Formation of SUS304/Aluminum Alloys Using Wire and Arc Additive Manufacturing
Abstract
:1. Introduction
2. Experimental Section
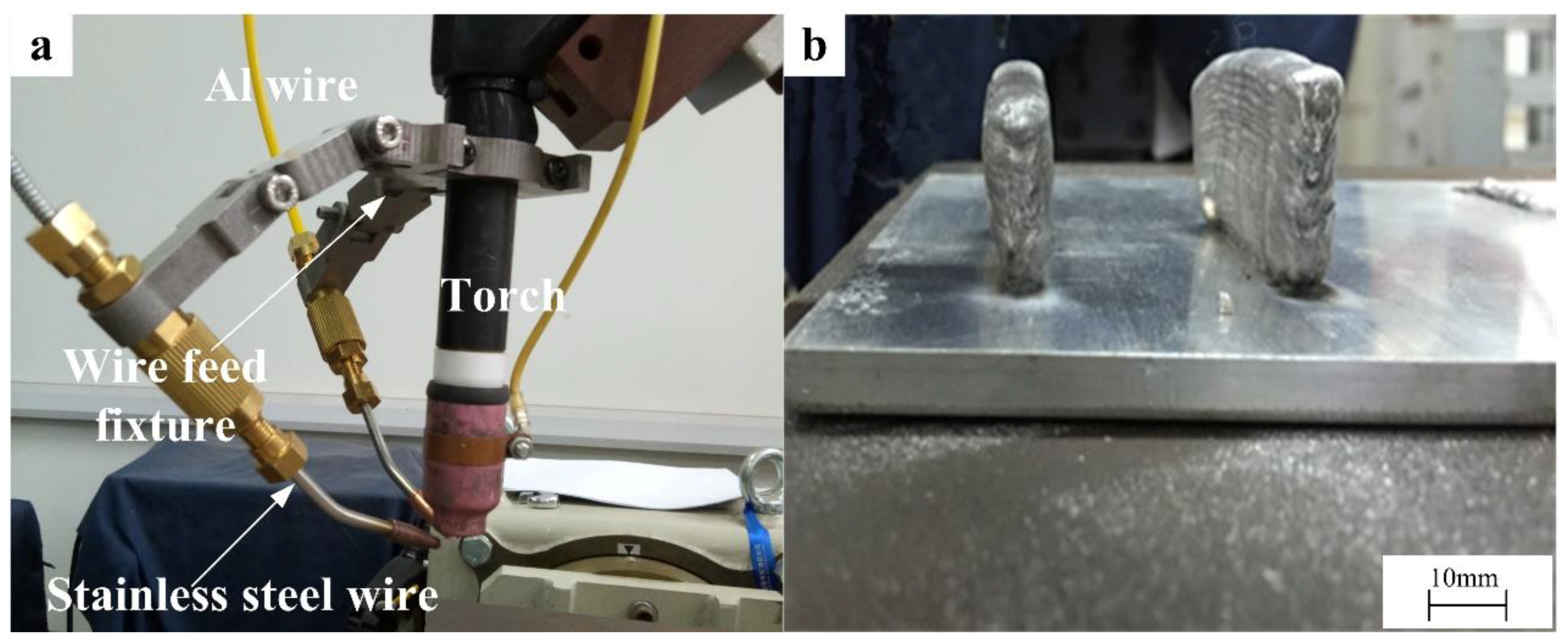
2.1. Materials and Fabrication Process
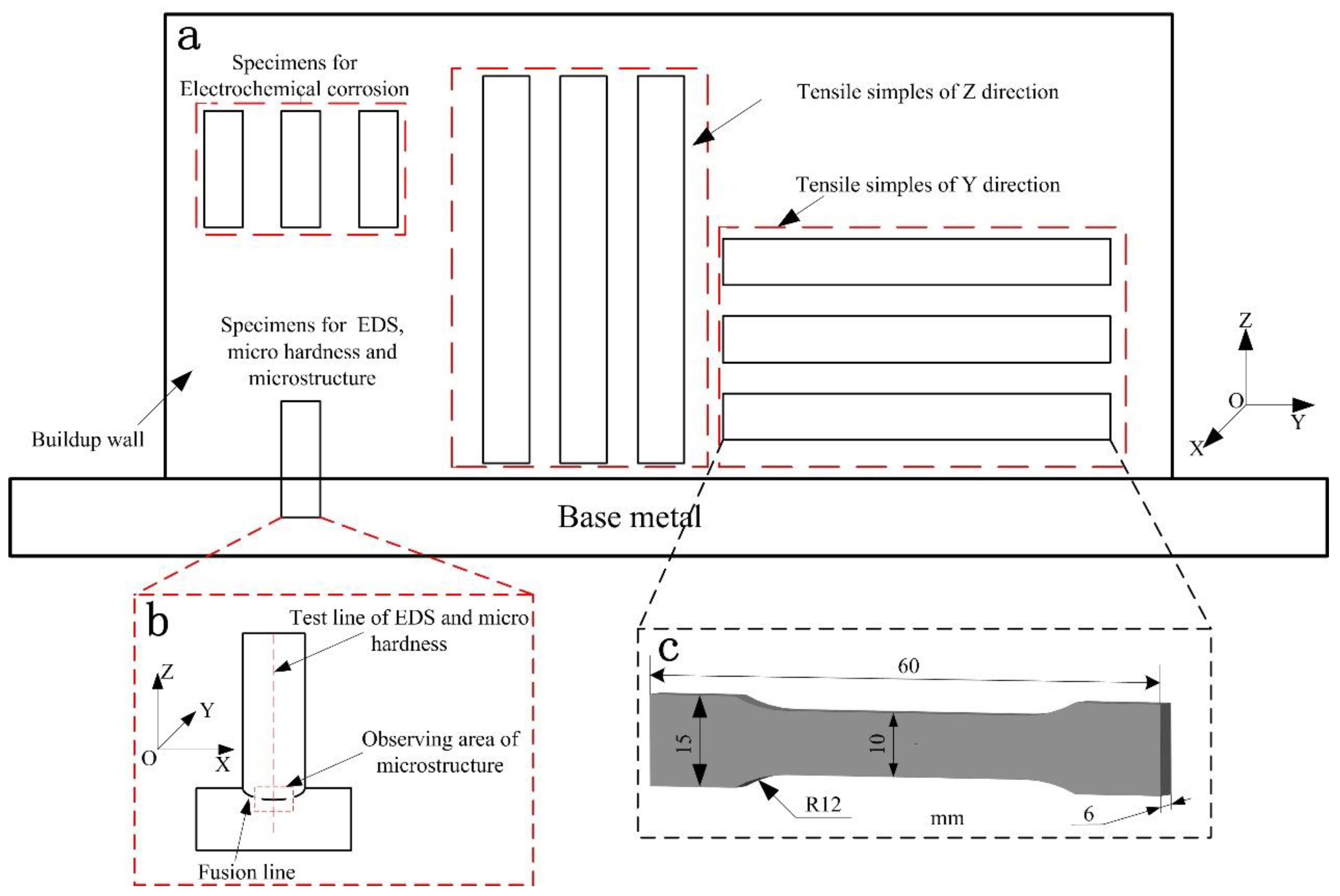
2.2. Material Characterization
