The Difference of Lamellar Structure Formation between Ti-45Al-5.4V-3.6Nb-Y Alloy and Ti-44Al-4Nb-4V-0.3Mo-Y Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
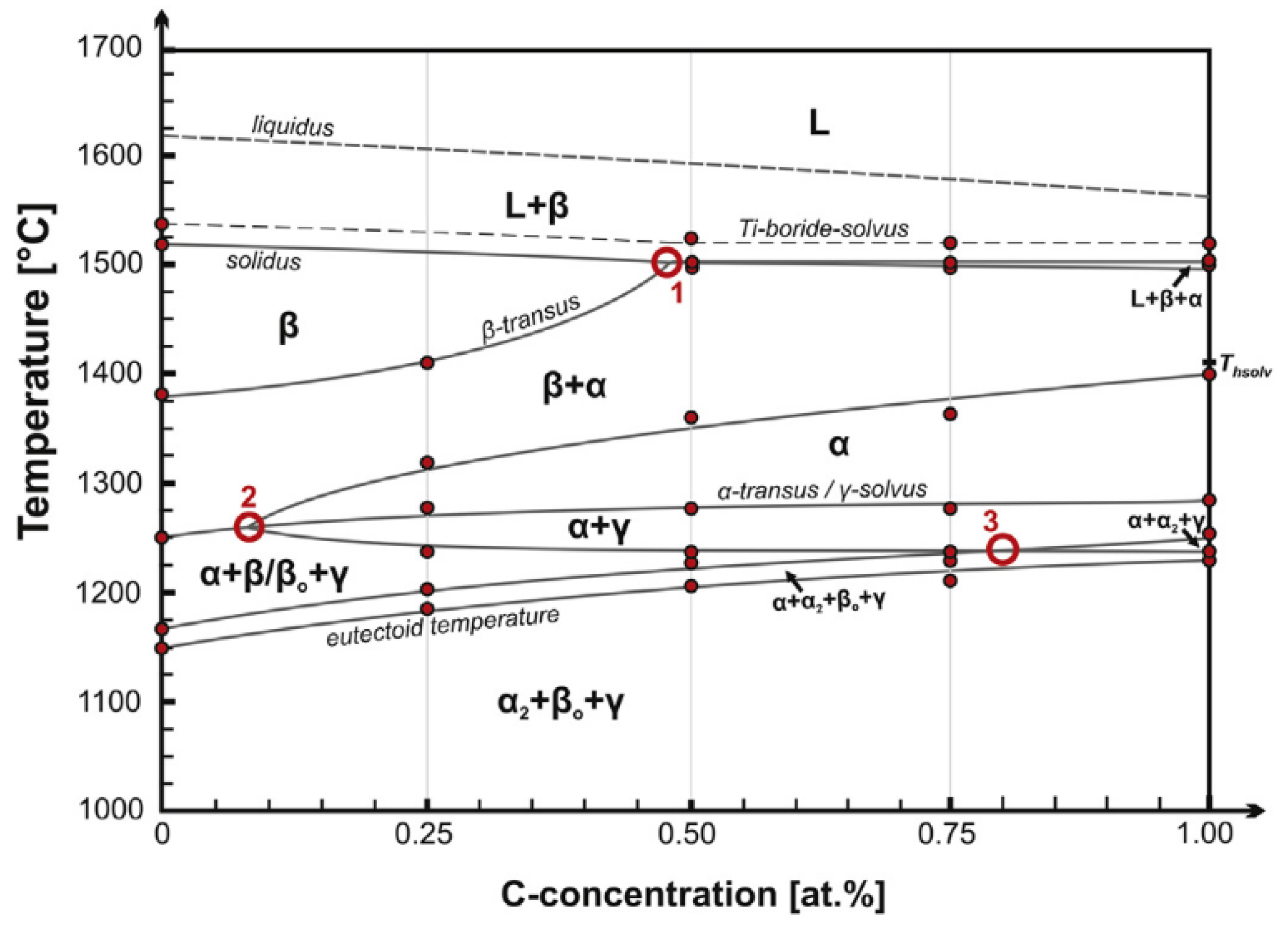
3.1. Ti-45Al-5.4V-3.6Nb-Y Alloy
3.2. Ti-44Al-4Nb-4V-0.3Mo-Y Alloy
4. Conclusions
- As a typical β-solidifying TiAl alloy, the as-forged microstructure of Ti-45Al-5.4V-3.6Nb-Y alloy is composed of γ-phase, β/β0-phase, and lamellar structure. The lamellar structure formation is influenced monotonically by temperature, when heat treated at different temperatures with the same holding time. At 1230 °C, the annealed structure consists of α2-phase, β/β0-phase, and γ phase, without lamellar structure. When the anneal temperature is elevated to 1260 °C, lamellar structure becomes one of components of the furnace cooled microstructure, instead of the single α2-phase. When the temperature is increased to 1300 °C, the microstructure changes to duplex structure, with more lamellar structure visible. The amount of lamellar structure increases with the increase of anneal temperature from 1260 °C to 1300 °C.
