Electrodeposition Kinetics of Ni/Nano-Y2O3 Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
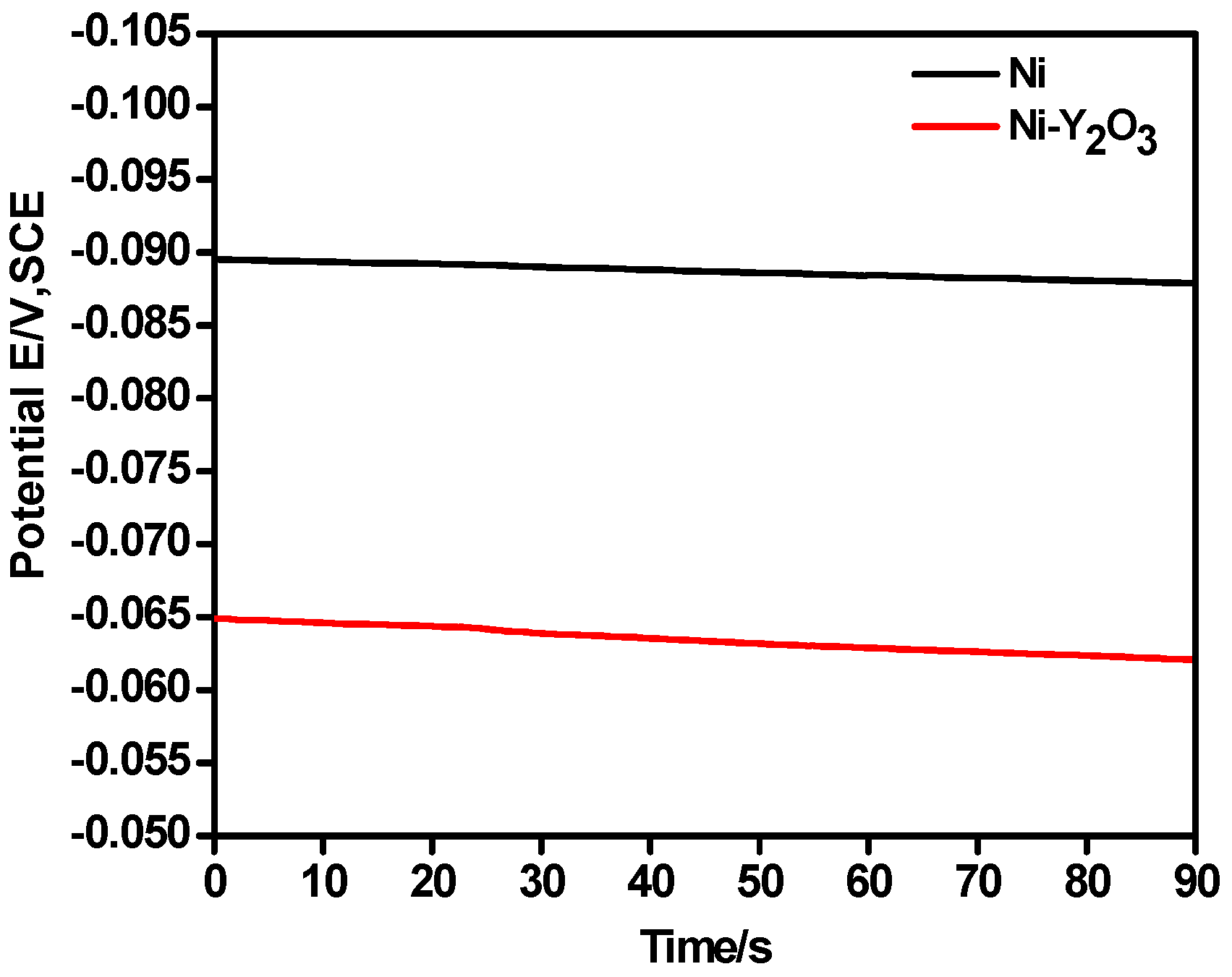
3.1. Linear Sweep Voltammetry (LSV)
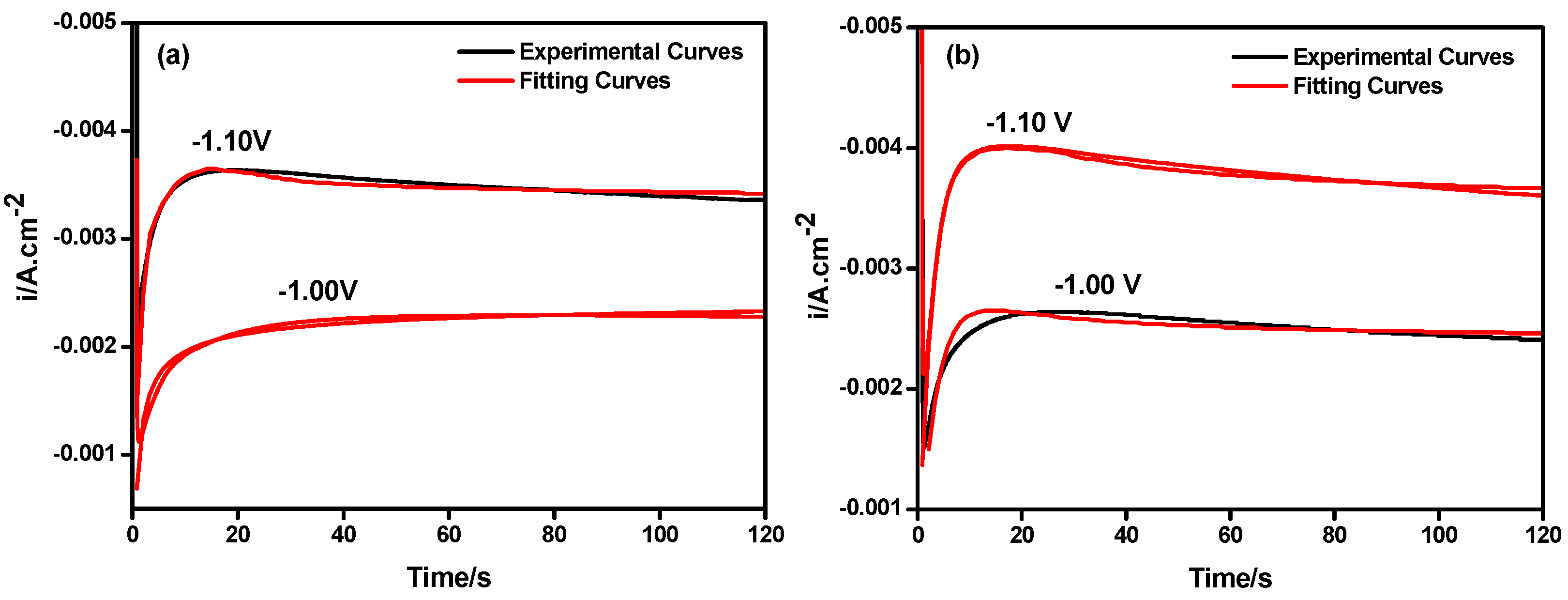
3.2. Chronoamperometry Study
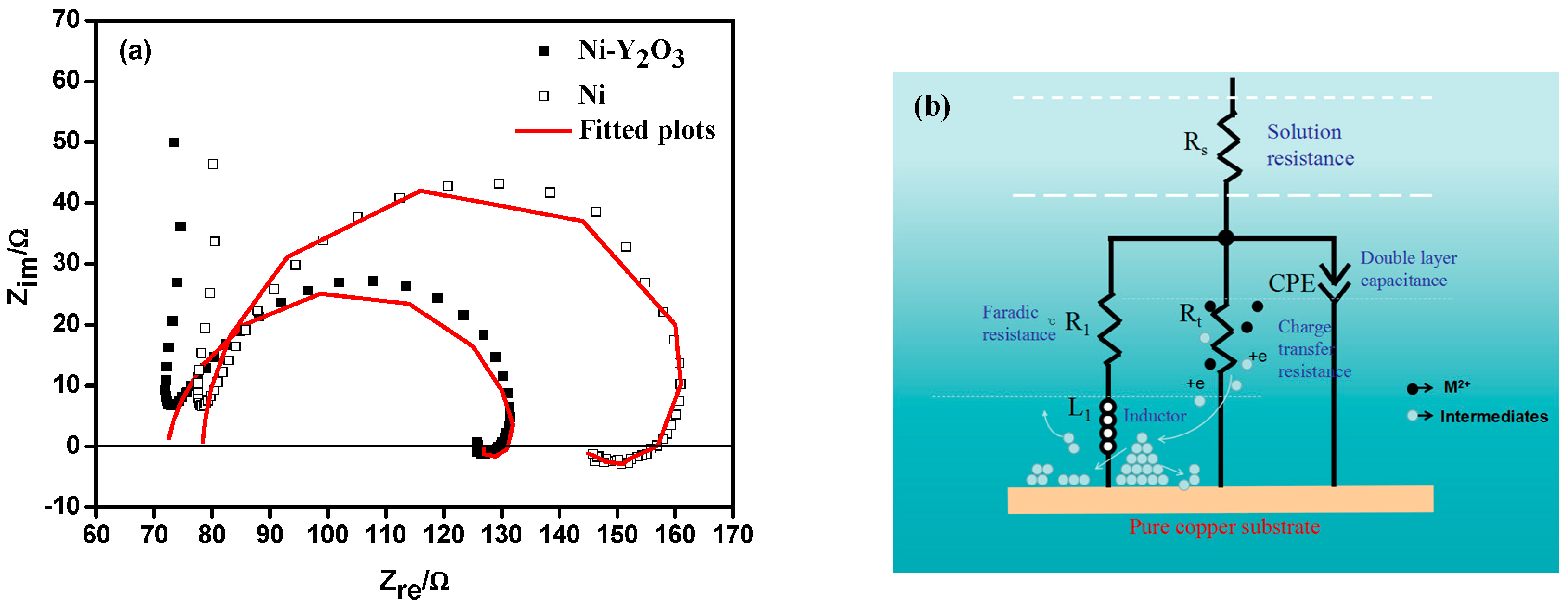
3.3. Electrochemical Impedance Spectroscopy (EIS) Studies
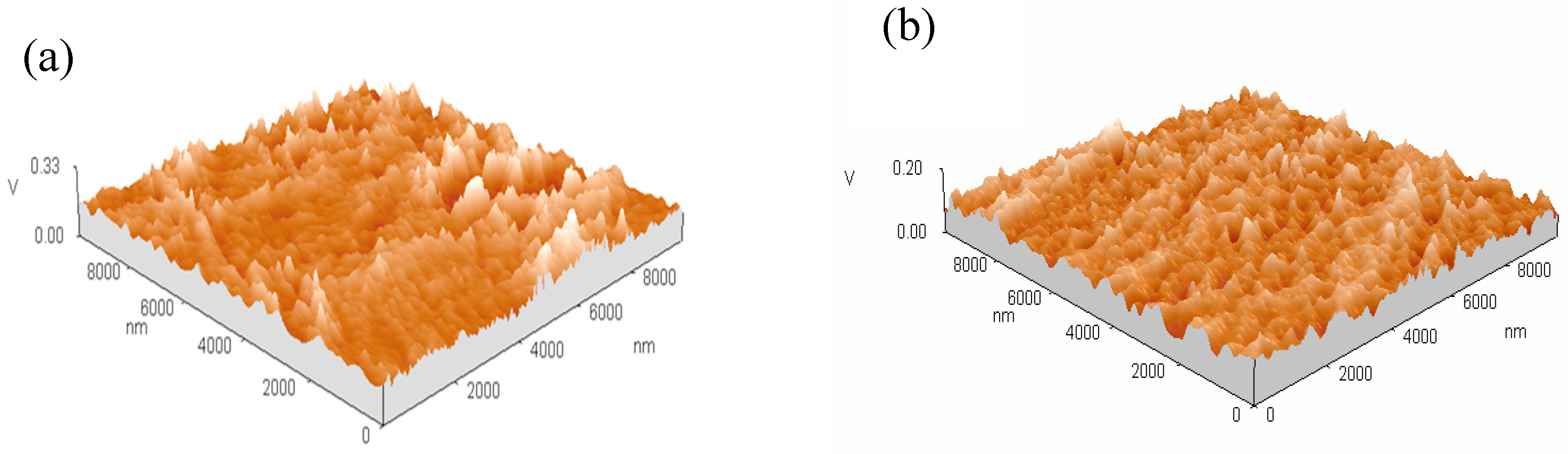
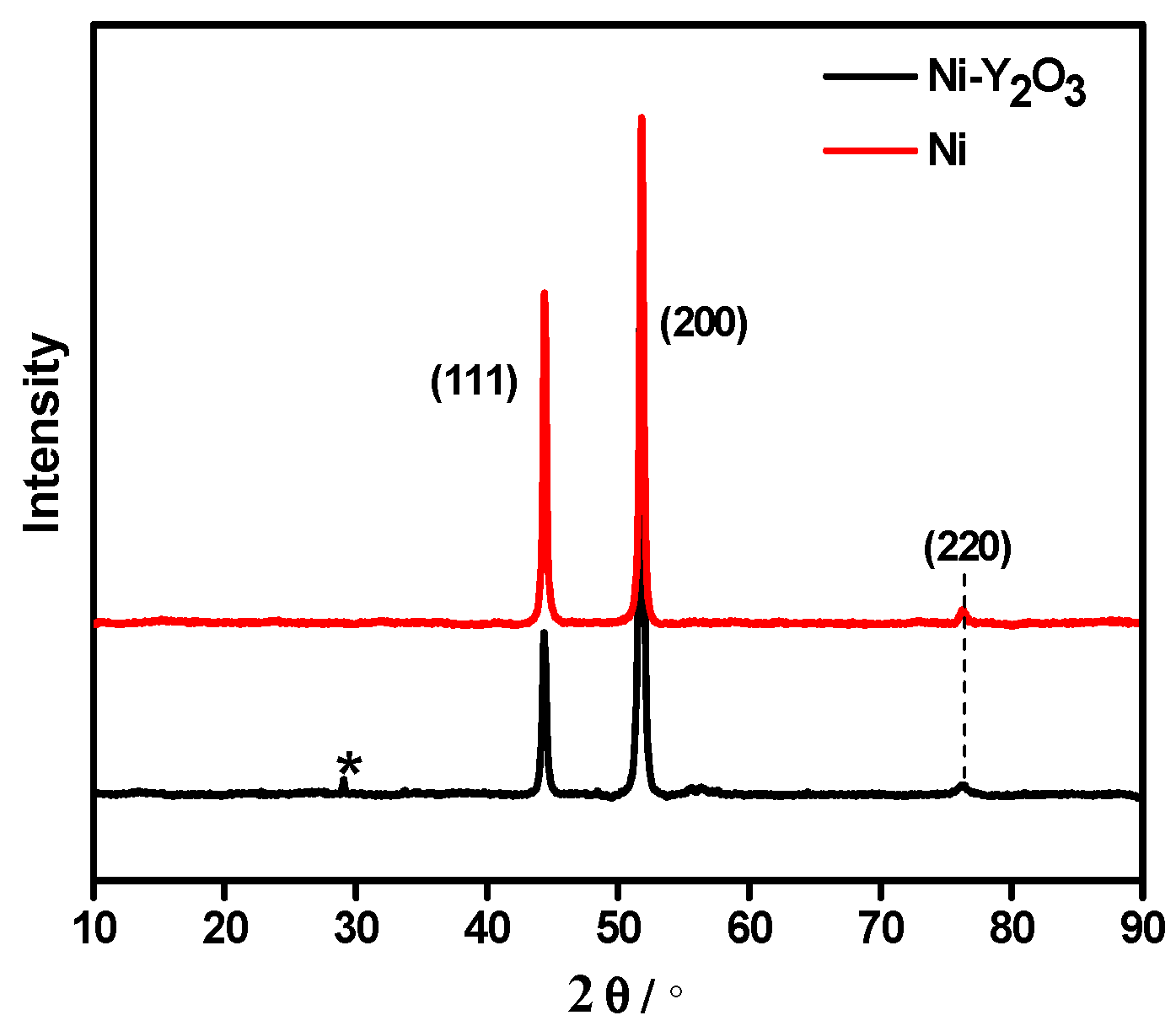
3.4. Microstructure of Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Musiani, M. Electrodeposition of composites: An expanding subject in electrochemical materials science. Electrochim. Acta 2000, 45, 3397–3402. [Google Scholar] [CrossRef]
- Walsh, F.C.; Leon, C.P.D. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Tudela, I.; Zhang, Y.; Pal, M.; Kerr, I.; Cobley, A.J. Ultrasound-assisted electrodeposition of composite coatings with particles. Surf. Coat. Technol. 2014, 259, 363–373. [Google Scholar] [CrossRef]
- Zhang, G.J.; Deng, Z.Y.; Kondo, N.; Yang, J.F.; Ohji, T. Reactive Hot Pressing of ZrB2-SiC Composites. J. Am. Ceram. Soc. 2010, 83, 2330–2332. [Google Scholar] [CrossRef]