3. Results and Discussion
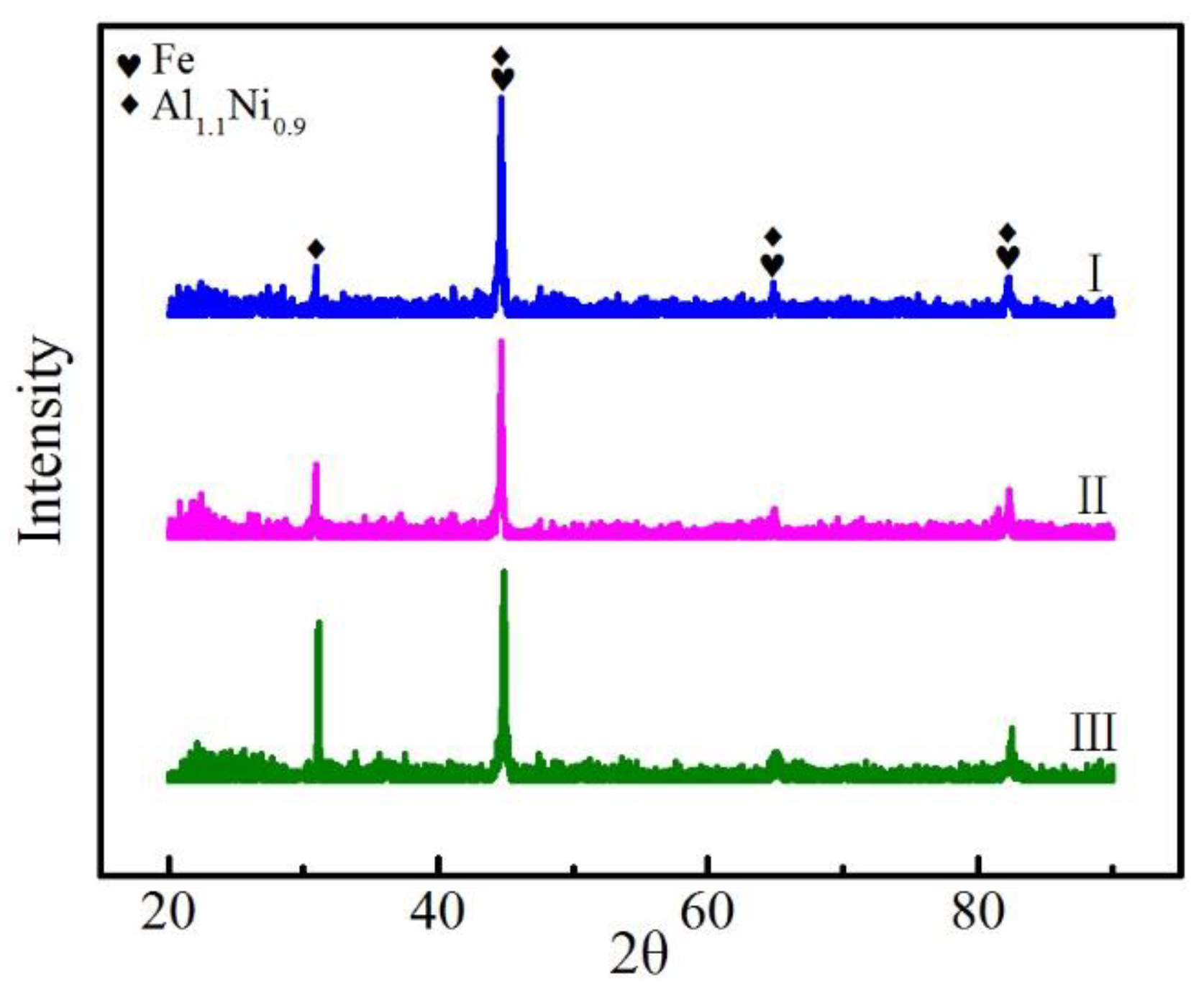
3.1. Quality of Fabrication and Phase Characterization
3.2. Microstructure
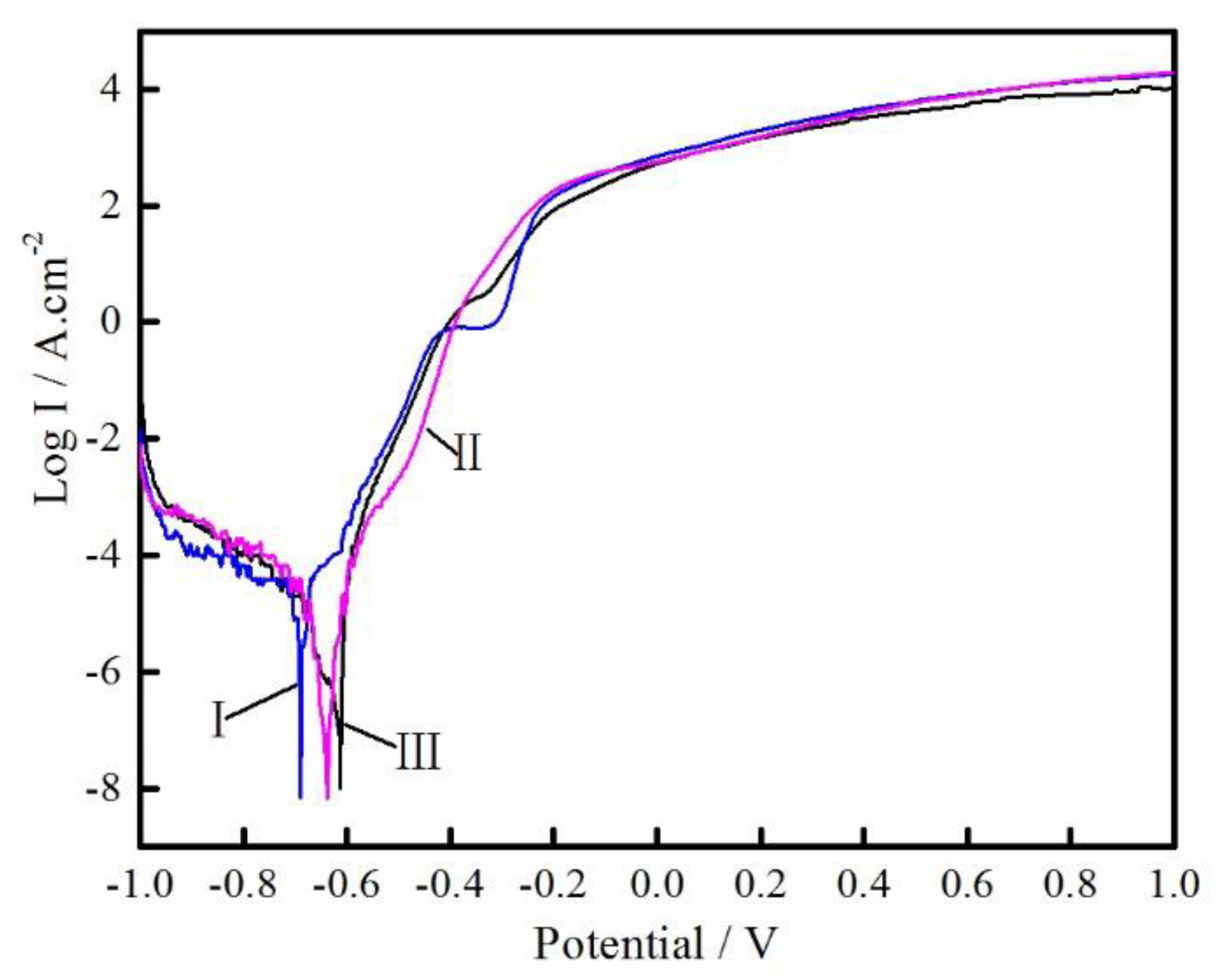
3.3. Electrochemical Corrosion
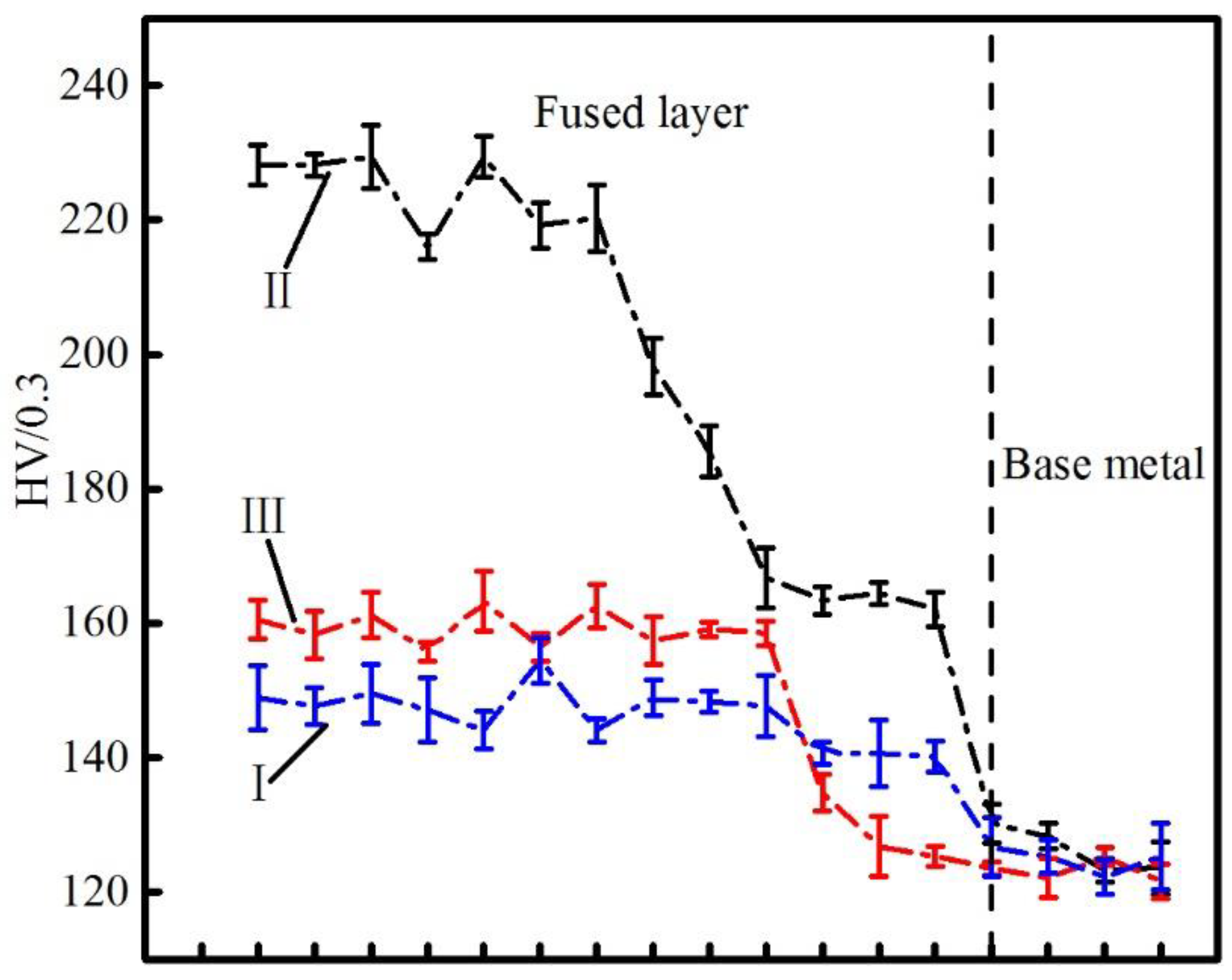
3.4. Micro Hardness
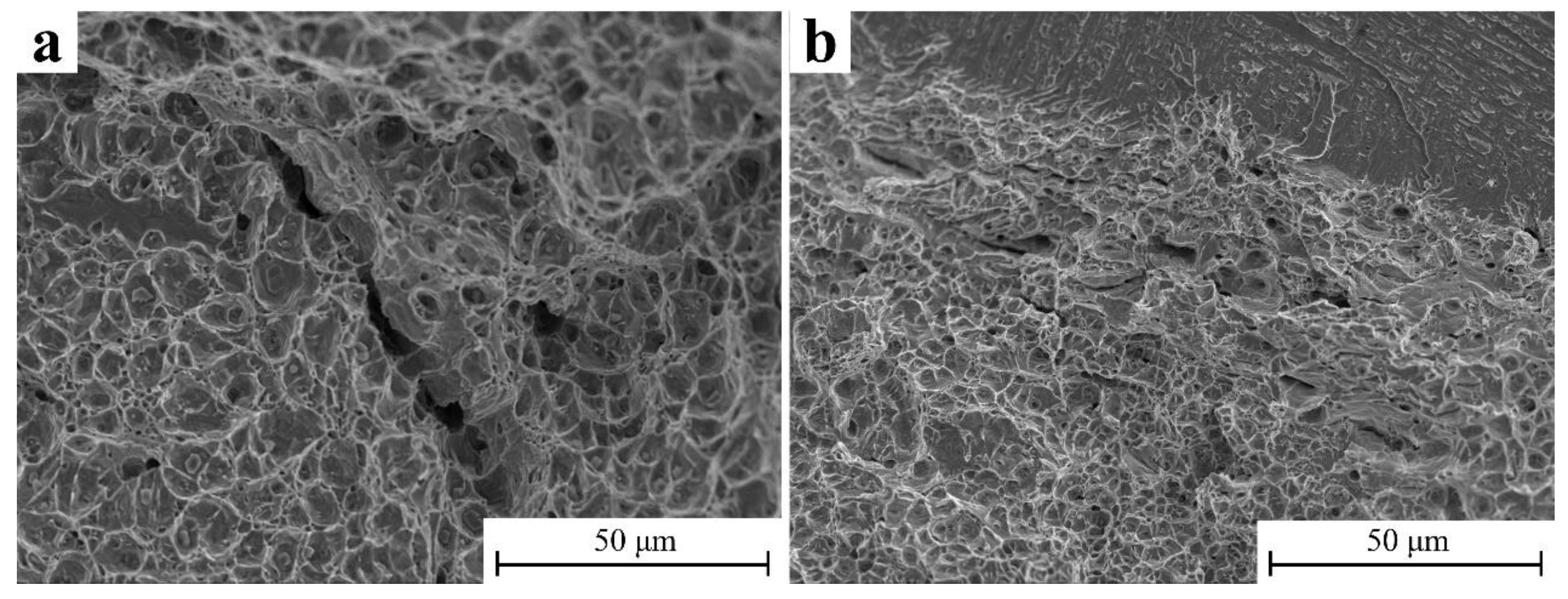
3.5. Tensile Properties
4. Conclusions
- (1)
- Columnar grains begin to grow from the area near the fusion line and transforms into equiaxed grains at the top of the buildup wall, and the grain size decreases with increasing travel speed.
- (2)
- The specimen deposited using pulse current shows the better resistance to electrochemical corrosion.
- (3)
- The hardness of the cladding layer is significantly greater than that of the base metal, which is related to the formation of intermetallic compounds, such as Al1.1Ni0.9.
- (4)
- This manufacturing method has little effect on the tensile strength of the material. The elongation in the Y direction and Z direction reach 10.25% and 9.94%, respectively.
- (5)
- The best parameters for fabricating SUS 304/AA 4043 alloy with WAAM are constant current of 150 A and scanning speed of 0.3 m/min.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mckamey, C.G.; DeVan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1779–1805. [Google Scholar] [CrossRef] [Green Version]
- Natesan, K. Corrosion Performance of Iron Aluminides; Argonne National Lab.: DuPage County, IL, USA, 1993.
