1. Introduction
Niobium pentoxide is an important oxide with potential applications in several fields, including Electrochromism and Catalysis [
1,
2]. It is considered as a catalyst material, being used to prepare some crystals with technological applications such as KNbO
3 and NaNbO
3 [
3]. The Nb
2O
5 is not a toxic compound so it can be used as a water decontaminator. This oxide has advantages in its photocatalytic performance because, during the synthesis process, it is possible to control variables such as structure and morphology. From the technological point of view, the niobium pentoxide is of remarkable importance because it presents energy absorption in the ultraviolet region, whereby this semiconductor can be used to protect materials from that radiation. Nb
2O
5 presents a series of structural phases (trigonal, hexagonal, monoclinic or orthorhombic) [
4] and consequently, it can present different physical characteristics such as a semiconductor or as a dielectric material, depending on the symmetry. Regarding these phases, many studies have demonstrated how it is possible to obtain a certain phase from a previous one. For instance, it is known that the trigonal L-Nb
2O
5Nb phase turns into a monoclinic high-temperature phase H-Nb
2O
5 close to 900 °C [
4]. Also, by heating niobium pentoxide at constant pressure, the sequence of phase transitions H-Nb
2O
5 → B-Nb
2O
5 → T-Nb
2O
5 is observed [
5] and, at a constant temperature, it was demonstrated that B-Nb
2O
5 and T-Nb
2O
5 constitute high pressure polymorphs of Nb
2O
5 [
6]. Interestingly, it is believed that many of the existent phases should be metastable or, at least, stabilized by impurities [
5]. A suitable combination of chemical, electronic and optical properties make this oxide useful in various applications [
7,
8,
9,
10]. However, this result is further needed in order to investigate the photoluminescence (PL) because the optical measurements provide important information about the electronic structure of niobium pentoxide [
11,
12,
13].
Among several physical properties, photoluminescence is important because it allows us to characterize a variety of material parameters. The PL emission produces information on the lifetime of the excited states, the identification of the surface interface and the impurity levels. It can also provide simultaneous information on intrinsic and extrinsic defects [
14,
15]. The niobate luminescence has been studied in crystalline materials and is strongly dependent upon the crystal structure [
16]. The most efficient luminescence occurs in niobyl groups as in the Nb-O group with a short bond distance, of approximately 0.17 nm [
17]. Structurally isolated NbO
6 octahedra are not efficient luminescent centers [
18], whereas isolated distorted niobate groups, such as in LaNbO
4, where the Nb(V) has a 4 + 2 coordination, provide very efficient centers. The closer the Nb-O-Nb angles are to 180 the more pronounced the effects of the NbO
6 condensation become [
16,
17,
19,
20,
21].
In this paper, we study the synthesis of the T-form of Nb2O5 through both the sol-gel and the Pechini methods, and analyze some aspects of the materials such as their morphology through scanning electron microscopy (SEM), photoluminescence (PL) and the behavior of samples under high pressure conditions.
2. Materials and Methods
The samples were obtained through both the sol-gel and the Pechini methods. The starting precursors were niobium (V) ethoxide, (Nb(OC
2H
5)
5 99.999% Alfa Aesar) and niobium clorure (NbCl
5 99% Sigma-Aldrich). The niobium pentoxide nanostructure materials obtained were calcined at 500 °C, 650 °C and 750 °C for 2 h [
19,
20].
On one hand, the XRD patterns of the samples synthesized through the Pechini method were obtained using an Xpert-PRO Panalytical diffractometer (Co K radiation, = 1.78901 Å, UPTC, Tunja, Colombia), operating at 40 mA. A step of 0.0133° in 1 min, in the 2 angle range of 10–90° was used. On the other hand, the XRD patterns of the samples synthesized by the sol-gel method were obtained using The Panalytical PW3373 equipment (Cu K radiation, = 1.540558 Å, Universidad de Antioquia, Medellin, Colombia), operating at 40 mA with s step of 0.05° in 50 s, in the 2 angle range of 5–80° was used. A field emission gun-scanning electron microscope (FEG-SEM JOEOL JSM 6701F, Universidad Nacional de Colombia, Bogota, Columbia) operating at 5.0 kV was used to verify the material morphology.