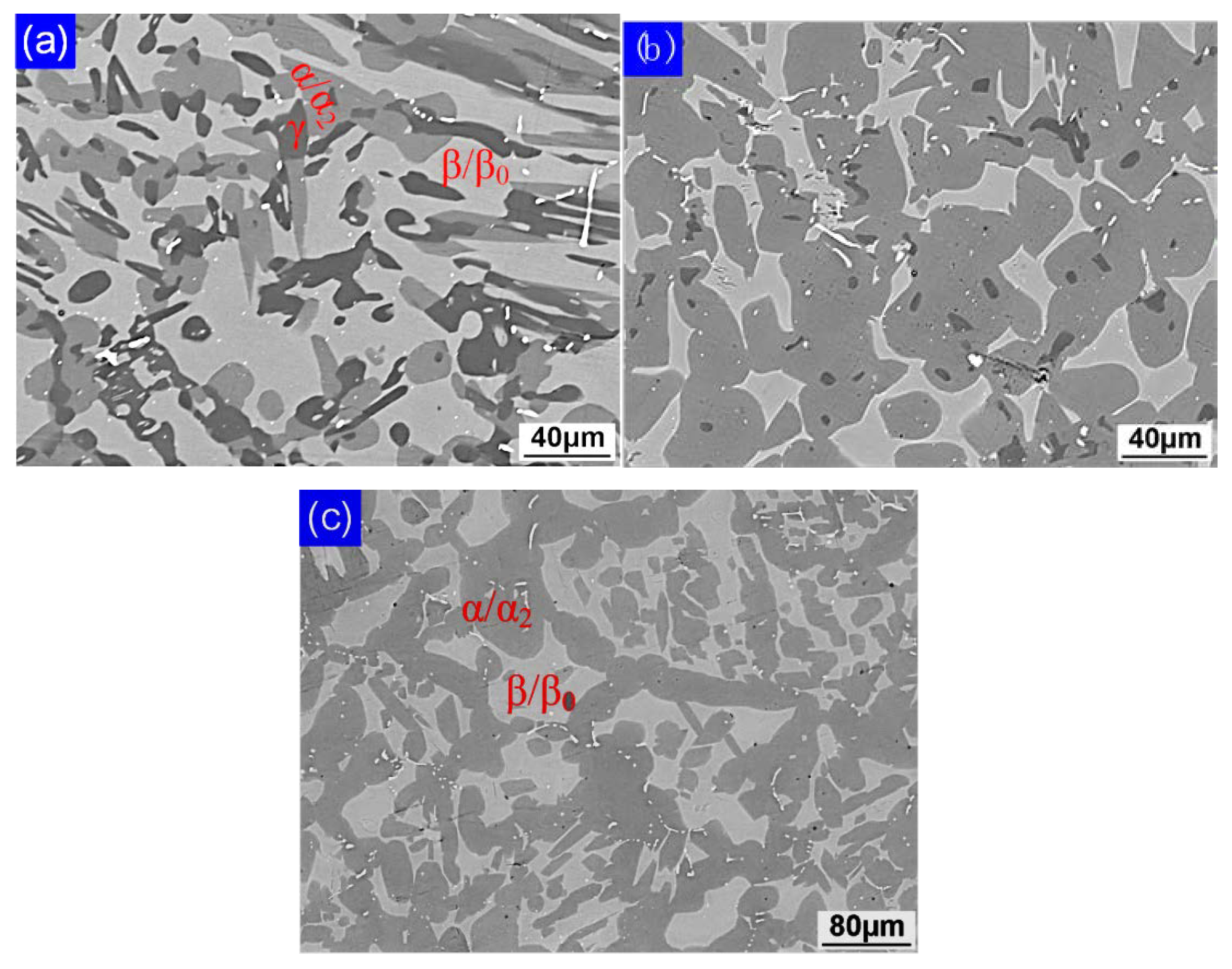
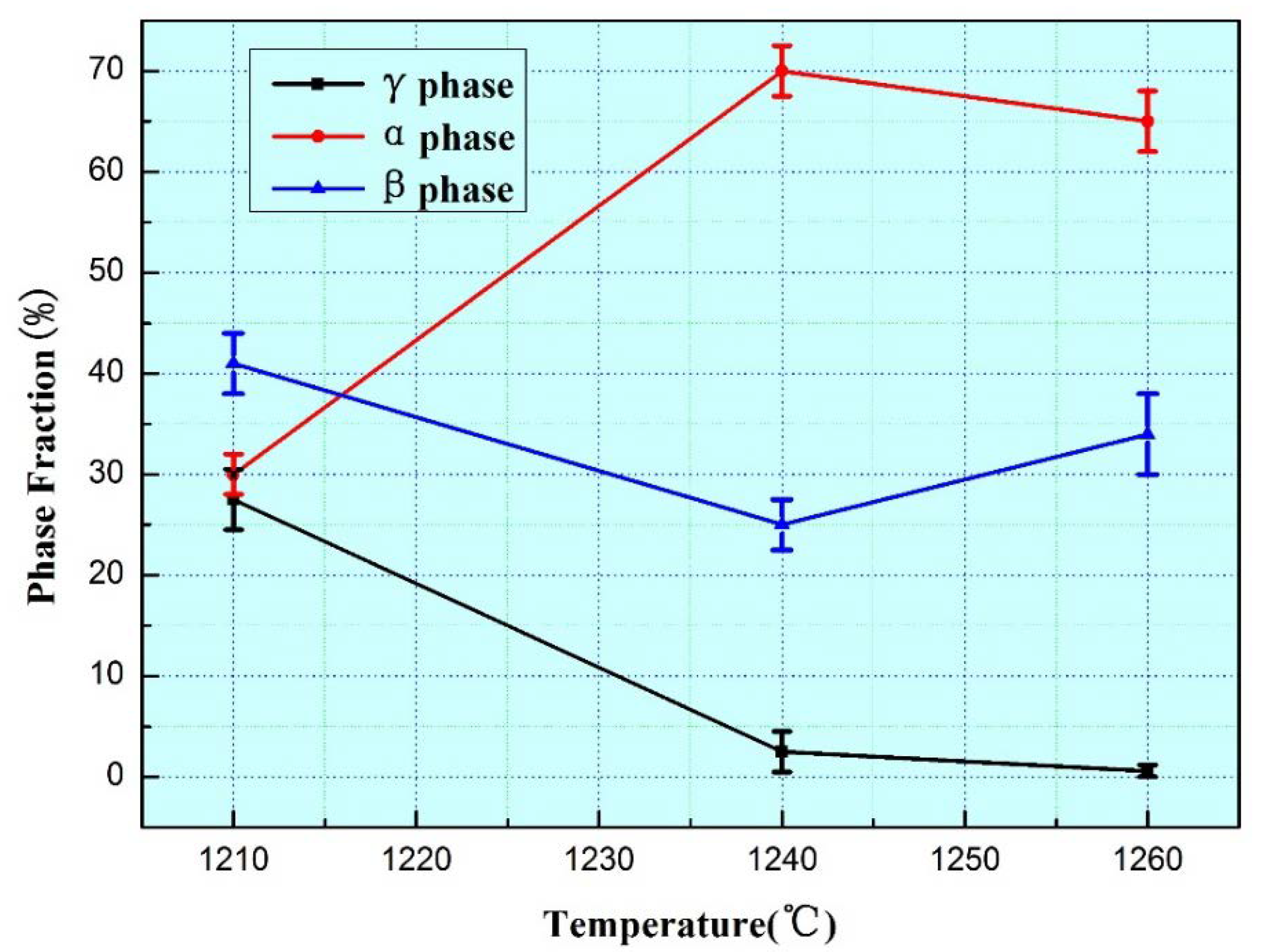
- Ti-44Al-4Nb-4V-0.3Mo-Y alloy has no α single-phase field in its solidification path. Different to the Ti-45Al-5.4V-3.6Nb-Y alloy, the formation of lamellar structure of Ti-44Al-4Nb-4V-0.3Mo-Y alloy is not only affected by the heat treatment temperature but also affected by β-phase stabilizing elements. When heat treatment temperature is elevated from 1210 °C to 1240 °C, the lamellar structure fraction decreases dramatically. However, a totally different tendency can be observed when temperature increases from 1240 °C to 1260 °C, which is that the volume fraction of lamellar structure clearly increases. Therefore, the heat treatment temperature, the segregation, and diffusivity of β-phase stabilizing elements are the main factors on the lamellar structure formation of Ti-44Al-4Nb-4V-0.3Mo-Y alloy.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, L.; Lin, J.; Li, J. Phase transformation mechanisms in a quenched Ti-45Al-8.5Nb-0.2W-0.2B-0.02Y alloy after subsequent annealing at 800 °C. J. Alloys Compd. 2017, 691, 60–66. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Song, L.; Kou, H.; Li, J.; Fu, H. Evolution of B2(ω) region in high-Nb containing TiAl alloy in intermediate temperature range. Intermetallics 2017, 82, 32–39. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Song, Z.W.; Han, J.C.; Zhang, C.J.; Lin, P.; Zhu, D.D.; Kong, F.T.; Chen, Y.Y. Effect of 2–6 at.