Structure and Physical Properties of NiO/Co3O4 Nanoparticles
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Mechanism of the Formation of Nanoparticles
3.2. Degree of Crystallization, Morphology and Phase Composition of Nickel-Cobalt Oxide Nanoparticles
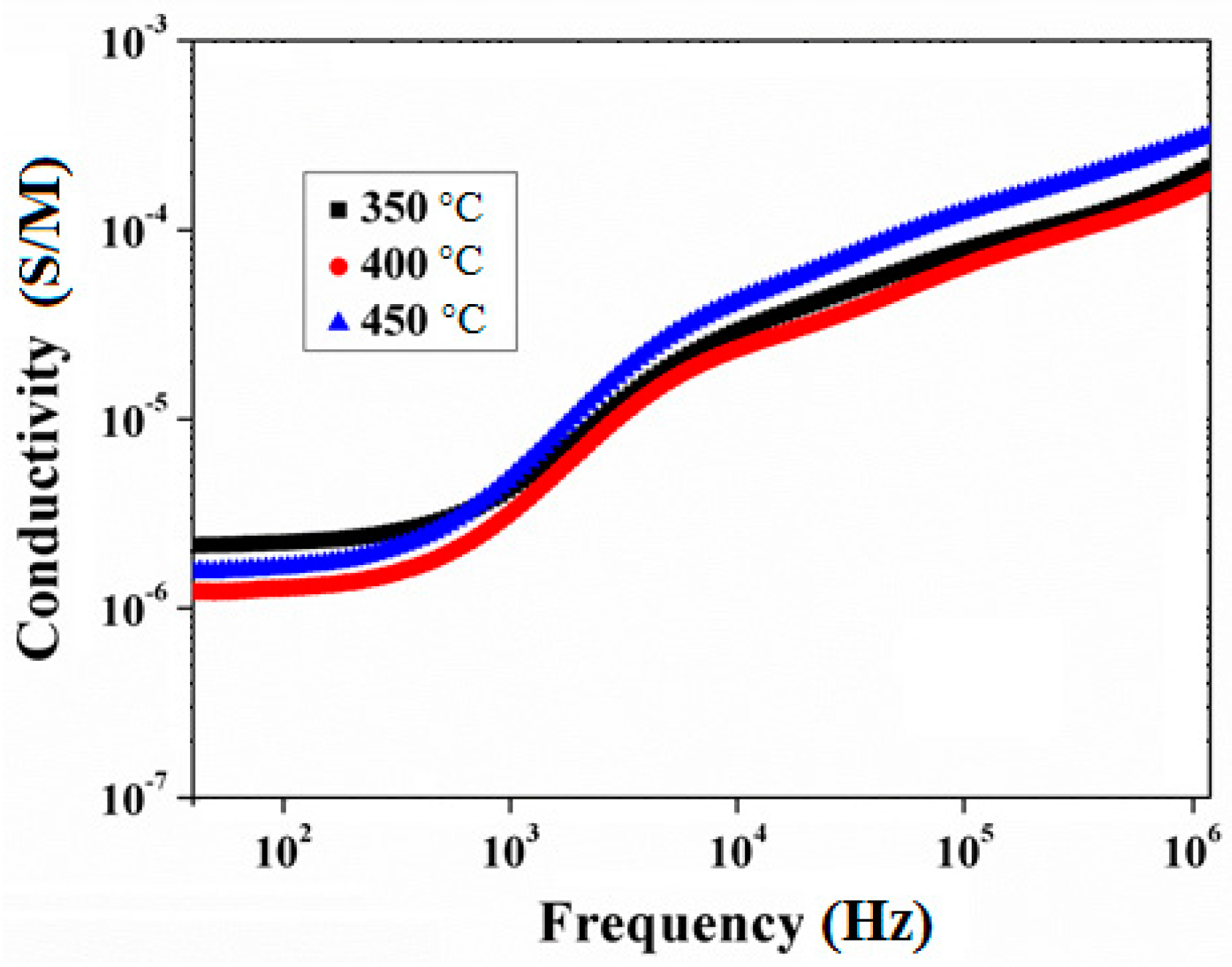
3.3. Electric and Dielectric Properties of Nickel-Cobalt Oxide Nanoparticles
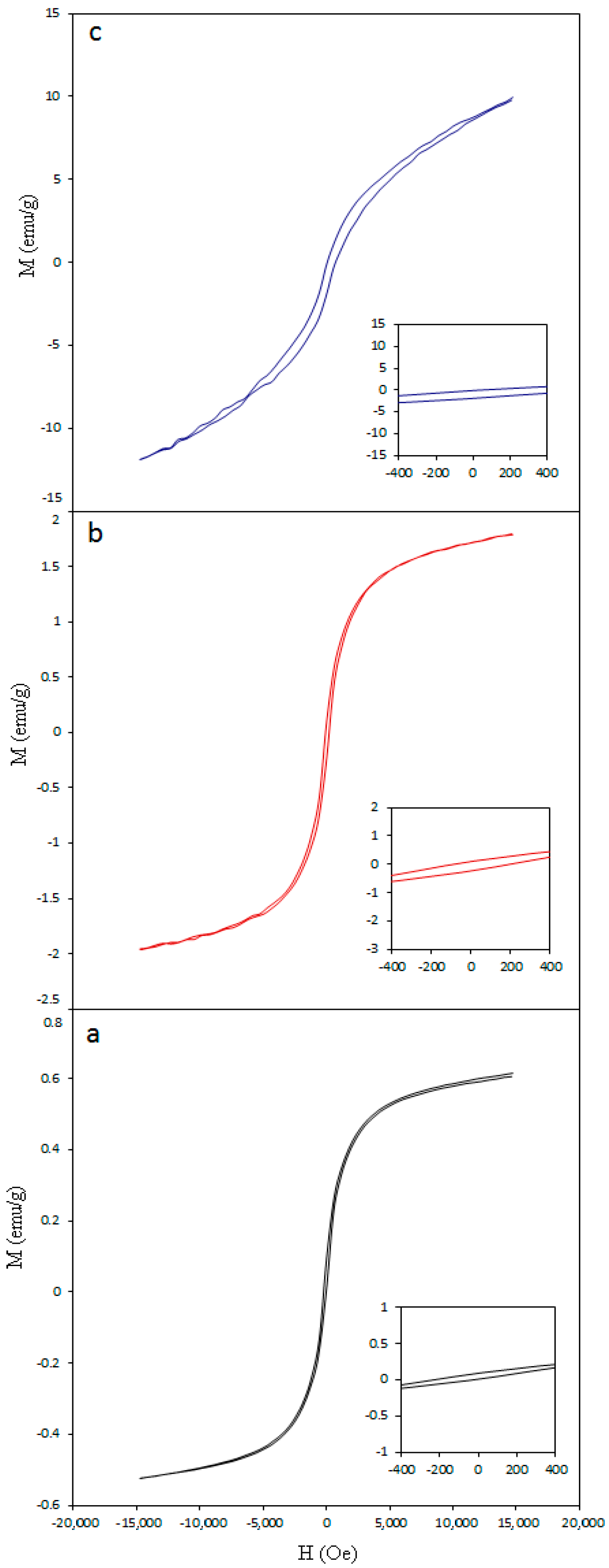
3.4. Magnetic Properties of Nickel-Cobalt Oxide Nanoparticles
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Li, Q.; Wang, L.S.; Hu, B.Y.; Yang, C.; Zhou, L.; Zhang, L. Preparation and characterization of NiO nanoparticles through calcination of malate gel. Mater. Lett. 2007, 61, 1615–1618. [Google Scholar] [CrossRef]
- Xin, X.; Zhe, L.; Zhou, B.; Huang, X.; Zhu, R.; Sha, X.; Zhang, Y.; Su, W. Effect of synthesis conditions on the performance of weakly agglomerated nanocrystalline NiO. J. Alloy. Compd. 2007, 427, 251–255. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chen, W.C.; Chang, K.H. How to achieve maximum utilization of hydrous ruthenium oxide for supercapacitors. J. Electrochem. Soc. 2004, 151, A281–A290. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Gibot, P.; Vidal, L. Original synthesis of chromium (III) oxide nanoparticles. J. Eur. Ceram. Soc. 2010, 30, 911–915. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Z.; Su, C.; Zhou, N.; Zhu, X.; He, H.; Zhang, C. Solvothermal synthesis of nanosheet-like lithium manganese phosphate cathode material with the improved electrochemical performance. Mater. Res. Express 2015, 2, 035004. [Google Scholar] [CrossRef]
- Kim, G.P.; Nam, I.; Park, S.; Park, J.; Yi, J. Preparation via an electrochemical method of graphene films coated on both sides with NiO nanoparticles for use as high-performance lithium ion anodes. Nanotechnology 2013, 24, 475402. [Google Scholar] [CrossRef] [PubMed]
- Rajjab, A. Structural, magnetic and dielectric behavior of Mg1−xCaxNiyFe2−yO4 nano-ferrites synthesized by the micro-emulsion method. Ceram. Int. 2014, 40, 3841–3846. [Google Scholar]
- Rashad, M.M.; Mohamed, R.M.; Ibrahim, M.A.; Ismail, L.F.M.; Abdel-Aal, E.A. Magnetic and catalytic properties of cubic copper ferrite nanopowders synthesized from secondary resources. Adv. Powder Technol. 2012, 23, 315–323. [Google Scholar] [CrossRef]