The PL measurements were performed with a monospec 27 monochromator (Thermal Jarrel AS, Franklin, MA, USA) coupled to a R446 photomultiplier (Hamamatsu photonics, Shizuoka, Japan). A Krypton ion laser (Coherent Innova 90 K, Silicon Valley, CA, USA) ( = 350 nm) was used as excitation source. Raman scattering spectra were obtained in the backscattering geometry with a Jobin-Yvon-T64000 spectrometer, coupled to a N2 cooled CCD device, using as excitation the 532 nm laser line of a Verdi laser, operating at 160 mW. In order for the Raman to reach a high pressure a National Bureau of Standards diamond anvil cell was used, having as compression medium an ethanol-methanol mixture (1:4) with a stainless steel gasket with 210 m thickness preindented to approximately 70 m for both samples investigated. The pressure was calibrated through the luminescence of ruby (Al2O3:Cr3+) through the R1 and R2 lines.
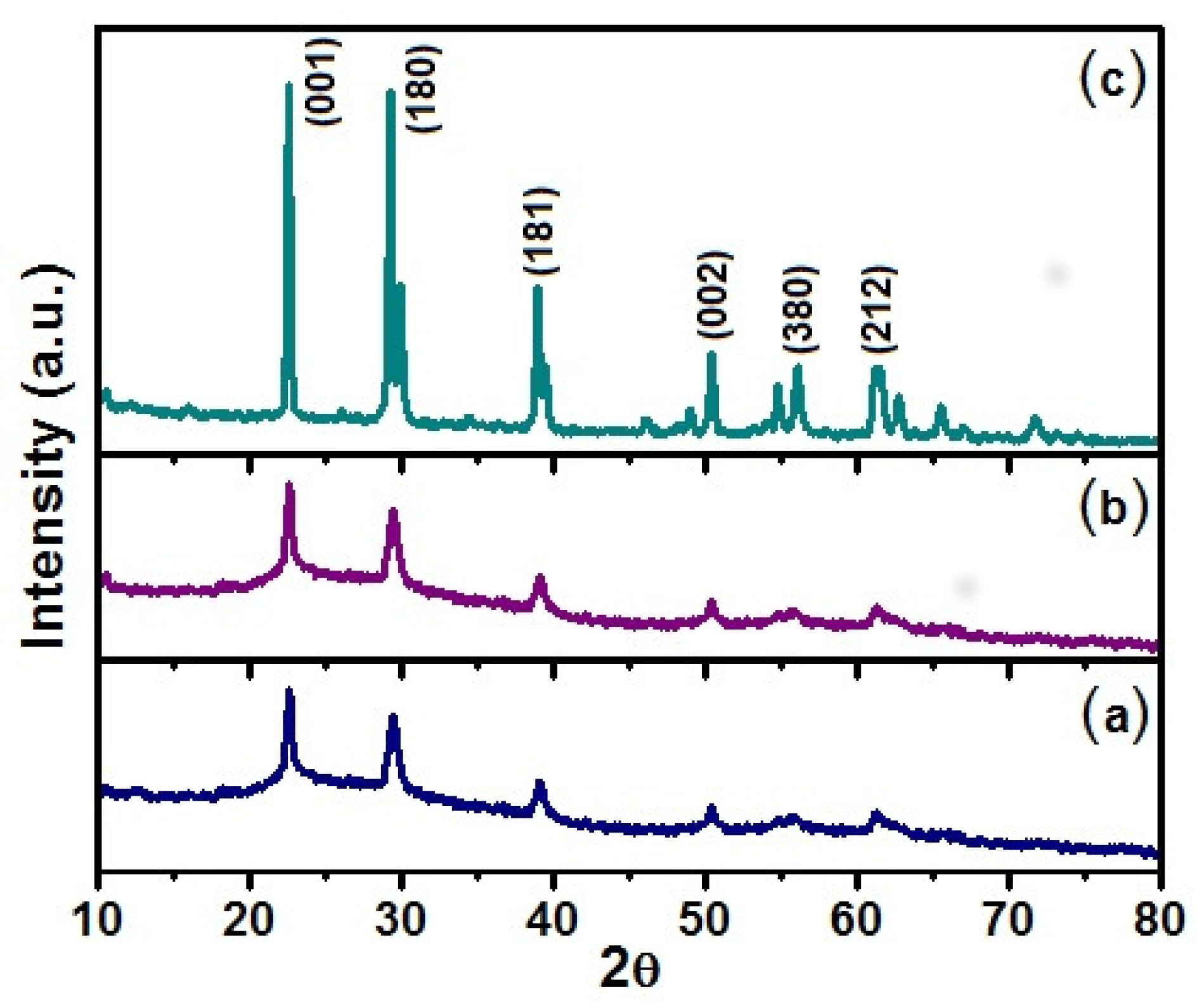
The X-ray diffractograms of
Figure 1 were recorded from solids obtained through the Pechini method submitted to three different thermal treatments: 500 °C, 650 °C and 750 °C. The (a) and (b) XRD patterns indicate that for the 500 °C and 650 °C treatments, the solids crystallize in a hexagonal structure with resolved peaks at: 2
= 22.6°, 28.5°, 36.7°, 46.2° and 56.1°, as reported in JCPDF028-0317. In the case (c), where the sample was heated up to 750 °C, it is found that the solid crystallizes in an orthorhombic structure with resolved peaks at: 2
= 22.6°, 28.8°, 37.1°, 46.0°, 50.9° and 56.3°, according to JCPDF027-1313. The orthorhombic structure, which we are particularly interested in due to its potential application, is composed by Nb atoms surrounded by oxygen atoms forming distorted octahedra or pentagonal bipyramids.
