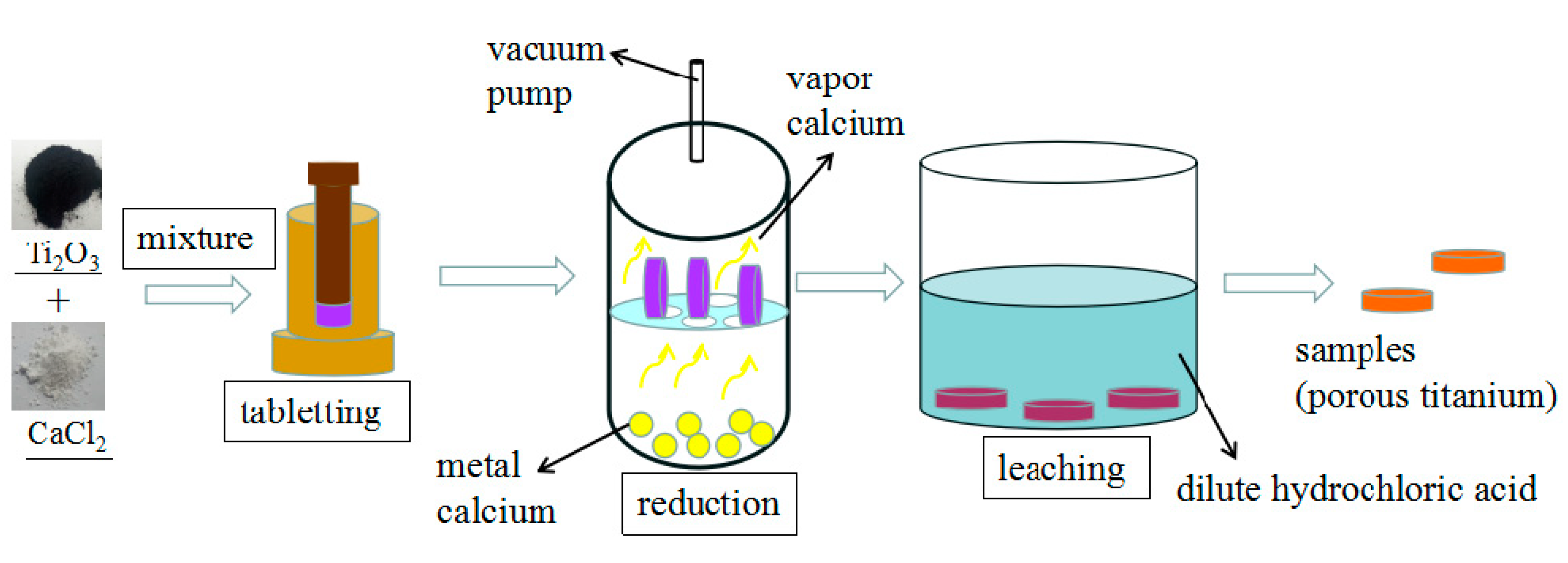
2.2.1. Preparation of Samples before Reduction
2.2.2. Preparation of Reduction Samples
2.3. Characterization
3. Results and Discussion
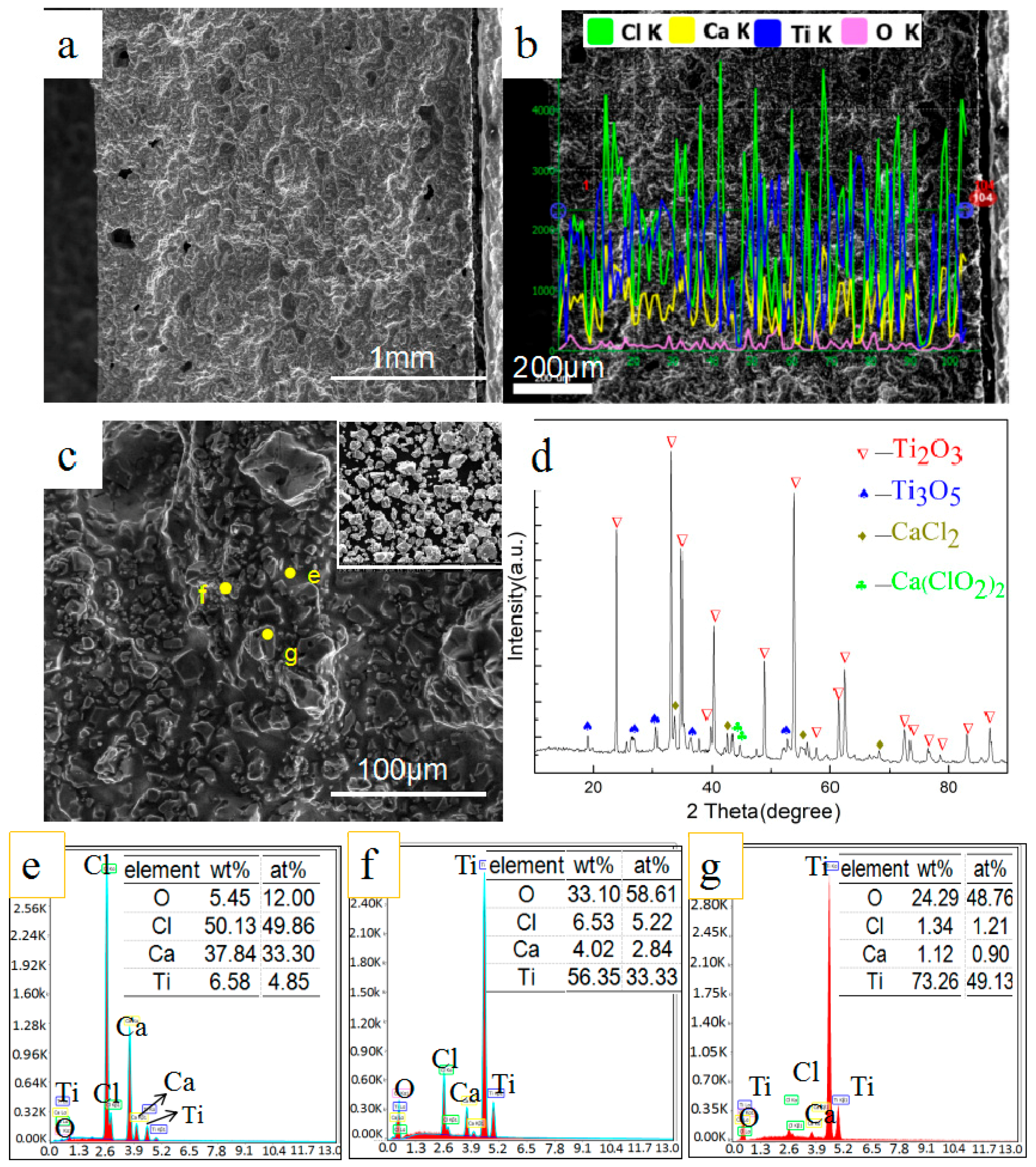
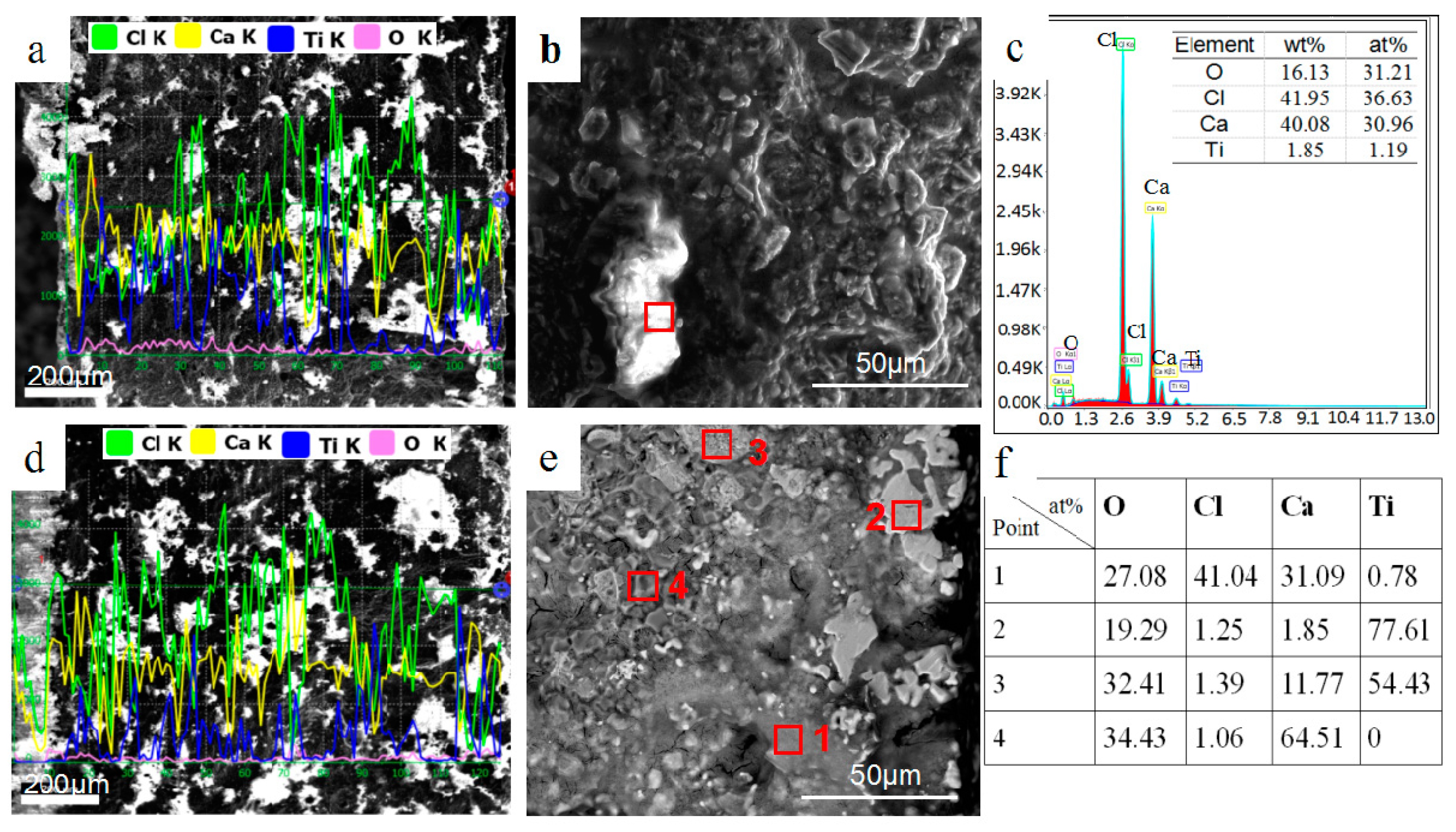
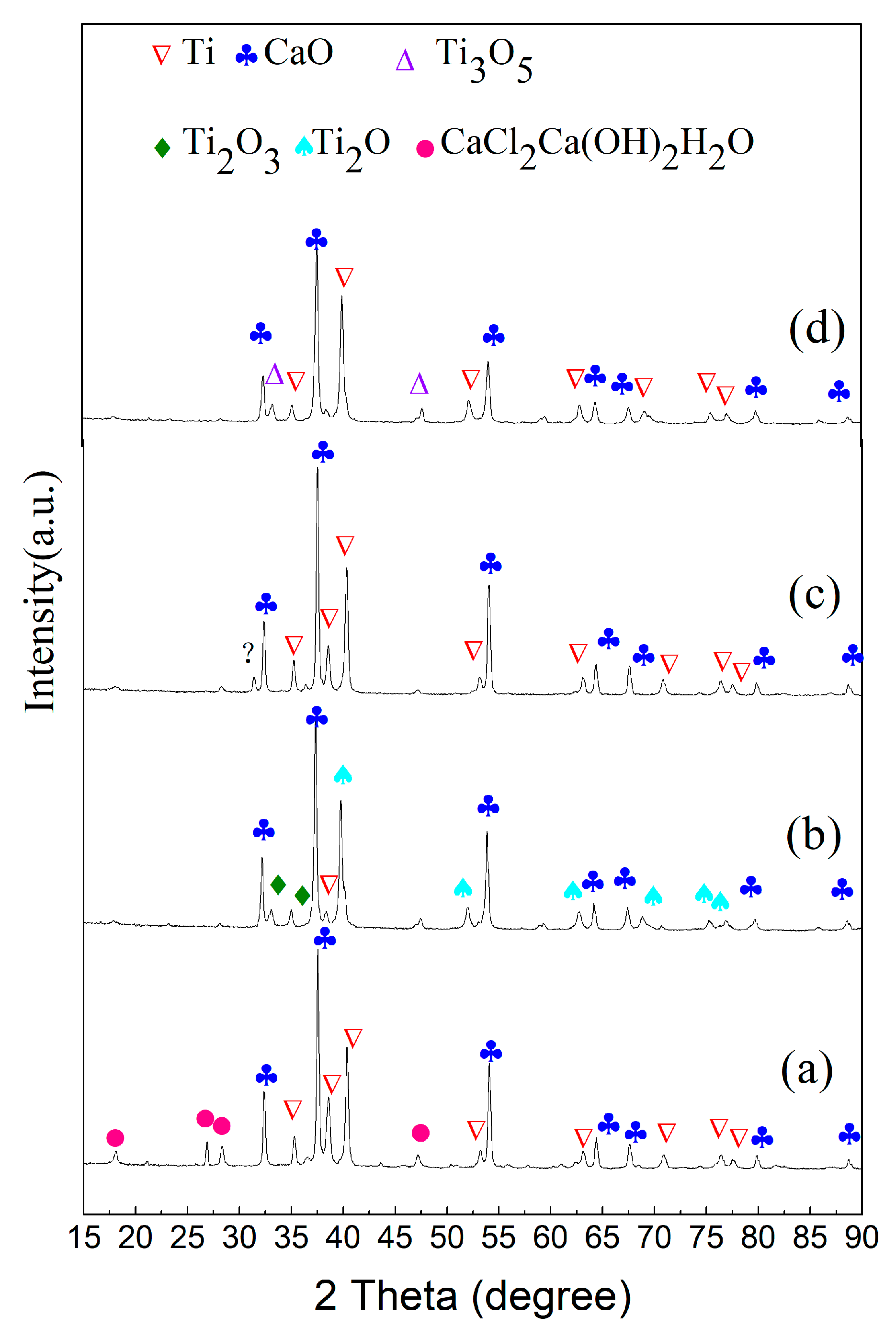
3.1. Phase and Microstructure of Preform Feeds before Reduction
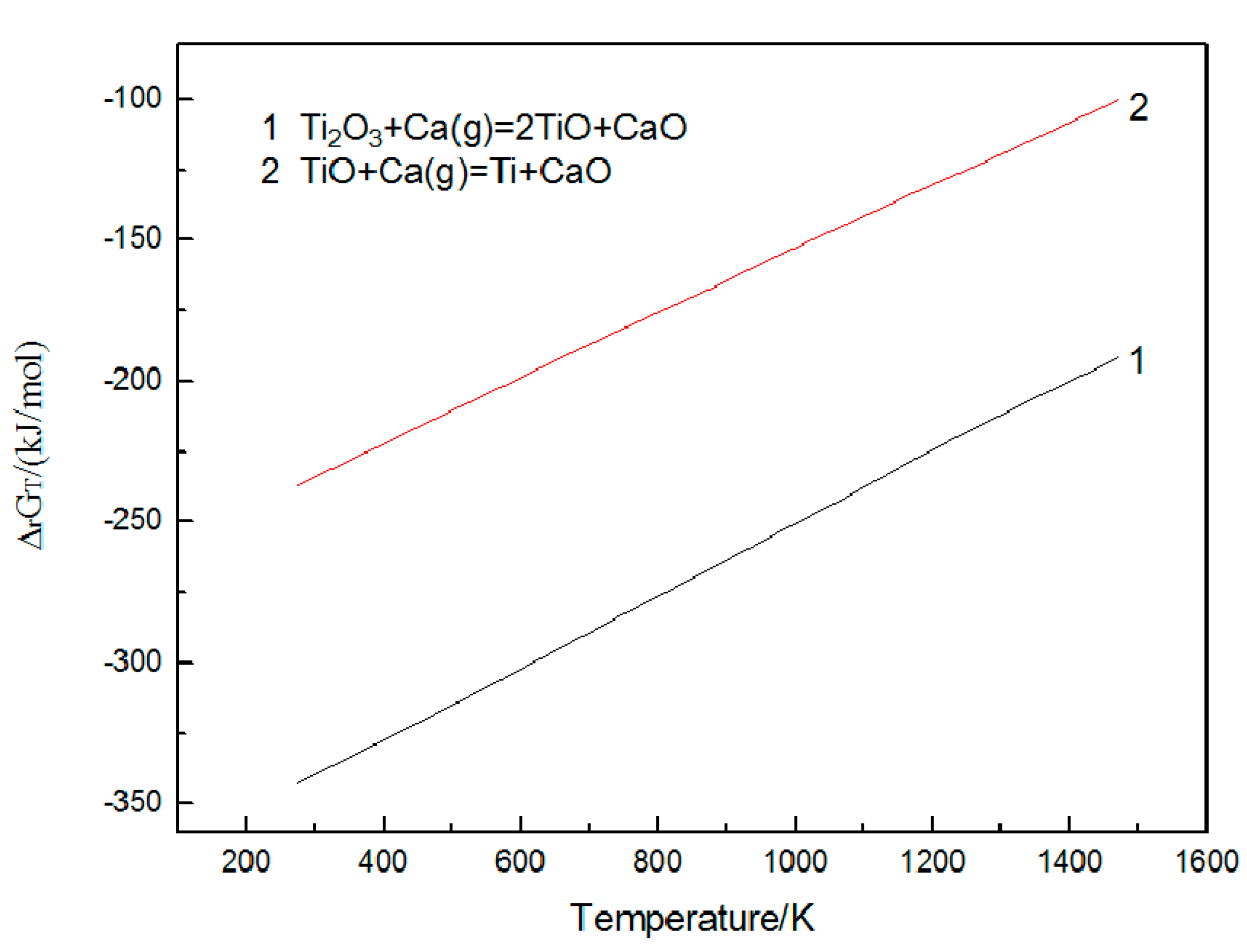
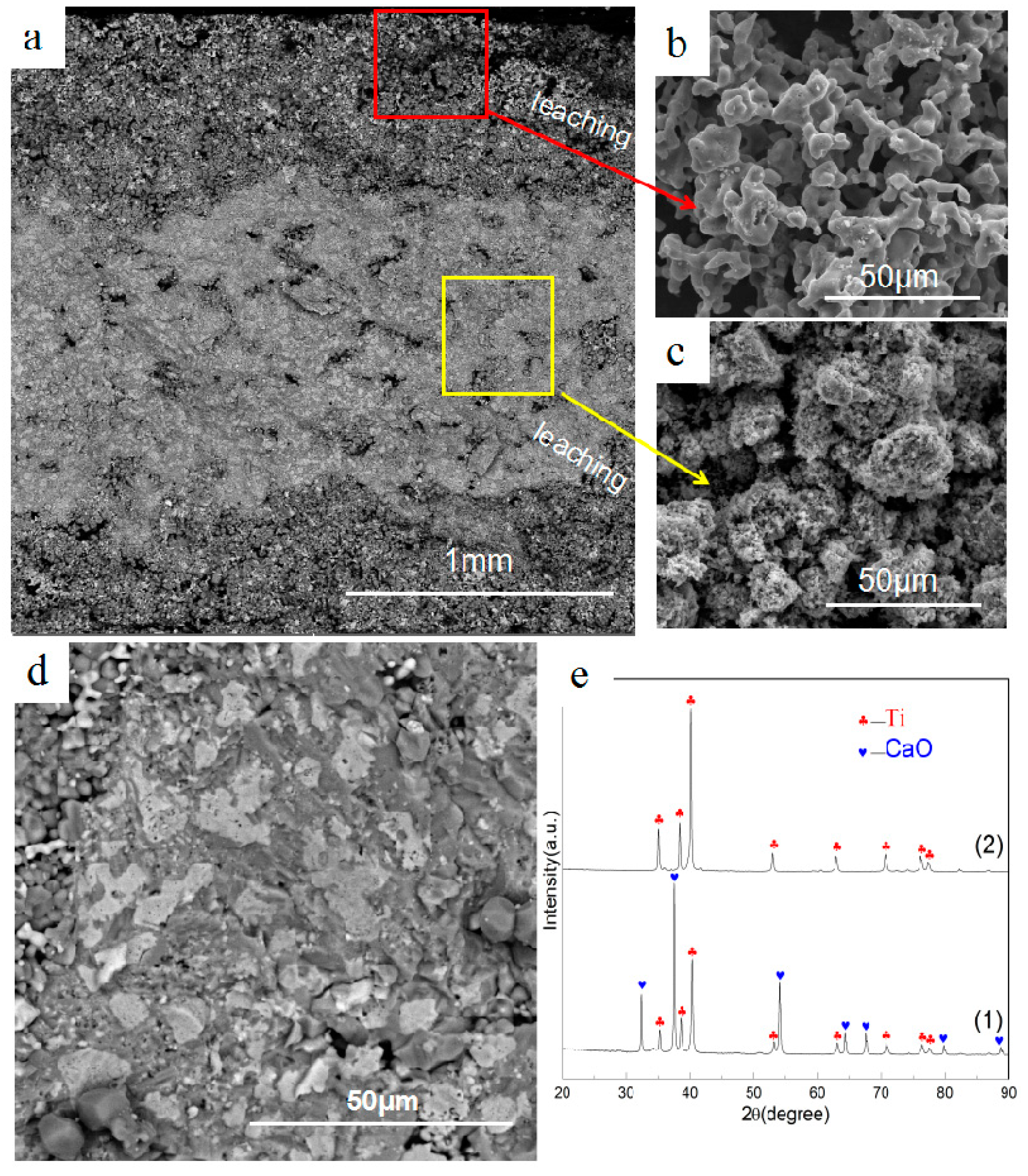
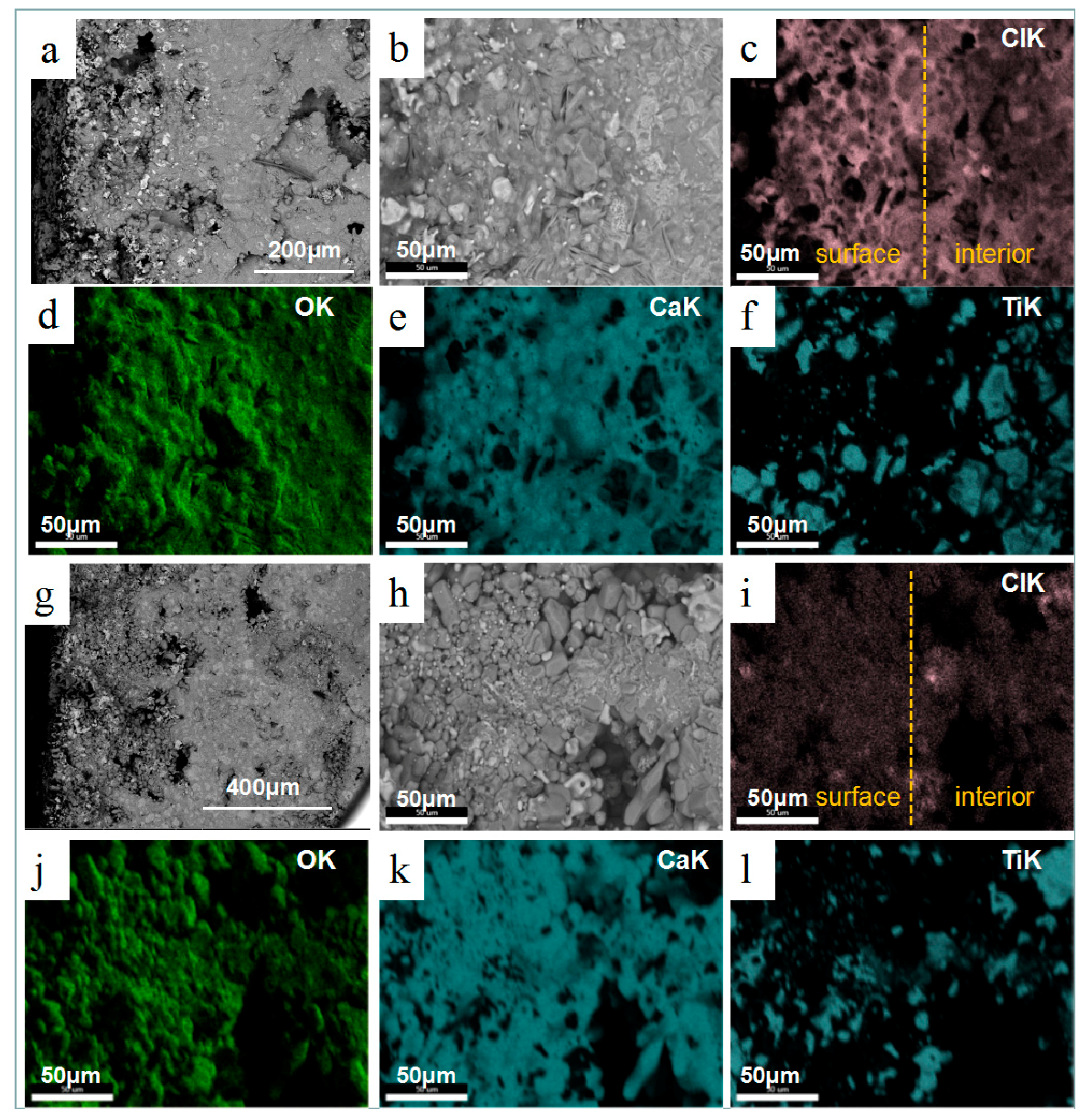
3.2. Effect of Calcium Chloride on Microstructure of Reduction Products
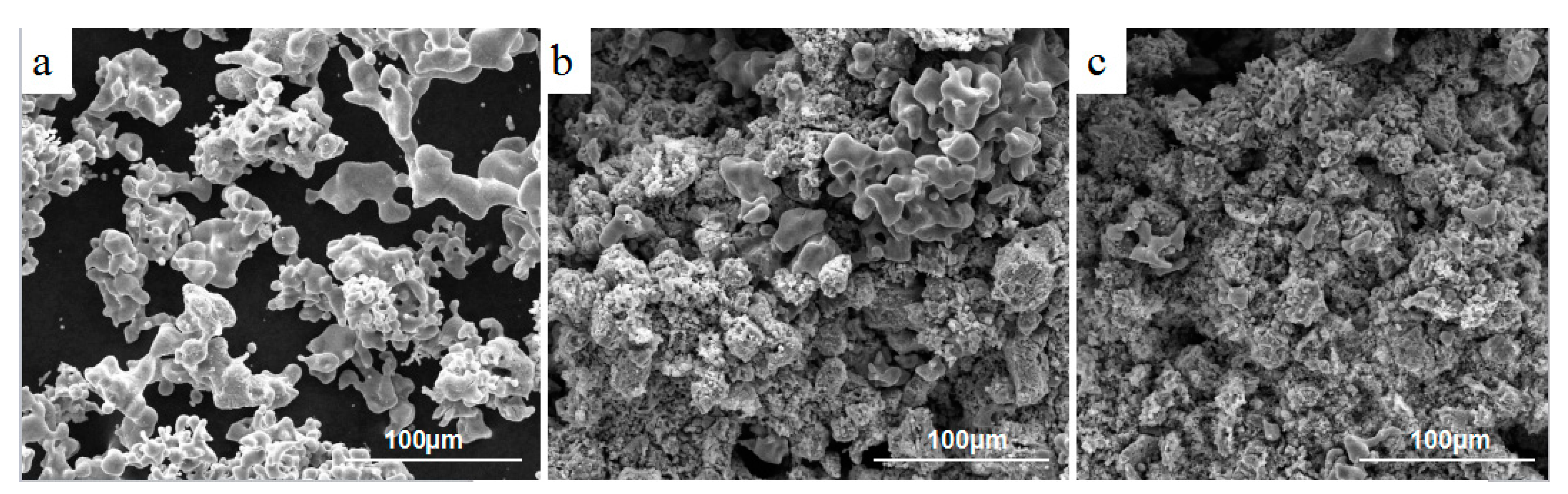
3.3. Effect of Calcium Chloride on the Size of Titanium Particles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.S.; Liang, K.M. Review Functional materials of porous metals made by P/M, electroplating and some other techniques. J. Mater. Sci. 2001, 36, 5059–5072. [Google Scholar] [CrossRef]