- Medeiros, A.M.L.; Miranda, M.A.R.; de Menezes, A.S.; Jardim, P.M.; da Silva, L.R.D.; Gouveia, S.T.; Sasak, J.M. Synthesis and Characterization of Cr2O3 Nanoparticles Obtained by Gelatin. J. Metastable Nanocrystalline Mater. 2004, 20–21, 399–406. [Google Scholar] [CrossRef]
- Mazarío, E.; Herrasti, P.; Morales, M.P.; Menéndez, N. Synthesis and characterization of CoFe2O4 ferrite nanoparticles obtained by an electrochemical method. Nanotechnology 2012, 23, 355708–355713. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.; Brogfeld, N.; Kim, J.; Parsons, J.G. Study of the thermodynamics of chromium(III) and chromium(VI) binding to iron(II/III) oxide or magnetite or ferrite and manganese(II) iron(III) oxide or jacob site or manganese ferrite nanoparticles. J. Colloid Interface Sci. 2013, 400, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Song, D.; Jiang, J.; Ma, A.; Zhang, L.; Cheng, L. Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy. Metals 2016, 6, 44. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Scudino, S.; Khoshkhoo, M.S.; Prashanth, K.G.; Mukhopadhyay, N.K.; Mishra, B.K.; Eckert, J. Synthesis and Characterization of NanocrystallineMg-7.4%Al Powders Produced by Mechanical Alloying. Metals 2013, 3, 58–68. [Google Scholar] [CrossRef]
- Vasoya, N.H.; Vanpariya, L.H.; Sakariya, P.N.; Timbadiya, M.D.; Pathak, T.K.; Lakhani, V.K.; Modi, K.B. Synthesis of nanostructured material by mechanical milling and study on structural property modifications in Ni0.5Zn0.5Fe2O4. Ceram. Int. 2010, 36, 947–954. [Google Scholar] [CrossRef]
- Mohamed, H.; Naseri, M.G.; Sadrolhosseini, A.R.; Dehzangi, A.; Kamalianfar, A.; Saion, E.B.; Zamiri, R.; Ahangar, H.A.; Majlis, B.Y. Silver Nanoparticle Fabrication by Laser Ablation in Polyvinyl Alcohol Solutions. Chin. Phys. Lett. 2014, 31, 077803. [Google Scholar] [CrossRef]
- Naseri, M.; Ghasemi, R. Structure and physical properties of Fe6O8/baFe6O11 nanostructure. J. Magn. Magn. Mater. 2016, 406, 200–206. [Google Scholar] [CrossRef]