% Mo addition on microstructural evolution of Ti-44Al alloy. J. Mater. Sci. Technol. 2018, 34, 1196–1204. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhang, C.J.; Du, Z.X.; Hou, Z.P.; Lin, P.; Kong, F.T.; Chen, Y.Y. Deformation behavior of high Nb containing TiAl based alloy in α+γ two phase field region. Mater. Des. 2016, 90, 225–229. [Google Scholar] [CrossRef]
- Zhou, H.T.; Kong, F.T.; Wang, X.P.; Chen, Y.Y. Hot deformation behavior and microstructural evolution of as-forged Ti-44Al-8Nb-(W, B, Y) alloy with nearly lamellar microstructure. Intermetallics 2017, 81, 62–72. [Google Scholar] [CrossRef]
- Han, J.C.; Dong, J.; Zhang, S.Z.; Zhang, C.J.; Xiao, S.L.; Chen, Y.Y. Microstructure evolution and tensile properties of conventional cast TiAl-based alloy with trace Ni addition. Mater. Sci. Eng. A 2018, 715, 41–48. [Google Scholar] [CrossRef]
- Erdely, P.; Staron, P.; Maawad, E.; Schell, N.; Klose, J.; Mayer, S.; Clemens, H. Effect of hot rolling and primary annealing on the microstructure and texture of a β-stabilised γ-TiAl based alloy. Acta Mater. 2017, 126, 145–153. [Google Scholar] [CrossRef]
- Klein, T.; Rashkova, B.; Holec, D.; Clemens, H.; Mayer, S. Silicon distribution and silicide precipitation during annealing in an advanced multi-phase γ-TiAl based alloy. Acta Mater. 2016, 110, 236–245. [Google Scholar] [CrossRef]