- Chou, S.N.; Huang, J.L.; Lii, D.F.; Lu, H.H. The mechanical properties of Al2O3/aluminum alloy composite manufactured by squeeze casting. J. Alloy. Compd. 2006, 419, 98–102. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J. Reactive thermal spraying of TiC-Fe composite coating by using asphalt as carbonaceous precursor. J. Mater. Sci. 2005, 40, 4149–4151. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Wang, D.; Price, T.; Zhang, D.; Keshri, A.K.; Chen, Y.; McCartney, D.G.; Shipway, P.H.; Agarwal, A. Microstructure and wear properties of aluminum/aluminum-silicon composite coatings prepared by cold spraying. Surf. Coat. Technol. 2009, 204, 503–510. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Wang, H.Y.; Ma, B.X.; Wang, Y.; Zhao, F. Fabrication of B4C particulate reinforced magnesium matrix composite by powder metallurgy. J. Alloy. Compd. 2005, 58, 3509–3513. [Google Scholar] [CrossRef]
- Zimmer, A.; Broch, L.; Boulanger, C.; Stein, N. Growth Mechanism during the Early Stages of electrodeposition of Bismuth telluride films. Electrochim. Acta 2015, 174, 376–383. [Google Scholar] [CrossRef]
- Tan, C.; Cui, H.; Hu, W.; Liu, Y. Influence of Nano-Al2O3 Particles on Nickel Electrocrystallization at Initial Stage. Rare Met. Mater. Eng. 2010, 39, 10–16. [Google Scholar]
- Tan, C.Y.; Liu, Y.; Zhao, X.S.; Zheng, Z.Q. Nickel co-deposition with SiC particles at initial stage. Trans. Nonferr. Metal. Soc. China 2008, 18, 1128–1133. [Google Scholar] [CrossRef]
- Kasturibai, S.; Kalaignan, G.P. Characterizations of electrodeposited Ni-CeO2 nanocomposite coatings. Mater. Chem. Phys. 2014, 147, 1042–1048. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.S.; Kim, D.J.; Kim, H.P.; Hwang, S.S. Effects of current density and agitation on co-deposition behaviour of electrodeposited Ni-TiO2 composite coating. Surf. Eng. 2015, 31, 673–678. [Google Scholar] [CrossRef]
- Ao, Z.H.; Xue, Y.J.; Li, X.H.; Li, J.S. Preparation of Ni-Nd2O3 Nanocomposite Coatings by Electrodeposition under Dual-Frequency Ultrasound. Adv. Mater. Res. 2012, 591–593, 1001–1005. [Google Scholar] [CrossRef]