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Wohlers, T. Developments in additive manufacturing. Manuf. Eng. 2010, 144, 54–59. [Google Scholar]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lv, Y.; Liu, Y.; Sun, Z.; Wang, K.; Li, Z.; Wu, Y.; Xu, B. Microstructural evolution and mechanical property of Ti-6Al-4V wall deposited by continuous plasma arc additive manufacturing without post heat treatment. J. Mech. Behav. Biomed. Mater. 2017, 69, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Zhou, F.; Ma, E. High tensile ductility in a nanostructured metal. Nature 2002, 419, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Vaerenbergh, J.V.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. A multi-bead overlapping model for robotic wire and arc additive manufacturing (WAAM). Robot. Comput. Integr. Manuf. 2015, 31, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Stallybrass, C.; Sauthoff, G. Ferritic Fe–Al–Ni–Cr alloys with coherent precipitates for high-temperature applications. Mater. Sci. Eng. A 2004, 387–389, 985–990. [Google Scholar] [CrossRef]
- Hadef, F.; Otmani, A.; Djekoun, A.; Grenèche, J.M. Nanocrystalline feal intermetallics obtained in mechanically alloyed Fe50Al40Ni10, powder. Superlattices Microstruct. 2011, 49, 654–665. [Google Scholar] [CrossRef]
- Ma, Y.; Cuiuri, D.; Hoye, N.; Li, H.; Pan, Z. The effect of location on the microstructure and mechanical properties of titanium aluminides produced by additive layer manufacturing using in-situ alloying and gas tungsten arc welding. Mater. Sci. Eng. A 2015, 631, 230–240. [Google Scholar] [CrossRef]
- Ma, Y.; Cuiuri, D.; Hoye, N.; Li, H.; Pan, Z. Effects of wire feed conditions on in situ alloying and additive layer manufacturing of titanium aluminides using gas tungsten arc welding. J. Mater. Res. 2014, 29, 2066–2071. [Google Scholar] [CrossRef]
- Shen, C.; Pan, Z.; Cuiuri, D.; Roberts, J.; Li, H. Fabrication of Fe-FeAl functionally graded material using the wire-arc additive manufacturing process. Metall. Mater. Transact. B 2016, 47, 763–772. [Google Scholar] [CrossRef]
- Shen, C.; Pan, Z.; Cuiuri, D.; Dong, B.; Li, H. In-depth study of the mechanical properties for Fe3Al based iron aluminide fabricated using the wire-arc additive manufacturing process. Mater. Sci. Eng. A 2016, 669, 118–126. [Google Scholar] [CrossRef]
- Lin, S.; Song, J.; Yang, C.; Ma, G. Microstructure analysis of interfacial layer with tungsten inert gas welding-brazing joint of aluminum alloy/stainless steel. Acta Metall. Sin. 2009, 45, 1211–1216. [Google Scholar]
- Liu, C.T.; George, E.P.; Maziasz, P.J.; Schneibel, J.H. Recent advances in B2 iron aluminide alloys: Deformation, fracture and alloy design. Mater. Sci. Eng. A 1998, 258, 84–98. [Google Scholar] [CrossRef]
- Crimp, M.A.; Vedula, K. Effect of boron on the tensile properties of B2 FeAl. Mater. Sci. Eng. 1986, 78, 193–200. [Google Scholar] [CrossRef]
- Mckamey, C.G.; Horton, J.A.; Liu, C.T. Effect of chromium on properties of Fe3Al. J. Mater. Res. 1989, 4, 1156–1163. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, Z.; Zhang, Z.; Huang, H. Mechanical properties of Fe3Al-based alloys with cerium addition. Scr. Metall. Mater. 1995, 33, 811–817. [Google Scholar]
- Mckamey, C.G.; Maziasz, P.J.; Goodwin, G.M.; Zacharia, T. Effects of alloying additions on the microstructures, mechanical properties and weldability of Fe3Al-based alloys. Mater. Sci. Eng. A 1994, 174, 59–70. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-feed additive manufacturing of metal components: Technologies, developments and future interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar] [CrossRef]