- Che, S.; Wang, J.; Chen, Q. Soft magnetic nanoparticles of BaFe12O19 fabricated under mild conditions. J. Phys. Condens. Mater. 2003, 15, L335–L339. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Naseri, M.G.; Halimah, M.K.; Dehzangi, A.; Kamalianfar, A.; Saion, E.B.; Majlis, B.Y. A comprehensive overview on the structure and comparison of magnetic properties of nanocrystalline synthesized by a thermal treatment method. J. Phys. Chem. Solids 2014, 75, 315–327. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiFe2O4 nanosheet via polymer assisted co-precipitation method. Mater. Lett. 2011, 65, 483–485. [Google Scholar] [CrossRef]
- Koebel, M.M.; Jones, L.C.; Somorjai, G.A. Preparation of size-tunable, highly monodisperse PVP-protected Pt-nanoparticles by seed-mediated growth. J. Nanopart. Res. 2008, 10, 1063–1069. [Google Scholar] [CrossRef]
- Roosen, A.R.; Carter, W.C. Simulations of microstructural evolution: Anisotropic growth and coarsening. Phys. A Stat. Mech. Appl. 1998, 261, 232–247. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: London, UK, 1978; p. 102. [Google Scholar]
- Naseri, M.G.; Saion, E.B.; Hashim, M.; Shaari, A.H.; Ahangard, H.A. Synthesis and characterization of zinc ferrite nanoparticles by a thermal treatment method. Solid State Commun. 2011, 151, 1031–1035. [Google Scholar] [CrossRef]
- Naseri, M. Optical and magnetic properties of monophasic cadmium ferrite (CdFe2O4) nanostructure prepared by thermal treatment method. J. Magn. Magn. Mater. 2015, 392, 107–113. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangard, H.A.; Hashim, M.; Shaari, A.H. Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2011, 323, 1745–1749. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangard, H.A.; Hashim, M.; Shaari, A.H. Simple preparation and characterization of nickel ferrite nanocrystals by a thermal treatment method. Powder Technol. 2011, 212, 80–88. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Shaari, A.H. Fabrication, characterization, and magnetic properties of copper ferrite nanoparticles prepared by a simple, thermal-treatment method. Mater. Res. Bull. 2013, 48, 1439–1446. [Google Scholar] [CrossRef]