- Xia, F.F.; Wu, M.H.; Wang, F.; Jia, Z.Y.; Wang, A.L.; Elkhoshkhany, N.; Hafnway, A.; Khaled, A. Electrodeposition and corrosion behavior of nano-structured Ni-WC and Ni-Co-WC composite coating. J. Alloy. Compd. 2016, 695, 1505–1514. [Google Scholar]
- Elkhoshkhany, N.; Hafnway, A.; Khaled, A. Electrodeposition and corrosion behavior of nano-structured Ni-WC and Ni-Co-WC composite coating. J. Alloy. Compd. 2016, 695. [Google Scholar] [CrossRef]
- Tudela, I.; Zhang, Y.; Pal, M.; Kerr, I.; Cobley, A.J. Ultrasound-assisted electrodeposition of thin nickel-based composite coatings with lubricant particles. Surf. Coat. Technol. 2015, 276, 89–105. [Google Scholar] [CrossRef]
- Cai, F.; Jiang, C.; Zhang, Z.; Ji, V. Synthesis and characterization of Ni-Al-Y2O3 composite coatings with different Y2O3 particle content. Ceram. Int. 2014, 40, 15105–15111. [Google Scholar] [CrossRef]
- Tian, L.; Xu, J. Electrodeposition and characterization of Ni-Y2O3 composite. Appl. Surf. Sci. 2011, 257, 7615–7620. [Google Scholar] [CrossRef]
- Ghaziof, S.; Kilmartin, P.A.; Gao, W. Electrochemical studies of sol-enhanced Zn-Ni-Al2O3 composite and Zn-Ni alloy coatings. J. Electroanal. Chem. 2015, 755, 63–70. [Google Scholar] [CrossRef]
