Leaching Process of Rare Earth Elements, Gallium and Niobium in a Coal-Bearing Strata-Hosted Rare Metal Deposit—A Case Study from the Late Permian Tuff in the Zhongliangshan Mine, Chongqing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
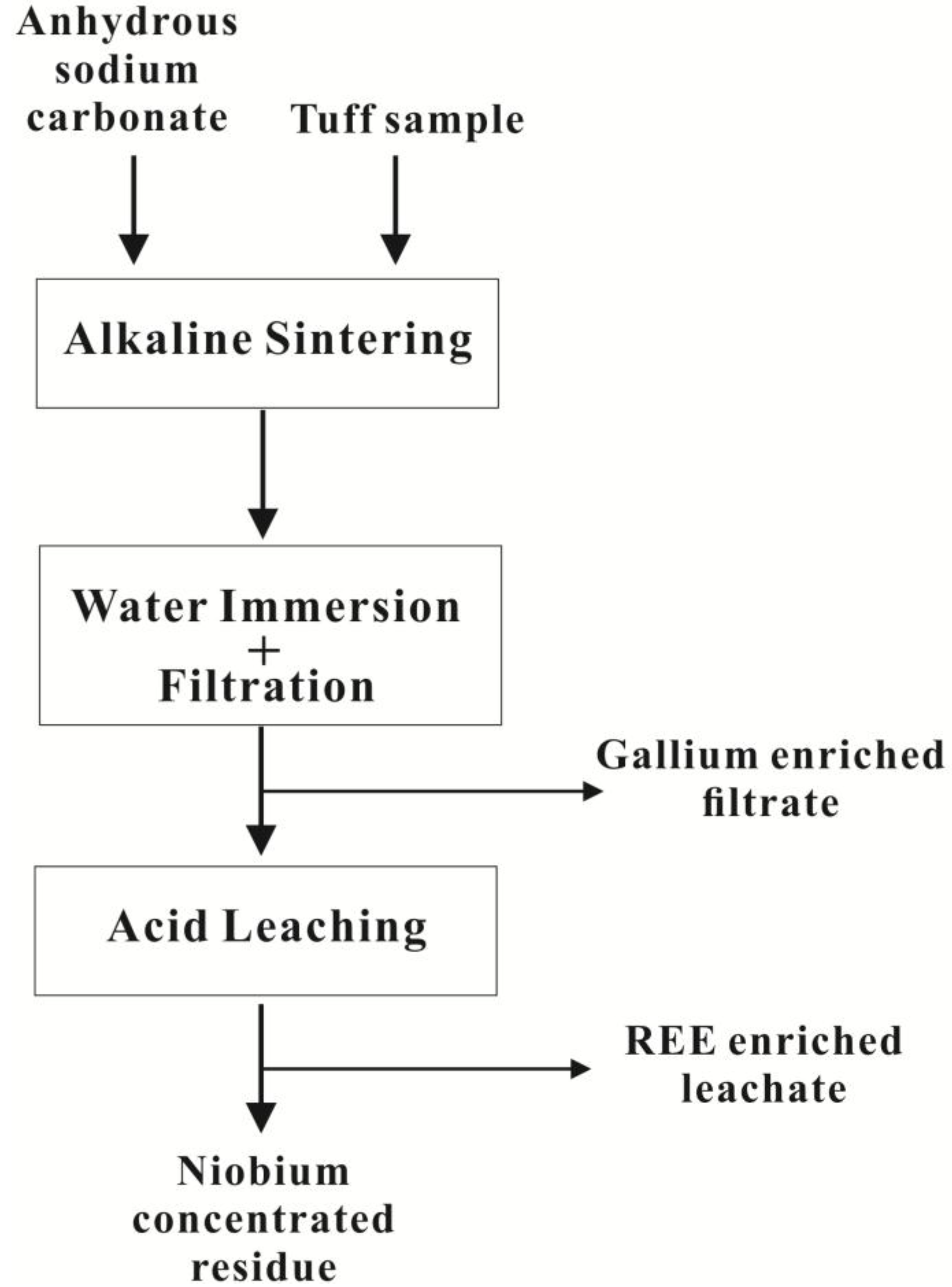
2.2. Experimental Procedure
2.3. Analytical Method
3. Results and Discussion
3.1. Tentative Experiment
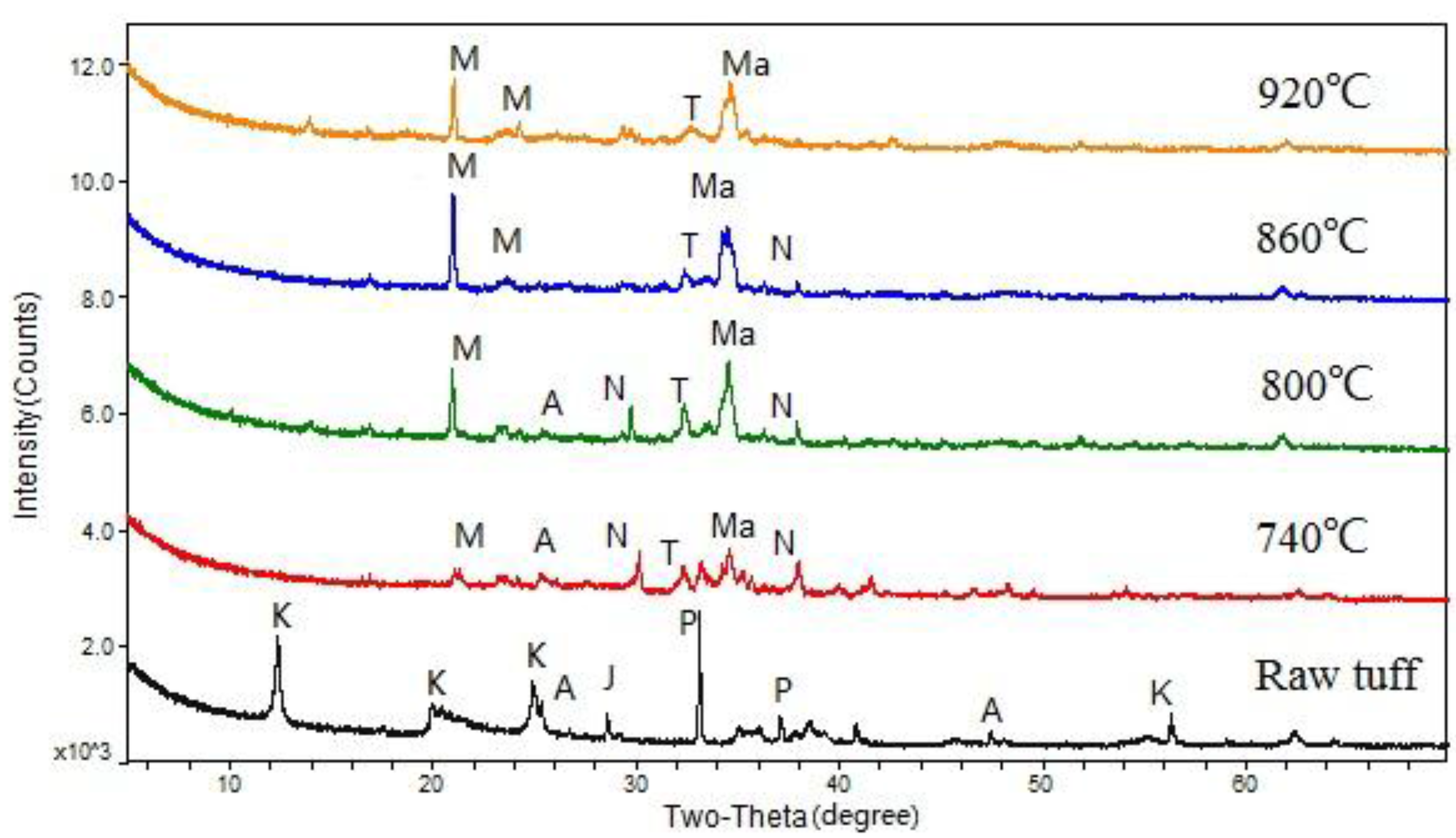
3.1.1. Alkaline Sintering
3.1.2. Water Immersion
3.1.3. Acid Leaching
3.2. Validation Experiment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seredin, V.; Finkelman, R. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Seredin, V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Seredin, V.; Dai, S.; Sun, Y.; Chekryzhov, I. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Seredin, V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Hower, J.; Granite, E.; Mayfield, D.; Lewis, A.; Finkelman, R. Notes on Contributions to the Science of Rare Earth Element Enrichment in Coal and Coal Combustion Byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Eskenazy, G. Rare earth elements in a sampled coal from the Pirin Deposit, Bulgaria. Int. J. Coal Geol. 1987, 7, 301–314. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.; Finkelman, R.; Seredin, V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundance, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.; Chekryzhov, I.; Seredin, V.; Nechaev, V.; Graham, I.; Hower, J.; Ward, C.; Ren, D.; Wang, X. Metalliferous coal deposits in East Asia (Primorye of Russia and South China): A review of geodynamic controls and styles of mineralization. Gondwana Res. 2016, 29, 60–82. [Google Scholar] [CrossRef]
- Dai, S.; Wang, P.; Ward, C.; Tang, Y.; Song, X.; Jiang, J.; Hower, J.; Li, T.; Seredin, V.; Wagner, N.; et al. Elemental and mineralogical anomalies in the coal-hosted Ge ore deposit of Lincang, Yunnan, southwestern China: Key role of N2-CO2-mixed hydrothermal solutions. Int. J. Coal Geol. 2015, 152, 19–46. [Google Scholar] [CrossRef]