- Naseri, M.G.; Ara, M.H.M.; Saion, E.B.; Shaari, A.H. Superparamagnetic magnesium ferrite nanoparticles fabricated by a simple, thermal-treatment method. J. Magn. Magn. Mater. 2014, 350, 141–147. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Khansari, A.; Davar, F. Synthesis and characterization of cobalt oxide nanoparticles by thermal treatment process. Inorg. Chim. Acta 2009, 362, 4937–4942. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Shaari, A.H.; Hashim, M. Simple synthesis and characterization of cobalt ferrite, nanoparticles by a thermal treatment method. J. Nano Mater. 2010, 2010, 1–8. [Google Scholar]
- Naseri, M.G.; Saion, E.B.; Zadeh, N.K. The amazing effects and role of PVP on the crystallinity, phase composition and morphology of nickel ferrite nanoparticles prepared by thermal treatment method. Int. Nano Lett. 2013, 3, 1–8. [Google Scholar]
- Naseri, M.G.; Kamari, H.M.; Dehzangi, A.; Kamalianfar, A.; Saion, E.B. Fabrication of a novel chromium-iron oxide (Cr2Fe6O12) nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2015, 389, 113–119. [Google Scholar] [CrossRef]
- Kamari, H.M.; Naseri, M.G.; Saion, E.B. A Novel Research on Behavior of Zinc Ferrite Nanoparticles in Different Concentration of Poly(vinyl pyrrolidone) (PVP). Metals 2014, 4, 118–129. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1996; Volume 7. [Google Scholar]
- Chisca, S.; Musteata, V.E.; Sava, I.; Bruma, M. Dielectric behavior of some aromatic polyimide films. Eur. Polym. J. 2011, 47, 1186–1197. [Google Scholar] [CrossRef]
- Soltani, N.; Dehzangi, A.; Saion, E.; Majlis, M.Y.; Zare, M.R.; Kharazmi, A.; Navasery, M. Influence of exposure time on structural, optical and electrical properties of zinc sulphide nanoparticles synthesized by microwave technique. Chalcogenide Lett. 2013, 10, 27–37. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341. [Google Scholar] [CrossRef]
- Tripathi, R.; Kumar, A.; Sinha, T.P. Dielectric properties of CdS nanoparticles synthesized by soft chemical route. Pramana 2009, 72, 969–978. [Google Scholar] [CrossRef]
- Wan, W.; Yu, D.; Xie, Y.; Guo, X.; Mao, Z.; Huang, L. Effects of Nanoparticle Surface Treatment on the Crystalline Morphology and Dielectric Property of Polypropylene/Calcium Carbonate Nanocomposites. In Proceedings of the 1st IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Zhuhai, China, 18–21 January 2006; pp. 387–390.