- Guglielmi, N. Codeposition of inert particles in a metallic matrix. J. Electrochem. Soc. 1972, 119, 1009. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, Y.Y.; Liang, Z.P.; Jin, H. Electrochemical Deposition and Nucleation/Growth Mechanism of Ni-Co-Y2O3 Multiple Coatings. Materials 2018, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Díaz-Morales, O.; Mostany, J.; Borrás, C.; Scharifker, B.R. Current transient study of the kinetics of nucleation and diffusion-controlled growth of bimetallic phases. J. Solid State Electrochem. 2013, 17, 345–351. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Scharifker, B.R.; Arce, E.M.; Romero-Romo, M. Nucleation and diffusion-controlled growth of electroactive centers: Reduction of protons during cobalt electrodeposition. Electrochim. Acta 2005, 50, 4736–4745. [Google Scholar] [CrossRef]
- Nowak, P.; Socha, R.P.; Kaisheva, M.; Fransaer, J.; Celis, J.P.; Stoinov, Z. Electrochemical investigation of the codeposition of SiC and SiO2 particles with nickel. J. Appl. Electrochem. 2004, 21, 429–437. [Google Scholar]
- Bapu, G.N.K.R.; Jayakrishnan, S. Oxidation characteristics of electrodeposited nickel-zirconia composites at high temperature. Mater. Chem. Phys. 2006, 96, 321–325. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Indyka, P.; Bigos, A.; Szczerba, M.J.; Kot, M. Ni-W/ZrO2 nanocomposites obtained by ultrasonic DC electrodeposition. Mater. Des. 2015, 80, 1–11. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]



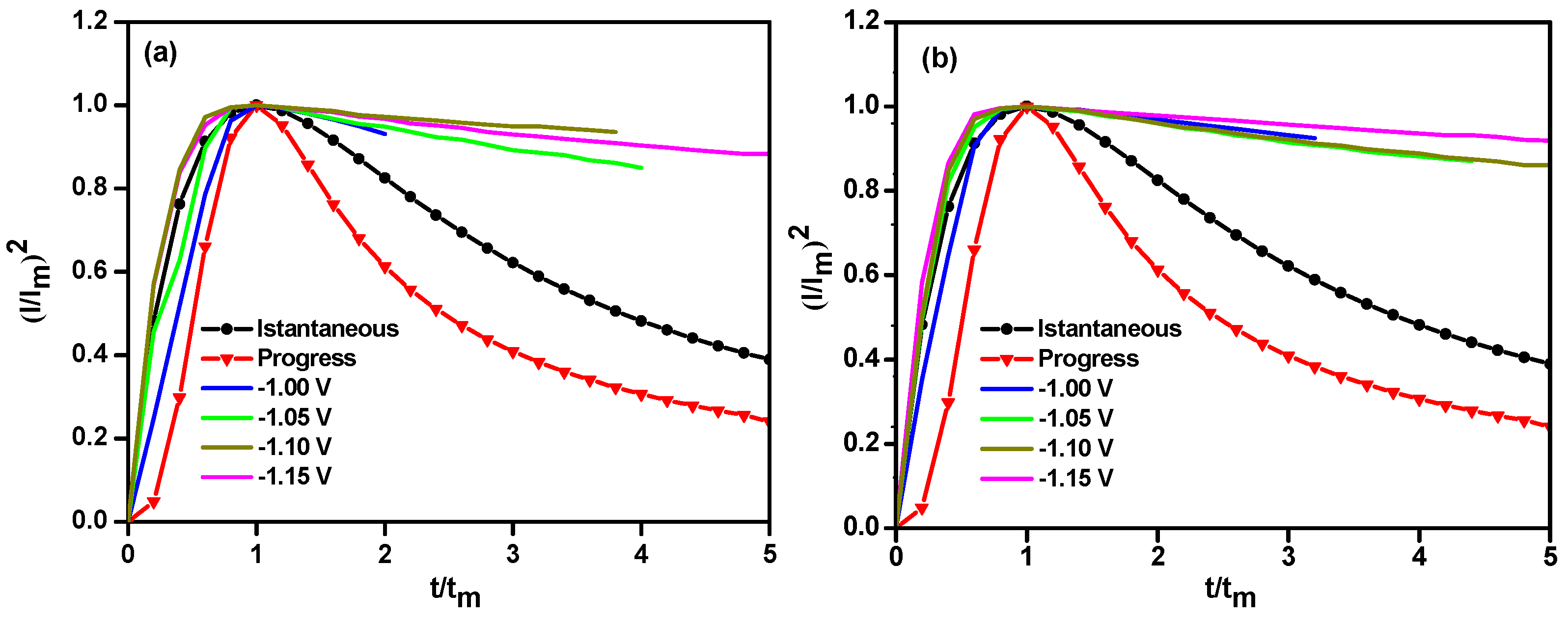
| Potential/V | Ni | Relaxation Time | Ni-Y2O3 | Relaxation Time |
|---|---|---|---|---|
| Im (A·cm−2) | tm (s) | Im (A·cm−2) | tm (s) | |
| −1.00 V | −0.00229 | 79.2 | −0.00260 | 35.5 |
| −1.05 V | −0.00307 | 29.7 | −0.00344 | 26.3 |
| −1.10 V | −0.00364 | 18.5 | −0.00402 | 16.5 |
| −1.15 V | −0.00427 | 14.9 | −0.00463 | 13.6 |
| Deposits | Potential | P1* (uA·cm−2) | P2 (s−1) | P3 (s−1) | P4 (uA·cm−2) | A (s−1) | N0 × 106 (cm−2) |
|---|---|---|---|---|---|---|---|
| Ni | −1.00 V | −2.34 | 0.16 | 2.19 | −1.66 | 2.19 | 1.21 |
| Ni | −1.10 V | −3.30 | 0.43 | 3.13 | −1.29 | 3.13 | 3.12 |
| Ni-Y2O3 | −1.00 V | −2.48 | 0.27 | 2.56 | −1.47 | 2.56 | 1.89 |
