A Study of the Heat Transfer Behavior of Mold Fluxes with Different Amounts of Al2O3
Abstract
:1. Introduction
2. Materials and Methods
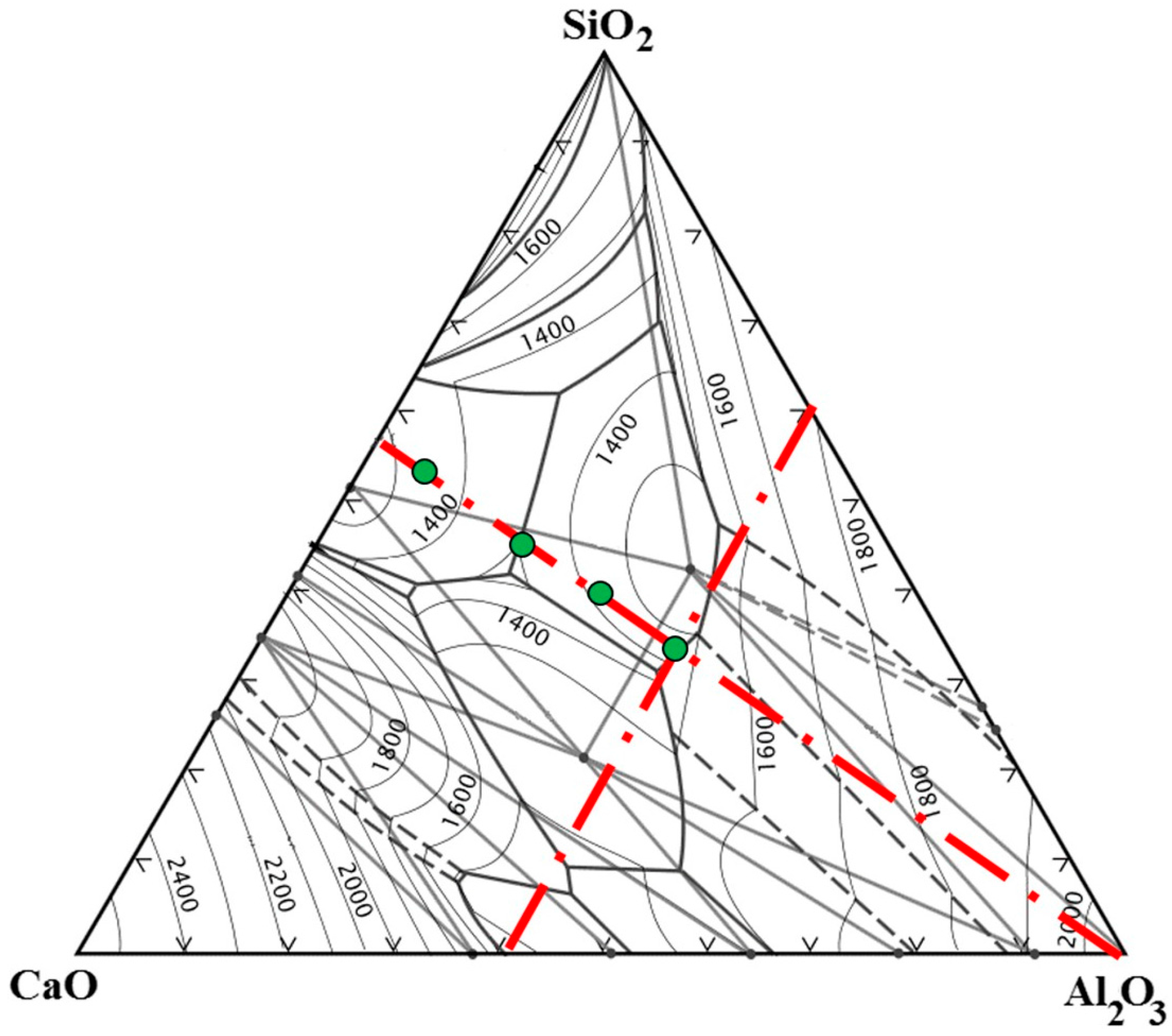