- Kato, K.; Yamamoto, A.; Ochiai, S.; Daigo, Y.; Isobe, T.; Matano, S.; Omori, K. Cell Proliferation, Corrosion Resistance and Mechanical Properties of Novel Titanium Foam with Sheet Shape. Mater. Trans. 2012, 53, 724–732. [Google Scholar] [CrossRef]
- Siegkas, P.; Tagarielli, V.L.; Petrinic, N.; Lefebvre, P. The compressive response of a titanium foam at low and high strain rates. J. Mater. Sci. 2011, 46, 2741–2747. [Google Scholar] [CrossRef]
- De Vasconcellos, L.M.; Leite, D.D.; Nascimento, F.O.; de Vasconcellos, L.G.; Graca, M.L.; Carvalho, Y.R.; Cairo, C.A. Porous titanium for biomedical applications: an experimental study on rabbits. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e407–e412. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Liu, P.; Tan, Q. Porous titanium materials with entangled wire structure for load-bearing biomedical applications. J. Mech. Behav. Biomed. Mater. 2012, 5, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.K.; Matsushita, T.; Takadama, H; Fukuda, A.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo1, T. Fabrication of Bioactive Porous Ti Metal with Structure Similar to Human Cancellous Bone by Selective Laser Melting. Bioceram. Dev. Appl. 2011, 1. [Google Scholar] [CrossRef]
- Shen, H.; Brinson, L.C. A numerical investigation of porous titanium as orthopedic implant material. Mech. Mater. 2011, 43, 420–430. [Google Scholar] [CrossRef]
- Esen, Z.; Şakir, B. Characterization of Ti-6Al-4V alloy foams synthesized by space holder technique. Mater. Sci. Eng. A 2011, 528, 3200–3209. [Google Scholar] [CrossRef]