- Jonscher, A.K. The universal dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Dutta, P.; Biswas, S.; de, S.K. Dielectric relaxation in polyaniline-polyvinyl alcohol composites. Mater. Res. Bull. 2002, 37, 193–200. [Google Scholar] [CrossRef]
- Soltani, N.; Dehzangi, A.; Kharazmi, A.; Saion, E.; Yunus, W.M.; Majlis, B.Y.; Zare, M.R.; Gharibshahi, E.; Khalilzadeh, N. Structural, optical and electrical properties of ZnS nanoparticles affecting by organic coating. Chalcogenide Lett. 2014, 11, 79–9. [Google Scholar]
- Tharayil, N.J.; Sagar, S.; Raveendran, R.; Vaidyan, A.V. Dielectric studies of nanocrystalline nickel-cobalt oxide. Phys. B Condens. Matter 2007, 399, 1–8. [Google Scholar] [CrossRef]
- Kraus, M.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. CO2 reforming of methane by the combination of dielectric-barrier discharges and catalysis. Phys. Chem. Chem. Phys. 2001, 3, 294–300. [Google Scholar] [CrossRef]
- Laokul, P.; Amornkitbamrung, V.; Seraphin, S.; Maensiri, S. Characterizationand magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr. Appl. Phys. 2011, 11, 101–108. [Google Scholar] [CrossRef]



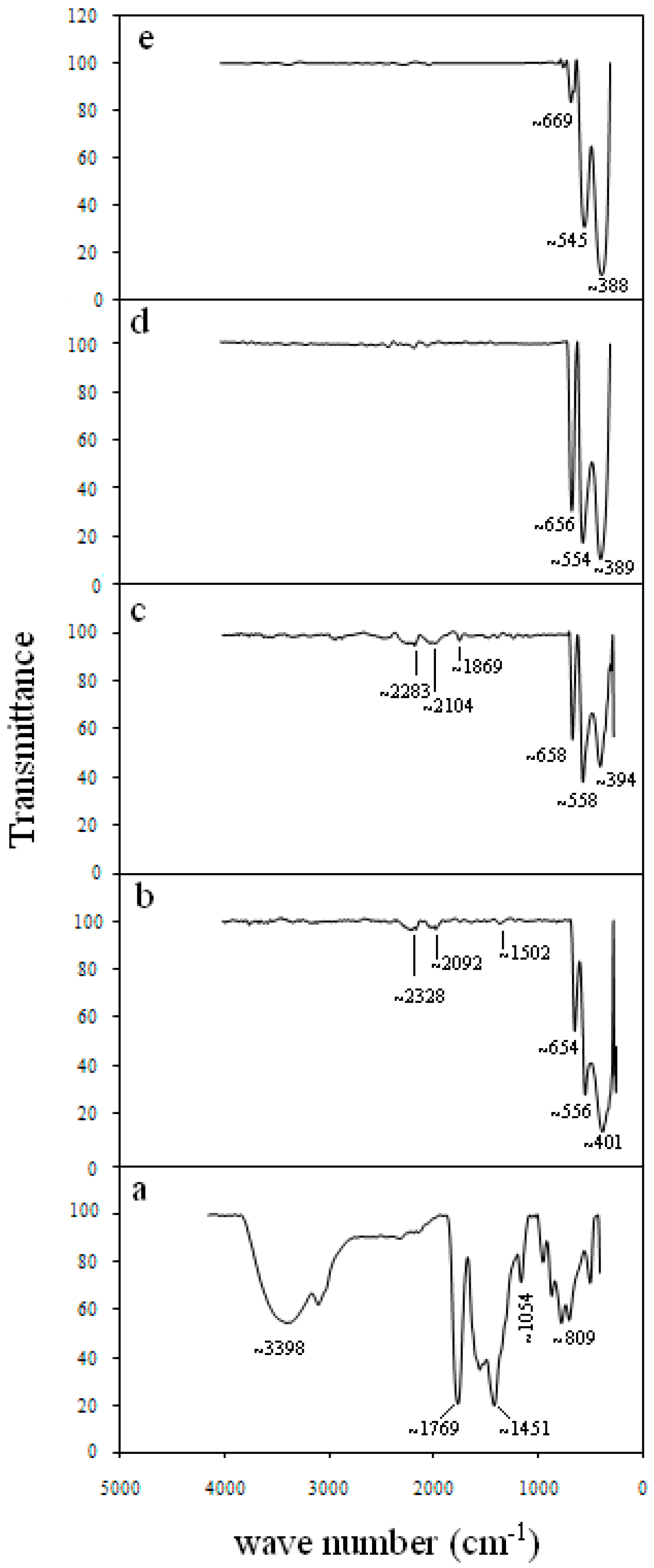


| Ni-Co Oxide Nanoparticles Calcined at (°C) | Average Particle Size XRD (nm) | Average Particle Size TEM (nm) | Wave Number (cm−1) | Saturation Magnetization Ms (emu/g) | Coercivity Field (Oe) | ||