2.1. Experimental Slags
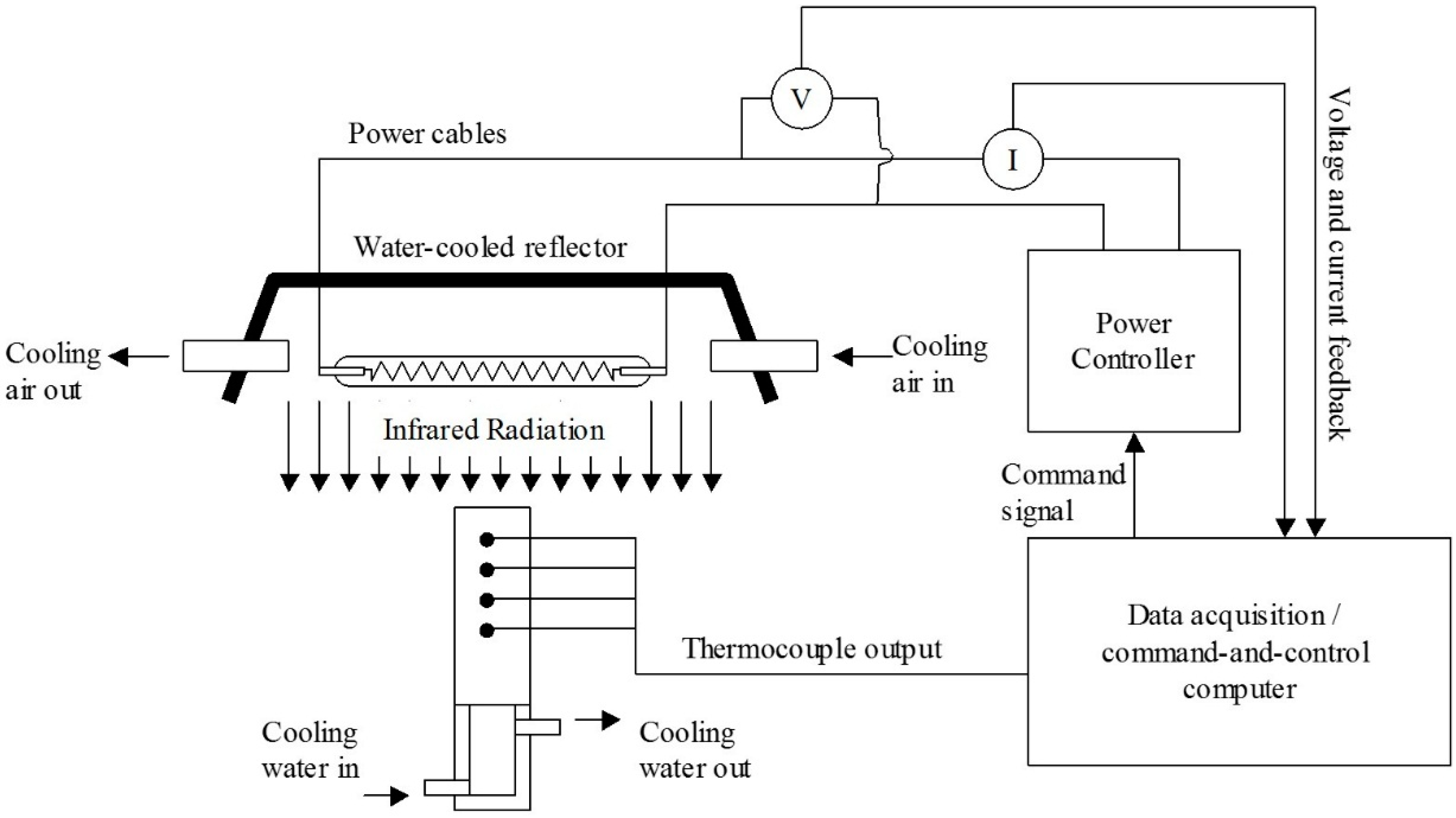