- Oh, I.H.; Nomura, N.; Chiba, A.; Murayama, Y.; Masahashi, N.; Lee, B.T.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Mater. Sci. Eng. C 2005, 25, 330–335. [Google Scholar]
- Xie, L.; Liao, X.; Yin, G.; Huang, Z.; Yan, D.; Yao, Y.; Liu, W.; Chen, X.; Gu, J. Structure, morphology and fibroblasts adhesion of surface-porous titanium via anodic oxidation. J. Mater. Sci. Mater. Med. 2010, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Kim, Y.H.; Chang, S.H.; Oh, I.H. Biocompatibility of Low Modulus Porous Titanium Implants Fabricated by Spark Plasma Sintering. Korean J. Mater. Res. 2007, 17, 107–114. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Moriyama, S.; Endo, M.; Kawakami, Y. Mechanical Property of Porous Titanium Produced by Spark Plasma Sintering. Key Eng. Mater. 2008, 385–387, 637–640. [Google Scholar] [CrossRef]
- Watanabe, T. The Sintering Phenomenon of Titanium Powders—A Discussion. Int. J Powder Met. Powder Technol. 1976, 12, 209–214. [Google Scholar]
- Ahn, M.K.; Jo, I.H.; Koh, Y.H.; Kim, H.E. Production of highly porous titanium (Ti) scaffolds by vacuum-assisted foaming of titanium hydride (TiH2) suspension. Mater. Lett. 2014, 120, 228–231. [Google Scholar] [CrossRef]
- Wan, H.L.; Xu, B.Q.; Dai, Y.N.; Yang, B.; Liu, D.C. Preparation of titanium powders by calciothermic reduction of titanium dioxide. J. Cent. South Univ. Technol. 2012, 19, 2434–2439. [Google Scholar] [CrossRef]
- Jia, J.G.; Xu, B.Q.; Yang, B.; Wang, D.; Liu, D.C. Preparation of Titanium Powders from TiO2 by Calcium Vapor Reduction. JOM 2013, 65, 630–635. [Google Scholar] [CrossRef]