- Feng, Y.P.; Gaztelumendi, N.; Fornell, J.; Zhang, H.Y.; Solsona, P.; Baró, M.D.; Suriñach, S.; Ibáñez, E.; Barrios, L.; Pellicer, E.; et al. Mechanical properties, corrosion performance and cell viability studies on newly developed porous Fe-Mn-Si-Pd alloys. J. Alloy. Compd. 2017, 724, 1046–1056. [Google Scholar] [CrossRef]
- Martina, F.; Mehnen, J.; Williams, S.W.; Colegrove, P.; Wang, F. Investigation of the benefits of plasma deposition for the additive layer manufacture of Ti–6Al–4V. J. Mater. Process. Technol. 2012, 212, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Chen, L.; He, E.; Davies, C.M.; Dear, J.P. Effect of filler metal feed rate and composition on microstructure and mechanical properties of fibre laser welded AA 2024-T3. J. Manuf. Process. 2017, 25, 26–36. [Google Scholar] [CrossRef] [Green Version]



| wt.% | C | Mn | P | S | Si | Cr | Ni | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| SUS 304 | ≤0.08 | ≤2.0 | ≤0.045 | ≤0.03 | ≤1.00 | ≤18.0 | ≤8.0 | - | - | Balance |
| AA 4043 | - | ≤0.05 | - | - | ≤6.0 | - | - | ≤0.3 | Balance | ≤0.8 |
| Low carbon steel | ≤0.22 | ≤1.4 | ≤0.045 | ≤0.05 | ≤0.35 | - | - | - | Balance | - |
| Number | Wire Feed Speed (mm/s−1) | Current Type | Welding Speed (m/min−1) | Arc Length (mm) | |
|---|---|---|---|---|---|
| SUS 304 | AA 4043 | ||||
| 1 | 10 | 10 | PC | 0.2 | 5 |
| 2 | 10 | 10 | PC | 0.3 | 5 |
| 3 | 10 | 10 | PC | 0.4 | 5 |
| 4 | 10 | 10 | CC | 0.2 | 5 |
| 5 | 10 | 10 | CC | 0.3 | 5 |
| 6 | 10 | 10 | CC | 0.4 | 5 |
| wt.% | I | II | III | |||
|---|---|---|---|---|---|---|
| Base Metal | Fused Layer | Base Metal | Fused Layer | Base Metal | Fused Layer | |
| Cr | 1.86 | 13.91 | 2.09 | 8.92 | 1.65 | 10.14 |
| Ni | 0.93 | 3.37 | 1.16 | 2.64 | 0.94 | 3.17 |
| Fe | 94.12 | 58.96 | 93.46 | 59.17 | 94.16 | 60.65 |
| Al | 1.47 | 21.16 | 1.64 | 26.41 | 1.61 | 23.33 |
| C | 0.63 | 0.51 | 0.67 | 0.54 | 0.60 | 0.50 |
| Si | 0.99 | 2.09 | 0.98 | 2.32 | 1.04 | 2.21 |
| Samples | Grain Size (μm) | |
|---|---|---|
| Equiaxed Grains | Columnar Grains | |
| I | 239.1 | 267.8 |
| II | 114.8 | 105.5 |
| III | 192 | 256.7 |
| Number | Tensile Direction | Y direction | Z direction |
|---|---|---|---|
| I | Ultimate tensile strength (MPa) | 488 ± 21 | 458 ± 15 |
| 0.2% yield strength (MPa) | 440 ± 12 | 392 ± 10 | |
| Elongation (%) | 10.2 ± 0.1 | 9.9 ± 0.1 | |
| II | Ultimate tensile strength (MPa) | 515 ± 15 | 485 ± 14 |
| 0.2% yield strength (MPa) | 465 ± 11 | 429 ± 9 | |
| Elongation (%) | 9.9 ± 0.2 | 9.4 ± 0.1 | |
| III | Ultimate tensile strength (MPa) | 503 ± 14 | 470 ± 12 |
| 0.2% yield strength (MPa) | 453 ± 10 | 405 ± 9 | |
| Elongation (%) | 10.0 ± 0.1 | 9.5 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Ao, S.; Cai, Y.; Zhang, W.; Luo, Z. Formation of SUS304/Aluminum Alloys Using Wire and Arc Additive Manufacturing. Metals 2018, 8, 595. https://doi.org/10.3390/met8080595
Hao Z, Ao S, Cai Y, Zhang W, Luo Z. Formation of SUS304/Aluminum Alloys Using Wire and Arc Additive Manufacturing. Metals. 2018; 8(8):595. https://doi.org/10.3390/met8080595
Chicago/Turabian StyleHao, Zhizhuang, Sansan Ao, Yangchuan Cai, Wei Zhang, and Zhen Luo. 2018. "Formation of SUS304/Aluminum Alloys Using Wire and Arc Additive Manufacturing" Metals 8, no. 8: 595. https://doi.org/10.3390/met8080595
APA StyleHao, Z., Ao, S., Cai, Y., Zhang, W., & Luo, Z. (2018). Formation of SUS304/Aluminum Alloys Using Wire and Arc Additive Manufacturing. Metals, 8(8), 595. https://doi.org/10.3390/met8080595