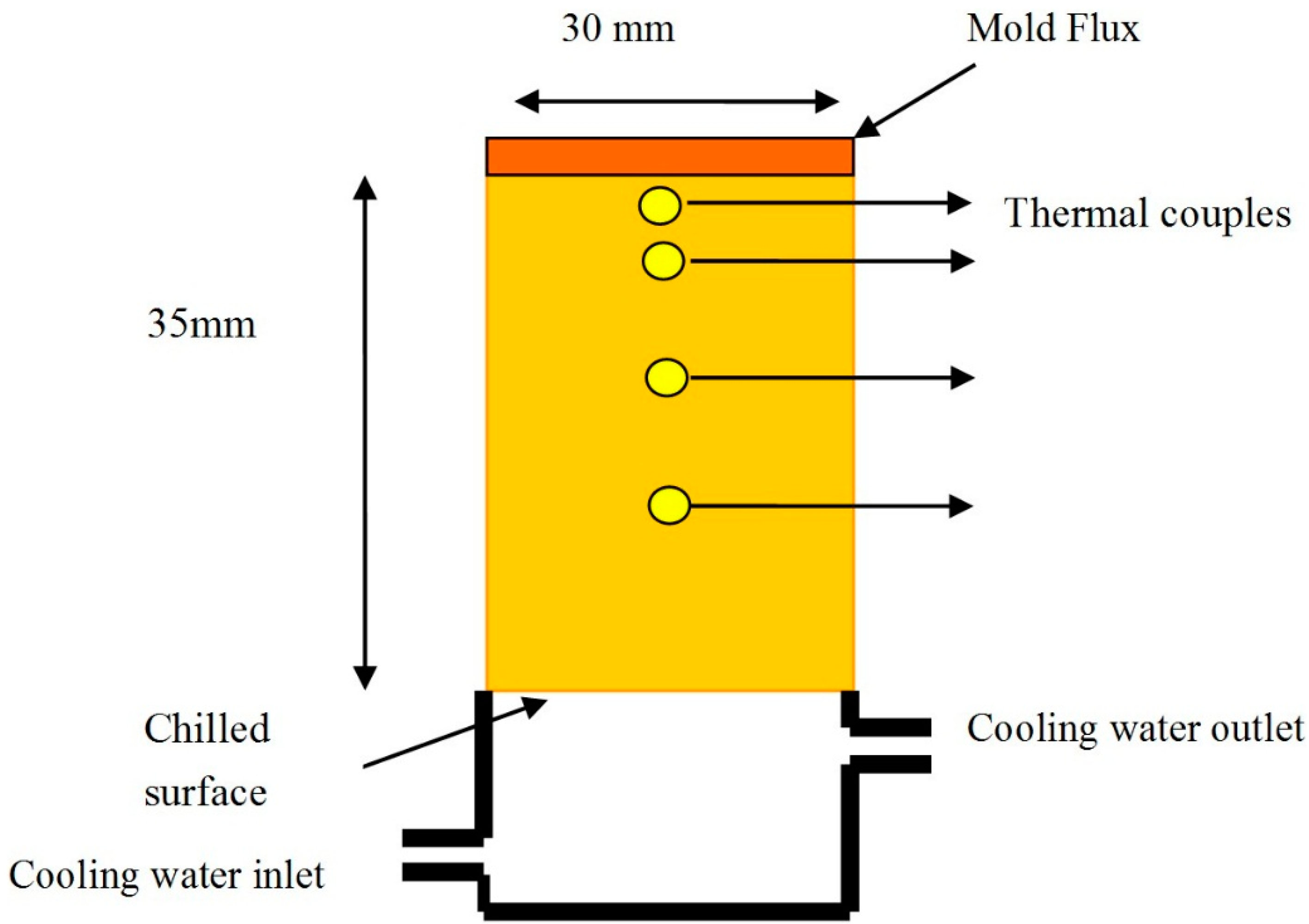
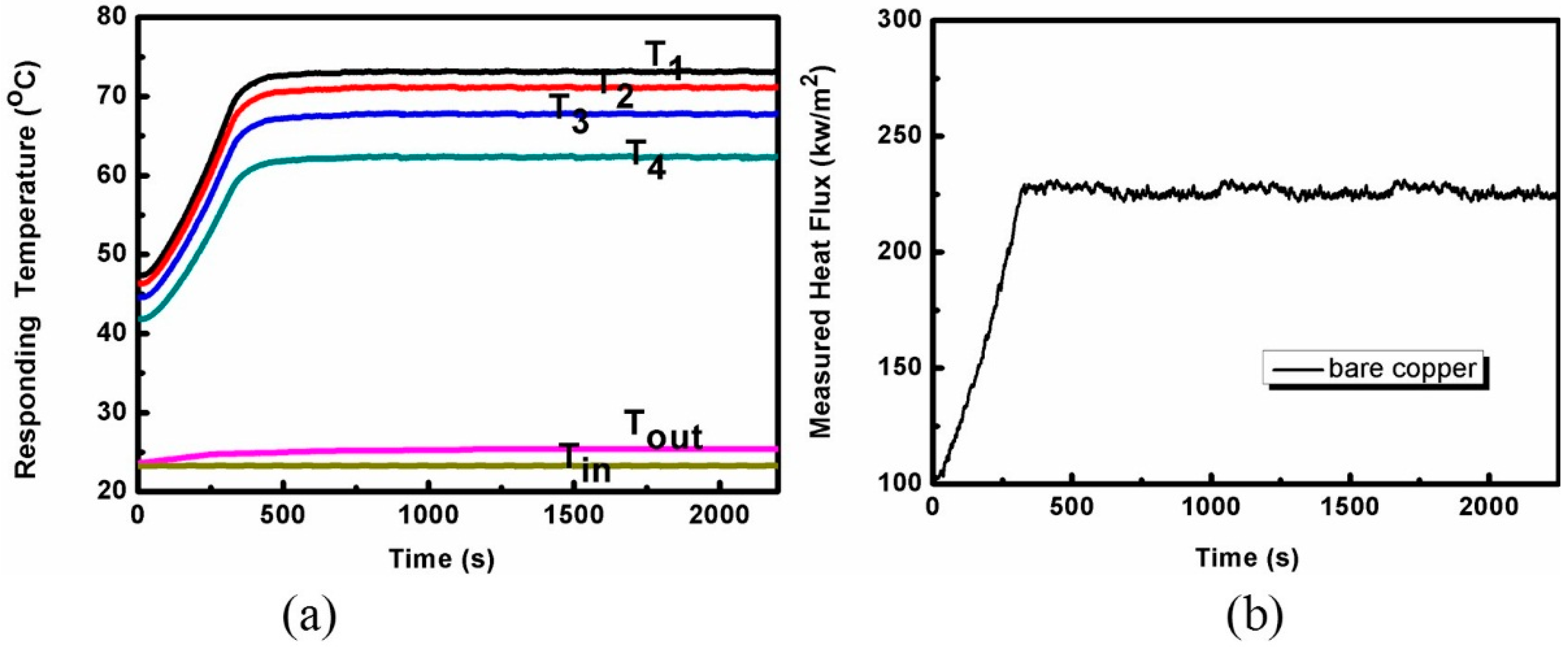
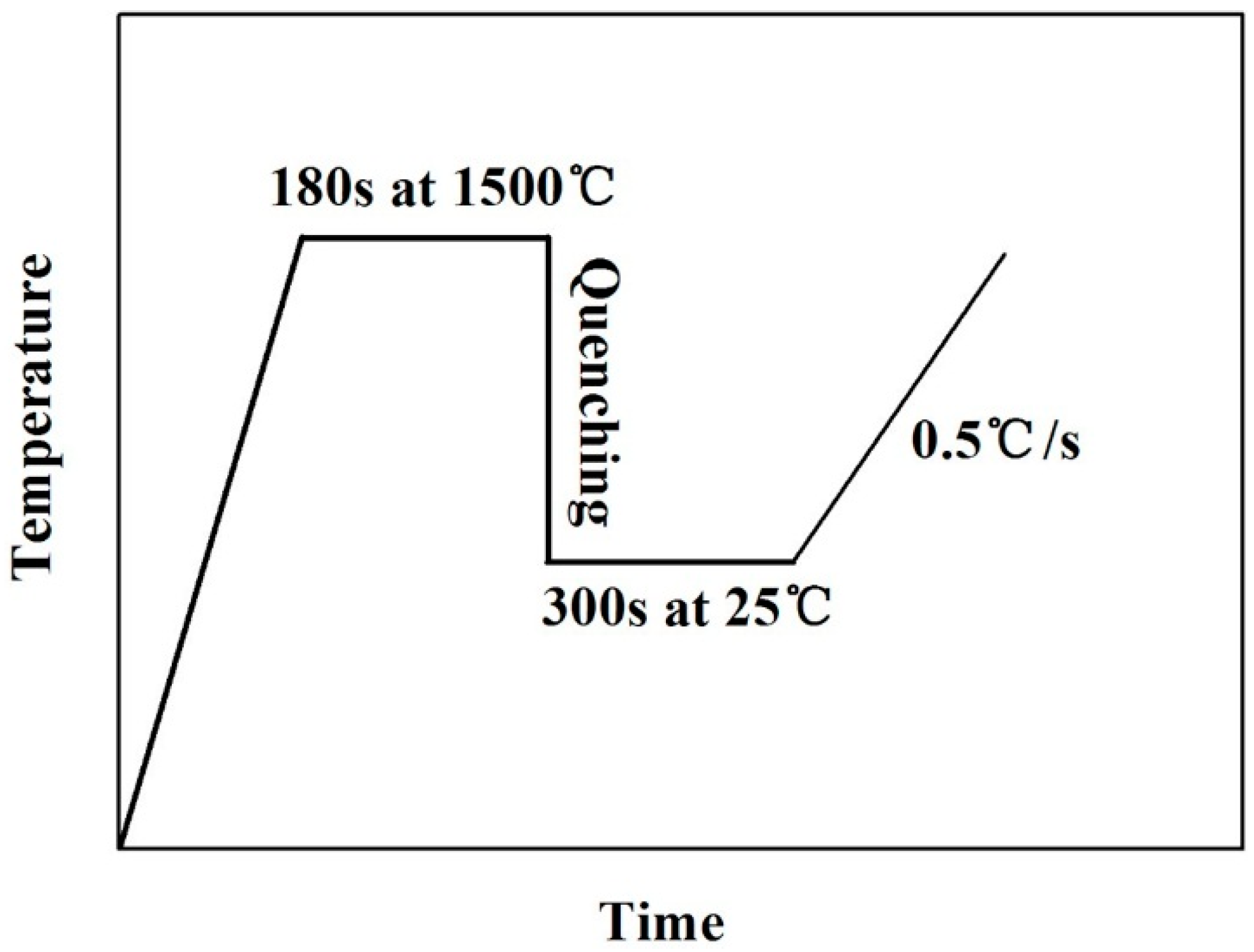