- Johnston, M.; Hower, J.; Dai, S.; Wang, P.; Xie, P.; Liu, J. Petrology and Geochemistry of the Harlan, Kellioka, and Darby Coals from the Louellen 7.5-Minute Quadrangle, Harlan County, Kentucky. Minerals 2015, 5, 894–918. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.; Ward, C.; Jiang, J.; Hower, J.; Song, X.; Jiang, Y.; Wang, X.; Gornostaev, T.; Li, X.; et al. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Graham, I.; Li, X.; Liu, H.; Song, X.; Hower, J.; Zhou, Y. Cryptic sediment-hosted critical element mineralization from eastern Yunnan Province, southwestern China: Mineralogy, geochemistry, relationship to Emeishan alkaline magmatism. Ore Geol. Rev. 2017, 80, 116–140. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Li, X.; Liu, H.; Zhang, B. New insights into the lowest Xuanwei Formation in eastern Yunnan Province, SW China: Implications for Emeishan large igneous province felsic tuff deposition and the cause of the end-Guadalupian mass extinction. Lithos 2016, 264, 375–391. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Zhang, M.; Wang, X.; Wang, J.; Song, X.; Jiang, Y.; Luo, Y.; Song, Z.; Yang, Z.; et al. A new type of Nb(Ta)–Zr(Hf)–REE–Ga polymetallic deposit in the late Permian coal-bearing strata, eastern Yunnan, southwestern China: Possible economic significance and genetic implications. Int. J. Coal Geol. 2010, 83, 55–63. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.; French, D.; Graham, I. Major and Trace Element Geochemistry of Coals and Intra-Seam Claystones from the Songzao Coalfield, SW China. Minerals 2015, 5, 870–893. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Li, S. Discovery of the superlarge gallium ore deposit in Jungar, Inner Mongolia, North China. Chin. Sci. Bull. 2006, 51, 2243–2252. [Google Scholar] [CrossRef]
- Seredin, V. From coal science to metal production and environmental protection: A new story of success. Int. J. Coal Geol. 2012, 90, 1–3. [Google Scholar] [CrossRef]