- Janschek, P. Wrought TiAl Blades. Mater. Today Proc. 2015, 2, S92–S97. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Gao, F.; Liu, Y.; Wang, Q. High tensile ductility and strength in the Ti-42Al-6V-1Cr alloy. J. Alloys Compd. 2017, 698, 898–905. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Lin, J.P.; Liang, Y.F.; Zhang, L.Q.; Shang, S.L.; Liu, Z.K. A novel hot pack rolling of high Nb-TiAl sheet from cast ingot. Intermetallics 2015, 67, 19–25. [Google Scholar] [CrossRef]
- Cao, G.; Fu, L.; Lin, J.; Zhang, Y.; Chen, C. The relationships of microstructure and properties of a fully lamellar TiAl alloy. Intermetallics 2000, 8, 647–653. [Google Scholar] [CrossRef]
- Wang, J.N.; Xie, K. Grain size refinement of a TiAl alloy by rapid heat treatment. Scr. Mater. 2000, 43, 441–446. [Google Scholar] [CrossRef]
- Jones, S.A.; Kaufman, M.J. Phase equilibria and transformations in intermediate titanium–aluminum alloys. Acta Metall. Mater. 1993, 41, 387–398. [Google Scholar] [CrossRef]
- Inui, H.; Oh, M.H.; Nakamura, A.A.; Yamaguchi, A.M. Ordered domains in TiAl coexisting with Ti3Al in the lamellar structure of Ti-rich TiAl compounds. Philos. Mag. A 1992, 66, 539–555. [Google Scholar] [CrossRef]
- Niu, H.Z.; Chen, Y.Y.; Zhang, Y.S.; Lu, J.W.; Zhang, W.; Zhang, P.X. Producing fully-lamellar microstructure for wrought beta-gamma TiAl alloys without single α-phase field. Intermetallics 2015, 59, 87–94. [Google Scholar] [CrossRef]