Figure 2 shows the XRD patterns of Nb
2O
5 obtained through the sol-gel method at (a) 650 °C and (b) 750 °C where the 2
peaks are compatible with orthorhombic structures. In other words, the diffractogram peaks from the samples obtained by sol-gel method are similar to those samples obtained by the Pechini method at 750 °C. In the synthesis of the two mentioned routes, Ammonia (NH
4OH) was used in the process of obtaining the samples.
3. Results and Discussion
Figure 3 shows the SEM images of niobium pentoxide synthesized at
T = 650 °C using NH
4OH through (a) sol-gel and (b) Pechini methods. The bar in
Figure 3a represents 1
m, while the bar in
Figure 3b represents 100 nm. We observe that the grains in the two figures have these dimensions as their characteristics. So, it is evident that the use of the Pechini method allows to synthesize samples with smaller particle sizes. In order to compare the materials synthesized at 650 °C and at 750 °C.
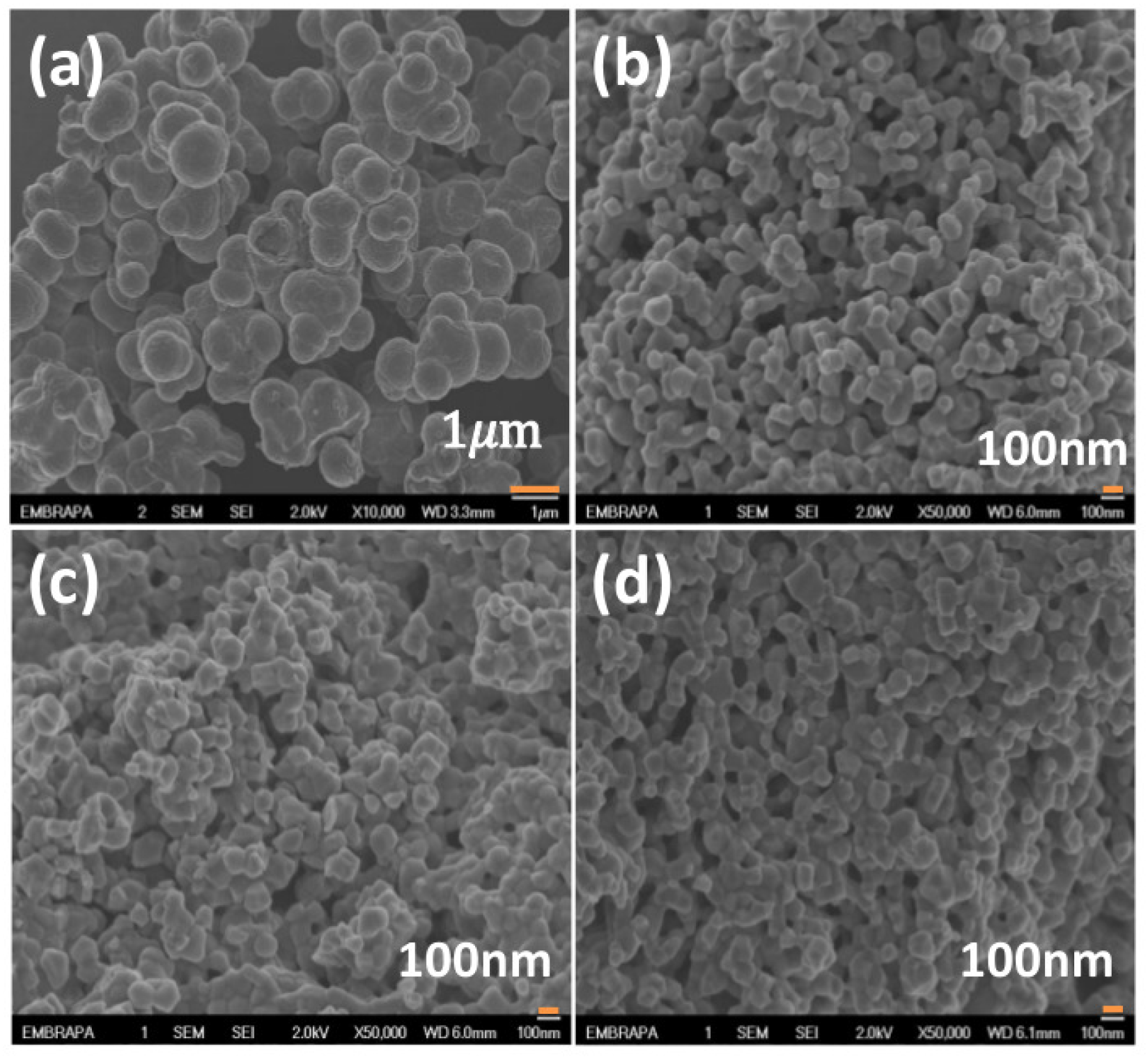
Figure 4 shows the SEM images of samples of Nb
2O
5 produced by different conditions at
T = 750 °C: (a) sol-gel, (b) Pechini (NH
4OH), (c) Pechini (HCl—hydrochloric acid) and (d) Pechini (HNO
3—nitric acid). From these images, the morphology of the particles can be observed. In
Figure 4, all the samples belong to the T-polymorph of niobium pentoxide. Again, the bar in
Figure 4a represents 1
m, while the bars in
Figure 4b–d represent 100 nm. Clearly the samples produced by the sol-gel method (NH
4OH) have higher particle sizes than those synthesized by the Pechini method for both temperatures (650 °C and 750 °C).
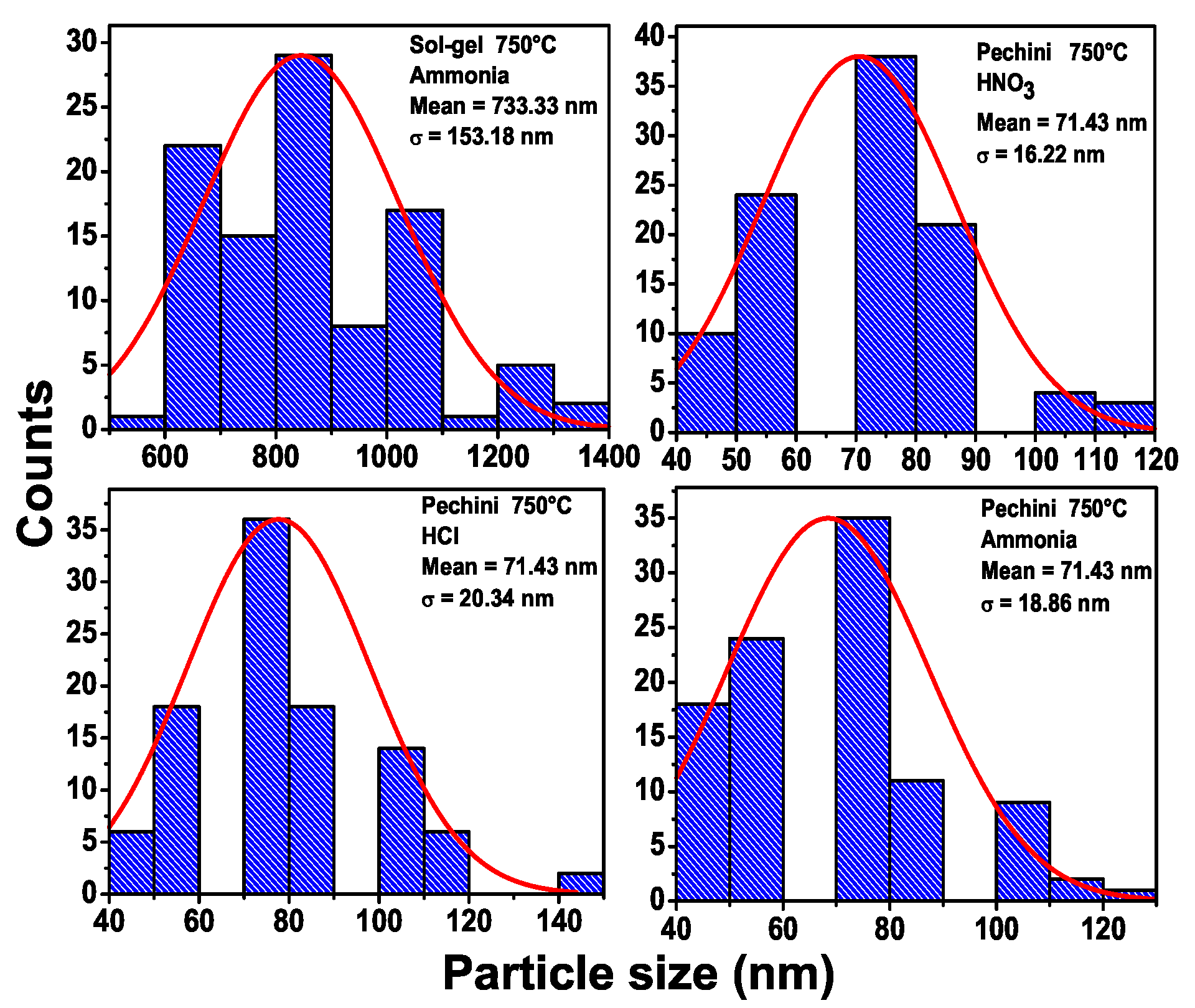
Figure 5 presents the number of particles vs. the size of the particles for several samples calcined at 750 °C for 2 h: (a) Sol-gel (NH