- Zou, J.; Tian, H.; Li, T. Geochemistry and Mineralogy of Tuff in Zhongliangshan Mine, Chongqing, Southwestern China. Minerals 2016, 6, 47. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.; Hower, J.; French, D.; Jia, S.; Hood, M.; Garrison, T. Mineralogical and geochemical compositions of Late Permian coals and host rocks from the Guxu Coalfield, Sichuan Province, China, with emphasis on enrichment of rare metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Hower, J.; Eble, C.; Dai, S.; Belkin, H. Distribution of rare earth elements in eastern Kentucky coals: Indicators of multiple modes of enrichment? Int. J. Coal Geol. 2016, 160–161, 73–81. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.; Ward, C.; Hower, J.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U-Se-Mo-Re-V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Deposita 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Hower, J.; Eble, C.; O’Keefe, J.; Dai, S.; Wang, P.; Xie, P.; Liu, J.; Ward, C.; French, D. Petrology, Palynology, and Geochemistry of Gray Hawk Coal (Early Pennsylvanian, Langsettian) in Eastern Kentucky, USA. Minerals 2015, 5, 592–622. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Hower, J.; Johnston, M.; Song, W.; Wang, P.; Zhang, S. Petrology, mineralogy, and chemistry of size-fractioned fly ash from the Jungar power plant, Inner Mongolia, China, with emphasis on the distribution of rare earth elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Zhuang, X.; Su, S.; Xiao, M.; Li, J.; Alastuey, A.; Querol, X. Mineralogy and geochemistry of the Late Permian coals in the Huayingshan coal-bearing area, Sichuan Province, China. Int. J. Coal Geol. 2012, 94, 271–282. [Google Scholar] [CrossRef]
- Dai, S.; Yang, J.; Ward, C.; Hower, J.; Liu, H.; Garrison, T.; French, D.; O’Keefe, J. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol. Rev. 2015, 70, 1–30. [Google Scholar] [CrossRef]