- Kainuma, R.; Fujita, Y.; Mitsui, H.; Ohnuma, I.; Ishida, K. Phase equilibria among α (hcp), β (bcc) and γ (L10) phases in Ti-Al base ternary alloys. Intermetallics 2000, 8, 855–867. [Google Scholar] [CrossRef]
- Hu, D.; Botten, R.R. Phase transformations in some TiAl-based alloys. Intermetallics 2002, 10, 701–715. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminum-titanium phase diagram. J. Phase Equilib. Diff. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Han, J.; Xiao, S.; Tian, J.; Chen, Y.; Xu, L.; Wang, X.; Jia, Y.; Du, Z.; Cao, S. Grain refinement by trace TiB2 addition in conventional cast TiAl-based alloy. Mater. Charact. 2015, 106, 112–122. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic beta-stabilized gamma-TiAl based alloy. Intermetallics 2014, 46, 173–184. [Google Scholar] [CrossRef]
- Gornakova, A.S.; Straumal, B.B.; Nekrasov, A.N.; Kilmametov, A.; Afonikova, N.S. Grain Boundary Wetting by a Second Solid Phase in Ti-Fe Alloys. J. Mater. Eng. Perform. 2018, 1–4. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Ivanisenko, Yu.; Gornakova, A.S.; Mazilkin, A.A.; Kriegel, M.J.; Fabrichnaya, O.B.; Baretzky, B.; Hahn, H. Phase Transformations in Ti–Fe Alloys Induced by High-Pressure Torsion. Adv. Eng. Mater. 2016, 17, 1835–1841. [Google Scholar] [CrossRef]
- Lo´pez, G.A.; Mittemeijer, E.J.; Straumal, B.B. Grain Boundary Wetting by a Solid Phase; Microstructural Development in a Zn-5 wt% Al Alloy. Acta Mater. 2004, 52, 4537–4545. [Google Scholar] [CrossRef]