4OH) the average size is 733.3 nm and (b) Pechini (HNO
3), (c) Pechini (HCl) and (d) Pechini (NH
4OH). For the Pechini method, the average size was 71.4 nm. This shows that the Pechini method must be used to obtain samples with smaller particle sizes. In the
Supplementary Material, we present the number of particles as function of the size of particles for the samples calcined at 650 °C. Again, it is possible to note that the Pechini method produces samples with smaller particle sizes 40 nm, while the sol-gel method produces samples with a size of 733.3 nm. The particle size through the Pechini method is similar to the reported in the work of Yun et al. [
21].
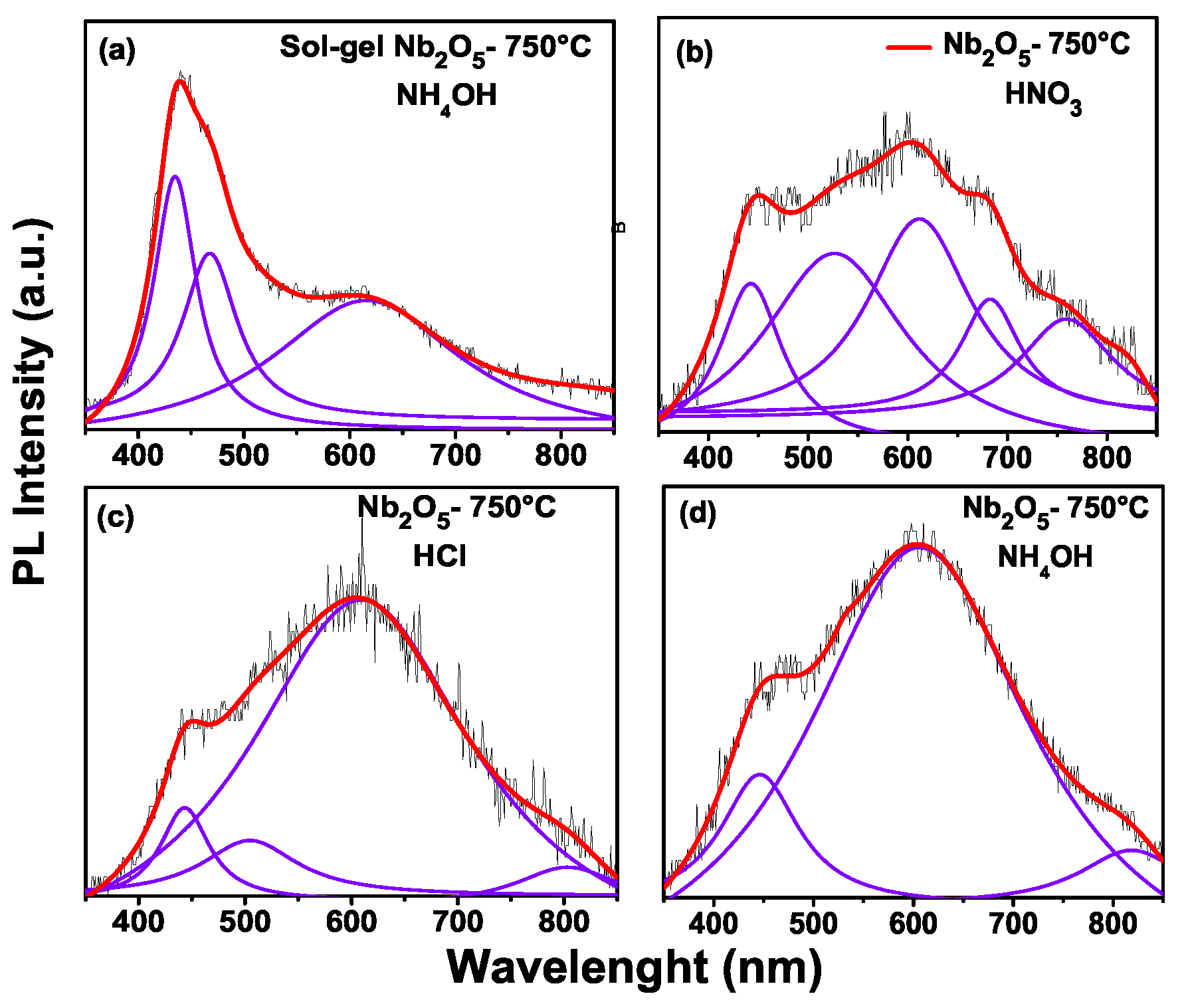
In photoluminescence, a change is observed with the increasing calcination temperature of the samples.