|---|---|---|---|---|---|---|---|
| ν1 | ν2 | ν3 | |||||
| 350 | 14 | 11 ± 4 | 401 | 556 | 654 | 0.61 | 98 |
| 400 | 17 | 12 ± 4 | 394 | 558 | 658 | 1.79 | 157 |
| 450 | 18.5 | 19 ± 3.5 | 389 | 554 | 656 | 9.66 | 714 |
| 500 | 32 | 35 ± 9 | 388 | 545 | 669 | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri, M.; Dehzangi, A.; Kamari, H.M.; See, A.; Abedi, M.; Salasi, R.; Goli-Kand, A.N.; Dianat, P.; Larki, F.; Abedini, A.; et al. Structure and Physical Properties of NiO/Co3O4 Nanoparticles. Metals 2016, 6, 181. https://doi.org/10.3390/met6080181
Naseri M, Dehzangi A, Kamari HM, See A, Abedi M, Salasi R, Goli-Kand AN, Dianat P, Larki F, Abedini A, et al. Structure and Physical Properties of NiO/Co3O4 Nanoparticles. Metals. 2016; 6(8):181. https://doi.org/10.3390/met6080181
Chicago/Turabian StyleNaseri, Mahmoud, Arash Dehzangi, Halimah Mohamed Kamari, Alex See, Mina Abedi, Reza Salasi, Ahmad Nozad Goli-Kand, Pouya Dianat, Farhad Larki, Alam Abedini, and et al. 2016. "Structure and Physical Properties of NiO/Co3O4 Nanoparticles" Metals 6, no. 8: 181. https://doi.org/10.3390/met6080181
APA StyleNaseri, M., Dehzangi, A., Kamari, H. M., See, A., Abedi, M., Salasi, R., Goli-Kand, A. N., Dianat, P., Larki, F., Abedini, A., Hassan, J., Far, A. K., & Majlis, B. Y. (2016). Structure and Physical Properties of NiO/Co3O4 Nanoparticles. Metals, 6(8), 181. https://doi.org/10.3390/met6080181