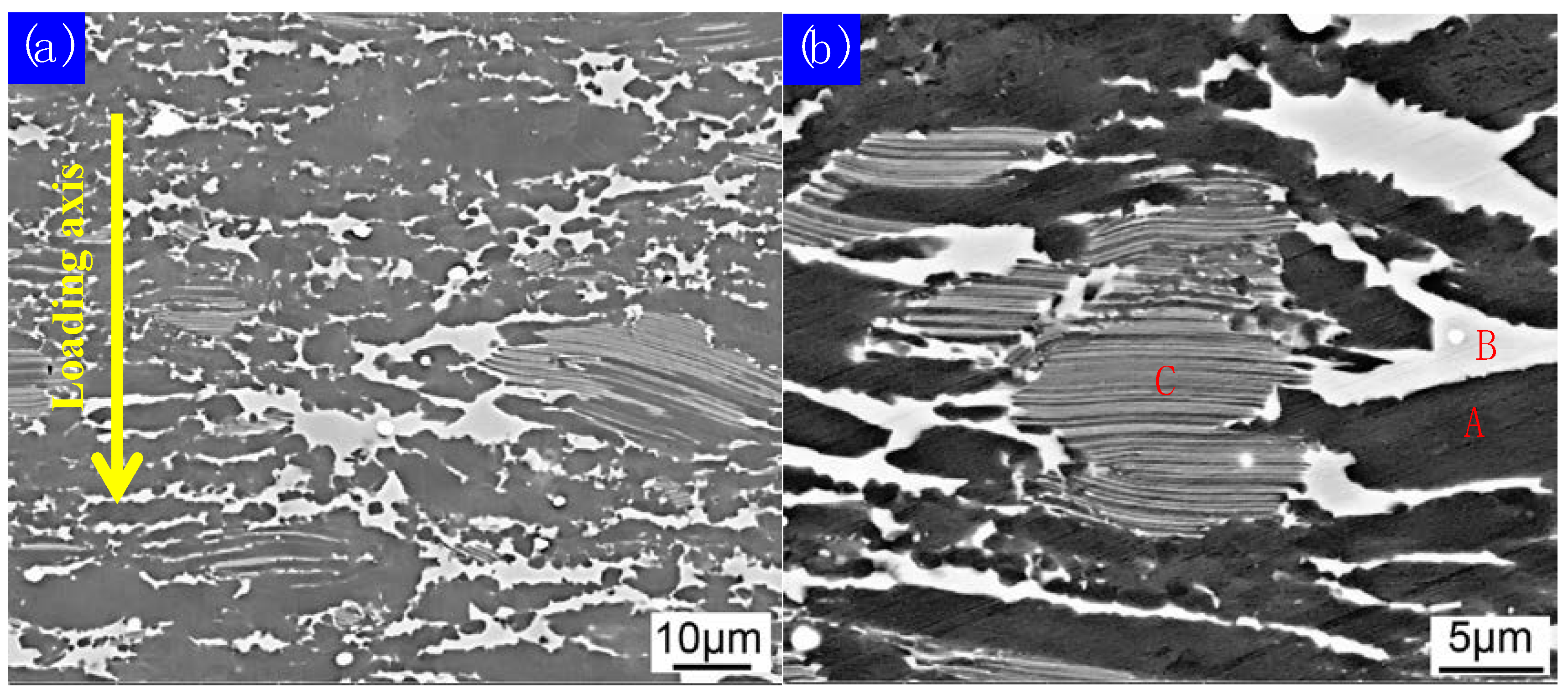


| Position | Ti (at.%) | Al (at.%) | V (at.%) | Nb (at.%) | Y (at.%) |
|---|---|---|---|---|---|
| A | 42 ± 0.63 | 50 ± 0.75 | 4 ± 0.12 | 4 ± 0.15 | 00.00 |
| B | 46 ± 0.78 | 39 ± 0.36 | 10 ± 0.23 | 4 ± 0.33 | 00.00 |
| C | 45 ± 0.55 | 46 ± 0.69 | 5 ± 0.18 | 4 ± 0.26 | 00.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Zhang, S.; Zhang, C.; Kong, F.; Chen, Y.; Yang, F. The Difference of Lamellar Structure Formation between Ti-45Al-5.4V-3.6Nb-Y Alloy and Ti-44Al-4Nb-4V-0.3Mo-Y Alloy. Metals 2018, 8, 566. https://doi.org/10.3390/met8080566
Han J, Zhang S, Zhang C, Kong F, Chen Y, Yang F. The Difference of Lamellar Structure Formation between Ti-45Al-5.4V-3.6Nb-Y Alloy and Ti-44Al-4Nb-4V-0.3Mo-Y Alloy. Metals. 2018; 8(8):566. https://doi.org/10.3390/met8080566
Chicago/Turabian StyleHan, Jianchao, Shuzhi Zhang, Changjiang Zhang, Fantao Kong, Yuyong Chen, and Fei Yang. 2018. "The Difference of Lamellar Structure Formation between Ti-45Al-5.4V-3.6Nb-Y Alloy and Ti-44Al-4Nb-4V-0.3Mo-Y Alloy" Metals 8, no. 8: 566. https://doi.org/10.3390/met8080566
APA StyleHan, J., Zhang, S., Zhang, C., Kong, F., Chen, Y., & Yang, F. (2018). The Difference of Lamellar Structure Formation between Ti-45Al-5.4V-3.6Nb-Y Alloy and Ti-44Al-4Nb-4V-0.3Mo-Y Alloy. Metals, 8(8), 566. https://doi.org/10.3390/met8080566