- Lei, X.; Xu, B.; Yang, G.; Shi, T.; Liu, D.; Yang, B. Direct calciothermic reduction of porous calcium titanate to porous titanium. Mater. Sci. Eng. C 2018, 91, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.H.; Oda, T.; Mitsuda, Y. Titanium powder production by preform reduction process (PRP). J. Alloys Compd. 2004, 364, 156–163. [Google Scholar] [CrossRef]
- Xu, B.; Yang, B.; Jia, J.; Liu, D.; Xiong, H.; Deng, Y. Behavior of calcium chloride in reduction process of titanium dioxide by calcium vapor. J. Alloys Compd. 2013, 576, 208–214. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yoshida, M.; Matsuura, S. Rapid reduction of titanium dioxide nano-particles by reduction with a calcium reductant. J. Phys. Chem. Solids 2014, 75, 1041–1048. [Google Scholar] [CrossRef]
- Dong, J.; Wei, Y.; Lu, C.; Zhou, S.; Li, B.; Ding, Z.; Wang, C.; Ma, B. Influence of Calcium Chloride Addition on Coal-Based Reduction Roasting of Low-Nickel Garnierite Ore. Mater. Trans. 2017, 58, 1161–1168. [Google Scholar] [CrossRef]

| Material | Form | Purity or Content/% | Producer |
|---|---|---|---|
| Ti2O3 | Powder | >99 | Aladdin Industrial Co., Ltd., Shanghai, China |
| Ca particles | Granular | >98 | Aladdin Industrial Co., Ltd., Shanghai, China |
| CaCl2 | Powder | >99 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| HCl | Aqueous | 3.5 a | Chuandong Chemical Group Co., Ltd., Chongqing, China |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Xu, B.; Wan, H.; Wang, F.; Yang, B.; Wang, Z. Effect of CaCl2 on Microstructure of Calciothermic Reduction Products of Ti2O3 to Prepare Porous Titanium. Metals 2018, 8, 698. https://doi.org/10.3390/met8090698
Yang G, Xu B, Wan H, Wang F, Yang B, Wang Z. Effect of CaCl2 on Microstructure of Calciothermic Reduction Products of Ti2O3 to Prepare Porous Titanium. Metals. 2018; 8(9):698. https://doi.org/10.3390/met8090698
Chicago/Turabian StyleYang, Guobo, Baoqiang Xu, Heli Wan, Fengkang Wang, Bin Yang, and Zhijun Wang. 2018. "Effect of CaCl2 on Microstructure of Calciothermic Reduction Products of Ti2O3 to Prepare Porous Titanium" Metals 8, no. 9: 698. https://doi.org/10.3390/met8090698
APA StyleYang, G., Xu, B., Wan, H., Wang, F., Yang, B., & Wang, Z. (2018). Effect of CaCl2 on Microstructure of Calciothermic Reduction Products of Ti2O3 to Prepare Porous Titanium. Metals, 8(9), 698. https://doi.org/10.3390/met8090698