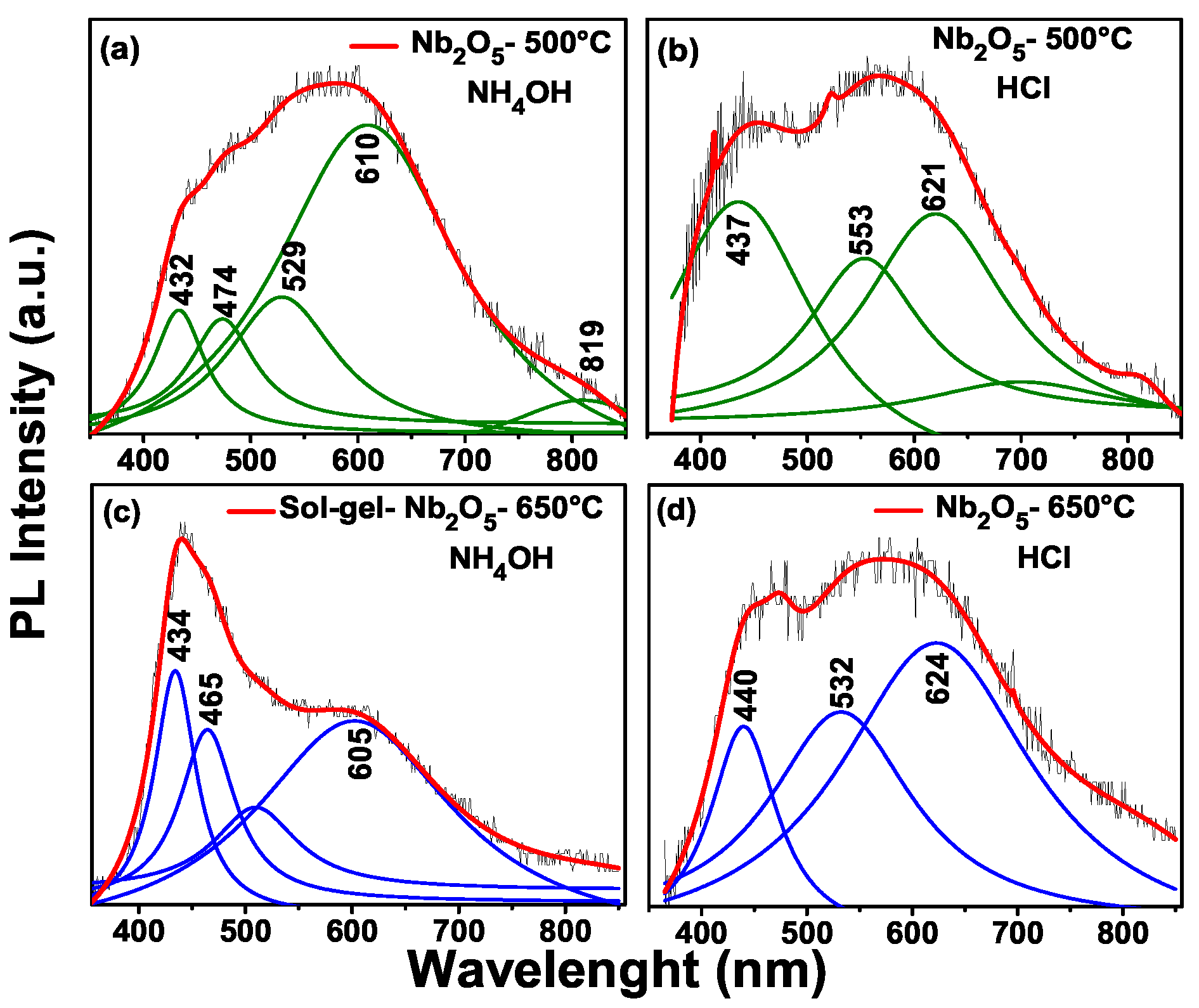
Figure 6 shows photoluminescence of: (a) sample to 500 °C through the pechini method with addition of NH
4OH. Here the peaks with an energy of 432 nm (2.87 eV), 474 nm (2.61 eV), 529 nm (2.34 eV), 610 nm (2.0 eV) and 819 nm (1.5 eV) are observed.