- Blissett, R.; Smalley, N.; Rowson, N. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- The U.S. Department of Energy. Available online: http://www.energy.gov/fe/articles/doe-selects-projects-enhance-its’s-research-recovery-rare-earth-elements-coal-and-coal (accessed on 2 December 2015).
- Zhang, B.; Liu, C.; Li, C.; Jiang, M. A novel approach for recovery of rare earths and niobium from Bayan Obo tailings. Miner. Eng. 2014, 65, 17–23. [Google Scholar] [CrossRef]
- Chi, R.; Tian, J. Chemical Metallurgy of Weathered Elution-Deposited Rare Earth Ores; Science press of China: Beijing, China, 2006; (In Chinese). ISBN 9787030178305. [Google Scholar]
- Li, G.; You, Z.; Sun, H.; Sun, R.; Peng, Z.; Zhang, Y.; Jiang, T. Separation of Rhenium from Lead-Rich Molybdenite Concentrate via Hydrochloric Acid Leaching Followed by Oxidative Roasting. Metals 2016, 6, 282. [Google Scholar] [CrossRef]
- Rozelle, P.; Khadilkar, A.; Pulati, N.; Soundarrajan, N.; Klima, M.; Mosser, M.; Miller, C.; Pisupati, S. A Study on Removal of Rare Earth Elements from U.S. Coal Byproducts by Ion Exchange. Metall. Mater. Trans. E 2016, 3E, 6–17. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud: A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Li, X.; Xiao, W.; Liu, W.; Liu, G.; Peng, Z.; Zhou, Q.; Qi, T. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferr. Met. Soc. 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Bai, G.; Teng, W.; Wang, X.; Qin, J.; Xu, P.; Li, P. Alkali desilicated coal fly ash as substitute of bauxite in lime-soda sintering process for aluminum production. Trans. Nonferr. Met. Soc. 2010, 20, 169–175. [Google Scholar] [CrossRef]
| Sample | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| T-1 | 35.69 | 29.84 | 9.43 | 0.2 | 0.13 | 0.12 | 0.12 |
| Y-1 | 37.53 | 31.88 | 6.89 | 0.2 | 0.12 | 0.13 | 0.17 |
| SD | 0.92 | 1.02 | 1.27 | 0 | 0.005 | 0.005 | 0.025 |
| Sample | MnO | TiO2 | P2O5 | REE (μg/g) | Ga (μg/g) | Nb (μg/g) | |
| T-1 | 0.023 | 2.58 | 0.062 | 1515 | 77 | 215 | |
| Y-1 | 0.01 | 2.97 | 0.051 | 1585 | 86 | 225 | |
| SD | 0.009 | 0.276 | 0.008 | 49.5 | 6.36 | 7.07 |