2.2. The Heat Transfer Test
2.3. The Initial Crystallization Temperature Test
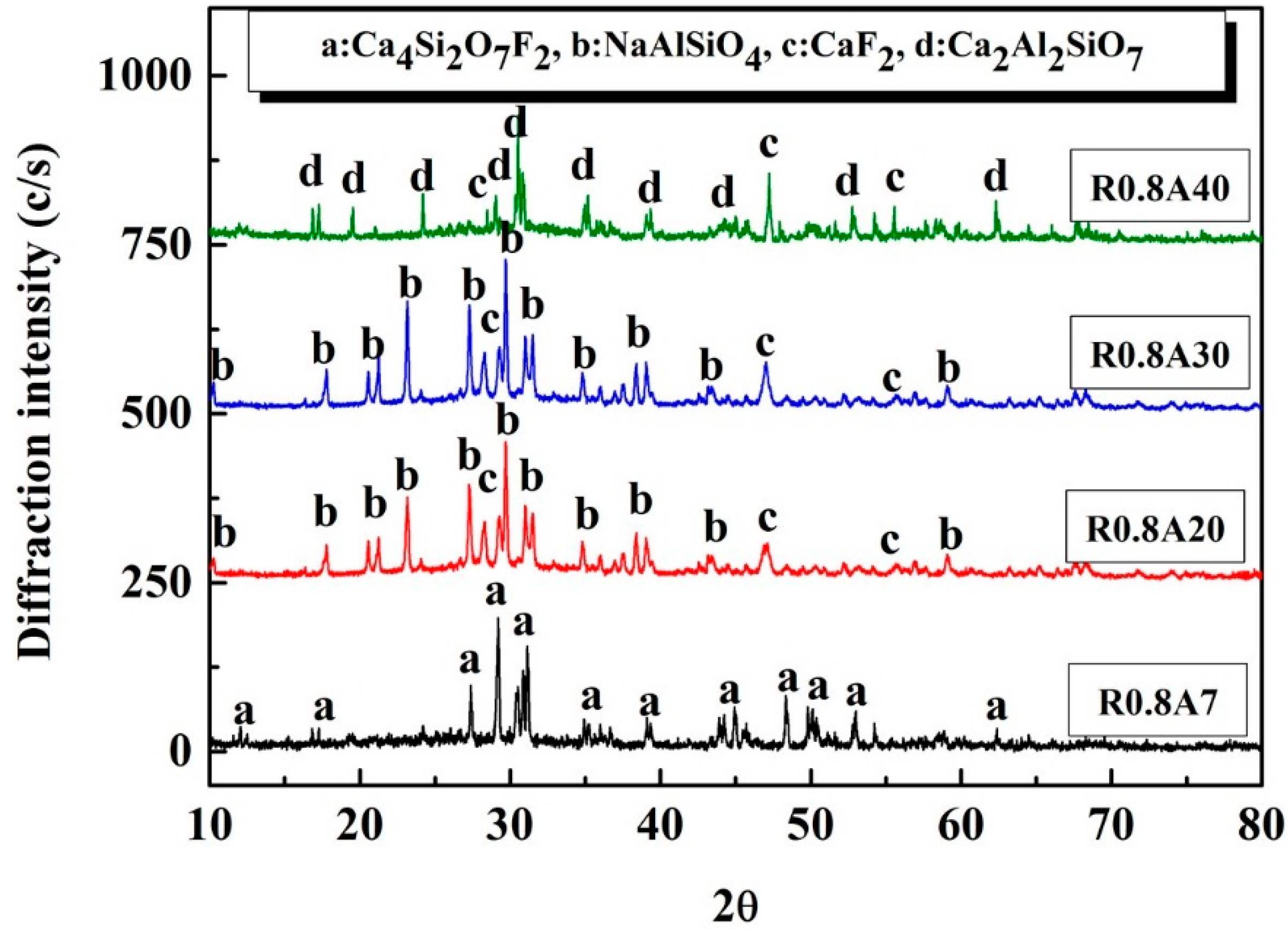
2.4. Phase Analysis
3. Results and Discussion