| Ni-Y2O3 | −1.10 V | −3.39 | 0.49 | 3.43 | −1.36 | 3.43 | 3.36 |
| Deposits | Potential (V) | Rs/Ω·cm2 | CPE1-P/F·cm2 | Rt/Ω·cm2 | R1/Ω·cm2 | L1/H·cm2 |
|---|---|---|---|---|---|---|
| Ni | −1.10 | 48.36 | 4.73 × 10−5 | 85.39 | 389.8 | 6.56 |
| Ni-Y2O3 | −1.10 | 43.26 | 4.98 × 10−5 | 57.74 | 226.8 | 5.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.; Liu, X.; Liang, Z.; Jin, H. Electrodeposition Kinetics of Ni/Nano-Y2O3 Composite Coatings. Metals 2018, 8, 669. https://doi.org/10.3390/met8090669
Zhou X, Wang Y, Liu X, Liang Z, Jin H. Electrodeposition Kinetics of Ni/Nano-Y2O3 Composite Coatings. Metals. 2018; 8(9):669. https://doi.org/10.3390/met8090669
Chicago/Turabian StyleZhou, Xinyu, Yiyong Wang, Xianglin Liu, Zhipeng Liang, and Hui Jin. 2018. "Electrodeposition Kinetics of Ni/Nano-Y2O3 Composite Coatings" Metals 8, no. 9: 669. https://doi.org/10.3390/met8090669
APA StyleZhou, X., Wang, Y., Liu, X., Liang, Z., & Jin, H. (2018). Electrodeposition Kinetics of Ni/Nano-Y2O3 Composite Coatings. Metals, 8(9), 669. https://doi.org/10.3390/met8090669