| Sample No. | Al | Ga | Nb | REE |
|---|---|---|---|---|
| 1 | 43.13 | 63.67 | 0.03 | 0.03 |
| 2 | 42.77 | 66.97 | 0.02 | 0.02 |
| 3 | 42.92 | 70.65 | 0.02 | 0.03 |
| 4 | 44.53 | 60.82 | 0.02 | 0.02 |
| 5 | 42.92 | 71.11 | 0.01 | 0.01 |
| 6 | 39.18 | 64.98 | 0.01 | 0.01 |
| 7 | 41.07 | 68.85 | 0.01 | 0.01 |
| 8 | 40.48 | 54.97 | 0.01 | 0.01 |
| 9 | 40.83 | 58.93 | 0.01 | 0.01 |
| Average | 41.98 | 64.55 | 0.02 | 0.02 |
| Standard Deviation | 1.58 | 5.19 | 0.01 | 0.01 |
| Level | A | B/°C | C/h | D/mol/L |
|---|---|---|---|---|
| 1 | 20 mL:1 g | 40 | 2 | 4 |
| 2 | 30 mL:1 g | 60 | 4 | 6 |
| 3 | 40 mL:1 g | 80 | 6 | 8 |
| Sample No. | Factors | Leaching Efficiency | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 85.56 |
| 2 | 1 | 2 | 2 | 2 | 84.97 |
| 3 | 1 | 3 | 3 | 3 | 83.65 |
| 4 | 2 | 1 | 2 | 3 | 71.82 |
| 5 | 2 | 2 | 3 | 1 | 79.82 |
| 6 | 2 | 3 | 1 | 2 | 80.71 |
| 7 | 3 | 1 | 3 | 2 | 81.79 |
| 8 | 3 | 2 | 1 | 3 | 84.59 |
| 9 | 3 | 3 | 2 | 1 | 80.85 |
| Іj | 254.18 | 239.17 | 250.86 | 246.23 | |
| ІІj | 232.35 | 249.38 | 237.64 | 247.47 | |
| Шj | 247.23 | 245.21 | 245.26 | 240.06 | |
| Kj | 3 | 3 | 3 | 3 | |
| Ij/Kj | 84.73 | 79.72 | 83.62 | 82.08 | |
| ІІj/Kj | 77.45 | 83.13 | 79.21 | 82.49 | |
| Шj/Kj | 82.41 | 81.74 | 81.75 | 80.02 | |
| Dj | 7.28 | 3.40 | 4.41 | 2.47 | |
| Sample No. | Factors | Leaching Efficiency (%) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 83.48 |
| 2 | 1 | 2 | 2 | 2 | 84.62 |
| 3 | 1 | 3 | 3 | 3 | 80.94 |
| 4 | 2 | 1 | 2 | 3 | 77.11 |
| 5 | 2 | 2 | 3 | 1 | 78.57 |
| 6 | 2 | 3 | 1 | 2 | 80.42 |
| 7 | 3 | 1 | 3 | 2 | 82.59 |
| 8 | 3 | 2 | 1 | 3 | 87.12 |
| 9 | 3 | 3 | 2 | 1 | 77.38 |
| Іj | 249.04 | 243.18 | 251.02 | 239.43 | |
| ІІj | 236.10 | 250.31 | 239.11 | 247.63 | |
| Шj | 247.09 | 238.74 | 242.10 | 245.17 | |
| Kj | 3 | 3 | 3 | 3 | |
| Ij/Kj | 83.01 | 81.06 | 83.67 | 79.81 | |
| ІІj/Kj | 78.70 | 83.44 | 79.70 | 82.54 | |
| Шj/Kj | 82.36 | 79.58 | 80.70 | 81.72 | |
| Dj | 4.31 | 3.86 | 3.97 | 2.73 | |