Figure 6b sample to 500 °C through the Pechini method with addition of HCl, with peaks at around of 432 nm (2.83 eV), 553 nm (2.24 eV) and 621 nm (2.0 eV). Therefore, in
Figure 6c for the sol-gel method with temperature at 650 °C, we have peaks at the following positions: 434 nm (2.8 eV), 465 nm (2.67 ev) and 605 nm ( 2.0 eV). Finally,
Figure 6d shows the photoluminescence for the Pechini (HCl) sample at 650 °C. The peak positions are: 440 nm (2.81 eV), 532 nm (2.33 eV) and 624 nm (1.98 eV). According to the work of Xiaofeng et al. [
22], the niobium oxides of low valence are oxidized with the annealing temperature, so the content of Nb
2O
5 is increased. In this Figure, the sol-gel method which has the maximum emission is in blue, with the Pechini being the orange, green and blue regions.
In
Figure 7a the photoluminescence for the sol-gel sample is presented, with the positions of peaks at: 435 nm (2.85 eV), 469 nm (2.64 eV) and 621 nm (1.99 eV). In
Figure 7b the highest intensity is in the peak position 612 nm (2.0 eV). The sample with an addition of HCl in the synthesis through the Pechini method is shown in
Figure 7d, with the highest peak being at 607 nm (2.0 eV). The ultraviolet emission band is at about 40 nm and some authors [
23] have attributed this to the near-bandgap emission from Nb
2O
5. The other intensity band is ascribed to structural defects such as distorted NbO
6 octahedral groups. The differences between the sol-gel and Pechini methods are the peak positions and the intensity of the photoluminescence. For the sol-gel method, the maximum intensity of the peak is related to the near band gap emission; however, for the Pechini method, the highest peak is related to the distortion of the octahedral groups. This can be seen in
Figure 6 and
Figure 7. In other words, for the sol-gel method, the maximum emission is shown in the violet wavelength (2.85 eV) and the maximum emission of Pechini is shown in orange (2.0 eV).
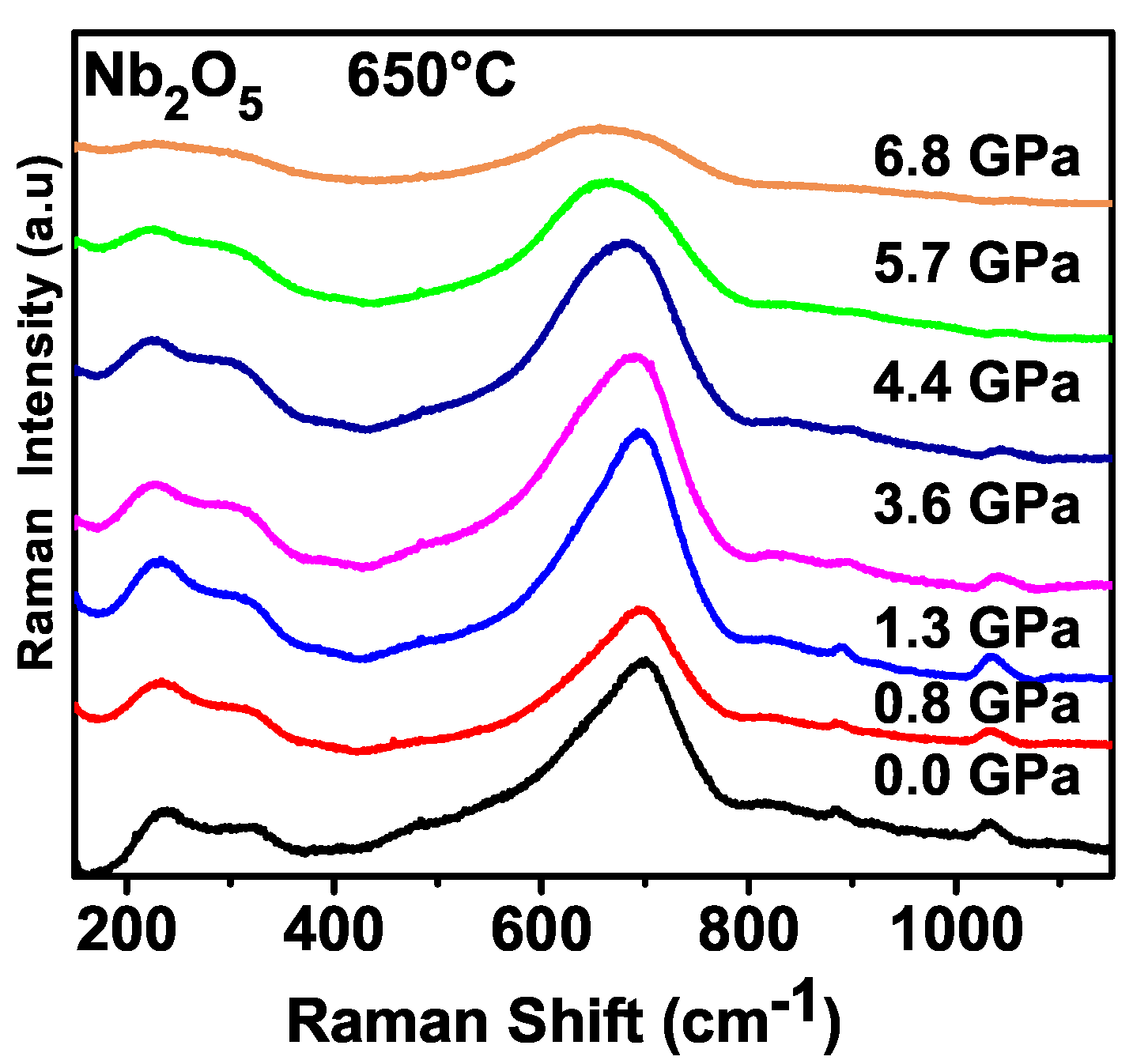
Figure 8 shows the Raman spectra of Nb
2O
5 obtained by the sol-gel method at 650 °C (NH
4OH) at several pressures recorded while at room temperature. All the pressure measurements started at ambient pressure, observing the following bands: Bands observed between 200 and 550 cm
−1 are assigned as bending of NbO
6 while the broad band close to 700 cm
−1 could be associated with stretching modes of NbO
6 [
24]. Moreover, an assignment was done for the H-Nb
2O
5 polymorph, where bands between 204 and 549 cm
−1 were assigned as bending vibrations of NbO