| Sample No. | Factors | Leaching Efficiency (%) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 3.2 |
| 2 | 1 | 2 | 2 | 2 | 1.01 |
| 3 | 1 | 3 | 3 | 3 | 2.46 |
| 4 | 2 | 1 | 2 | 3 | 6.87 |
| 5 | 2 | 2 | 3 | 1 | 1.36 |
| 6 | 2 | 3 | 1 | 2 | 2.88 |
| 7 | 3 | 1 | 3 | 2 | 3.32 |
| 8 | 3 | 2 | 1 | 3 | 11.02 |
| 9 | 3 | 3 | 2 | 1 | 1.74 |
| Ij | 6.67 | 13.39 | 17.10 | 6.30 | |
| ІІj | 11.11 | 13.39 | 9.62 | 7.21 | |
| Шj | 16.08 | 7.08 | 7.14 | 20.35 | |
| Kj | 3 | 3 | 3 | 3 | |
| Ij/Kj | 2.22 | 4.46 | 5.70 | 2.10 | |
| ІІj/Kj | 3.70 | 4.46 | 3.21 | 2.40 | |
| Шj/Kj | 5.36 | 2.36 | 2.38 | 6.78 | |
| Dj | 3.14 | 2.10 | 3.32 | 4.68 | |
| Experiment Step | REE | Ga | Nb |
|---|---|---|---|
| Water immersion | <0.4 | 67.31 | <1 |
| Acid leaching | 85.81 | 79.71 | <1 |
| Total leaching | 85.81 | 93.37 | <1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Tian, H.; Wang, Z. Leaching Process of Rare Earth Elements, Gallium and Niobium in a Coal-Bearing Strata-Hosted Rare Metal Deposit—A Case Study from the Late Permian Tuff in the Zhongliangshan Mine, Chongqing. Metals 2017, 7, 174. https://doi.org/10.3390/met7050174
Zou J, Tian H, Wang Z. Leaching Process of Rare Earth Elements, Gallium and Niobium in a Coal-Bearing Strata-Hosted Rare Metal Deposit—A Case Study from the Late Permian Tuff in the Zhongliangshan Mine, Chongqing. Metals. 2017; 7(5):174. https://doi.org/10.3390/met7050174
Chicago/Turabian StyleZou, Jianhua, Heming Tian, and Zhen Wang. 2017. "Leaching Process of Rare Earth Elements, Gallium and Niobium in a Coal-Bearing Strata-Hosted Rare Metal Deposit—A Case Study from the Late Permian Tuff in the Zhongliangshan Mine, Chongqing" Metals 7, no. 5: 174. https://doi.org/10.3390/met7050174
APA StyleZou, J., Tian, H., & Wang, Z. (2017). Leaching Process of Rare Earth Elements, Gallium and Niobium in a Coal-Bearing Strata-Hosted Rare Metal Deposit—A Case Study from the Late Permian Tuff in the Zhongliangshan Mine, Chongqing. Metals, 7(5), 174. https://doi.org/10.3390/met7050174