6. Bands between 615 and 995 cm
−1 were assigned as Nb–O stretching of NbO
6. Although the symmetry of the sample studied in this work, corresponding to the T-polymorph, is different from the symmetry of the work of Reference [
24], we can understand that the bending and stretching vibrations in the orthorhombic polymorph must be in the same spectral range.
With increased pressure, we notice some modifications in the Raman spectrum. For example, the bands observed between 200 and 400 cm
−1 maintain their intensities up to approximately 4.4 GPa but above this pressure, the intensities decrease. The bands observed between 200 and 400 cm
−1, as occurs with the other bands in the Raman spectrum of niobium pentoxide, maintain intensity up to 4.4 GPa. After this pressure, the value of intensity of the bands diminishes in such a way that, in the spectrum recorded at 6.8 GPa, the peaks have very small intensities. The decreasing of intensity of the Raman bands is a point that deserves a brief discussion. Moreover, in general terms, when a material is submitted to compression, its Raman bands decrease in intensity and the linewidth increases. This is understood mainly as a consequence of the disorder introduced by the compression [
25,
26]. So, we can understand that some disorder is being introduced in the sample due to the compression. Additionally, we observe that the frequency of the stretching mode of Nb–O diminishes in compression, i.e., the energy of this mode presents a red shift. The softening of the stretching vibration Nb–O can be interpreted as a strengthening of interactions between NbO
6 octahedra. We have also obtained the pressure Raman spectra of Nb
2O
5 synthesized by the sol-gel method at 750 °C (NH
4OH); the same behavior of the sample obtained at 650 °C is observed (See
Supplementary Material).
Although we observed evidence of disorder produced by the application of pressure, there is no evidence that the material had undergone any kind of phase transition, and we can understand that the orthorhombic phase of the sample is stable, up to the highest pressure reached in our experiments. Note that a transition H-Nb
2O
5 → T-Nb
2O
5 was previously observed at 0.5 GPa (although the temperature necessary for the occurrence of this transition is higher than 1000 °C as shown in the work of Reference [
5]). To our knowledge, the present study is the first high pressure investigation of the T-polymorph of Nb
2O
5. Up to the highest pressure at which it was possible to record the Raman spectrum, the material seems to be stable.