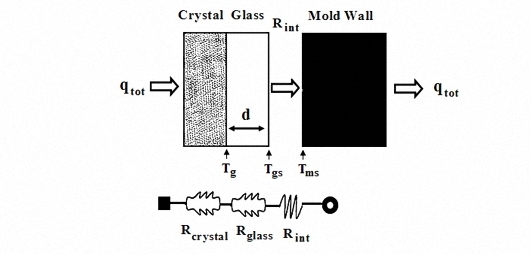
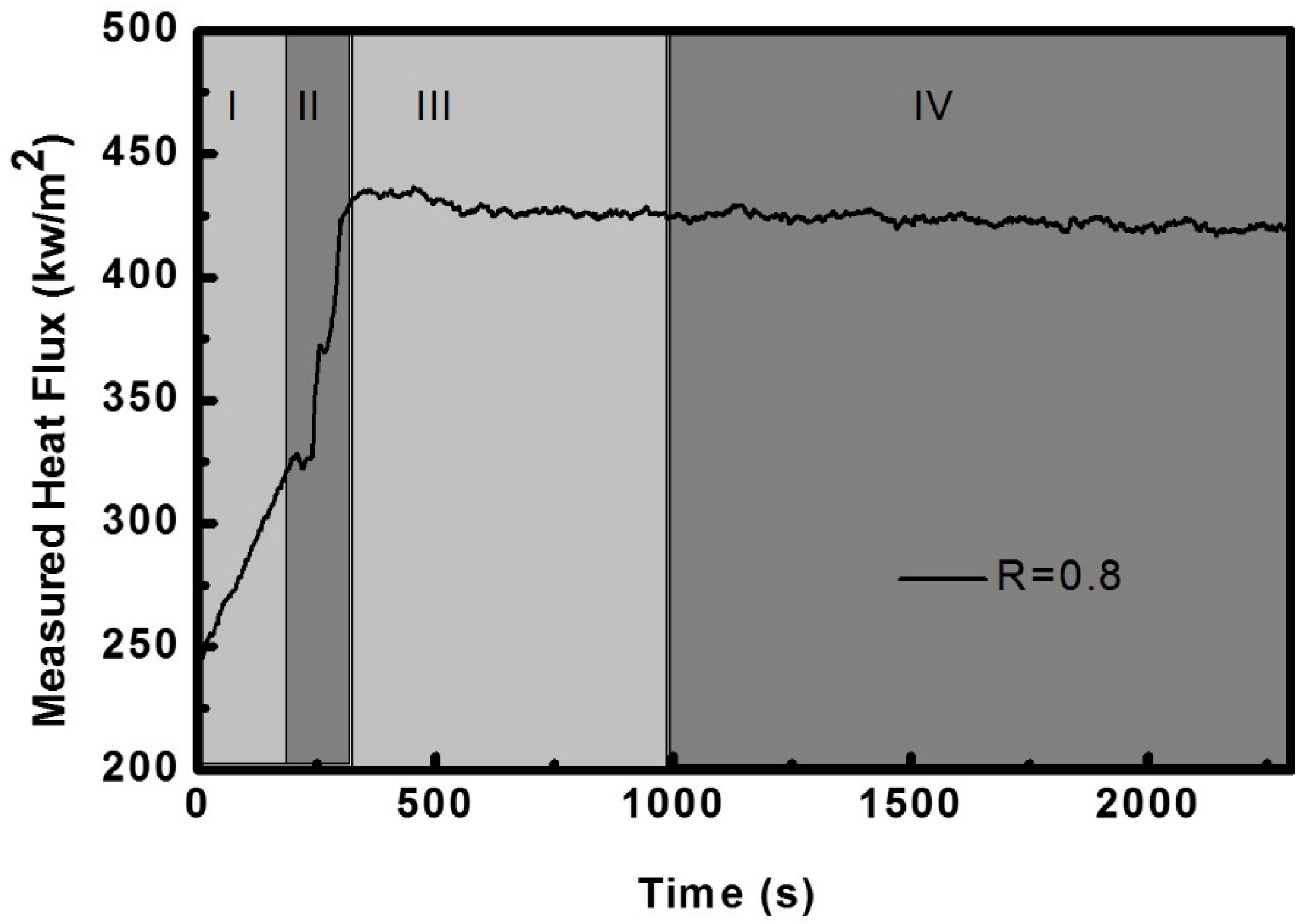
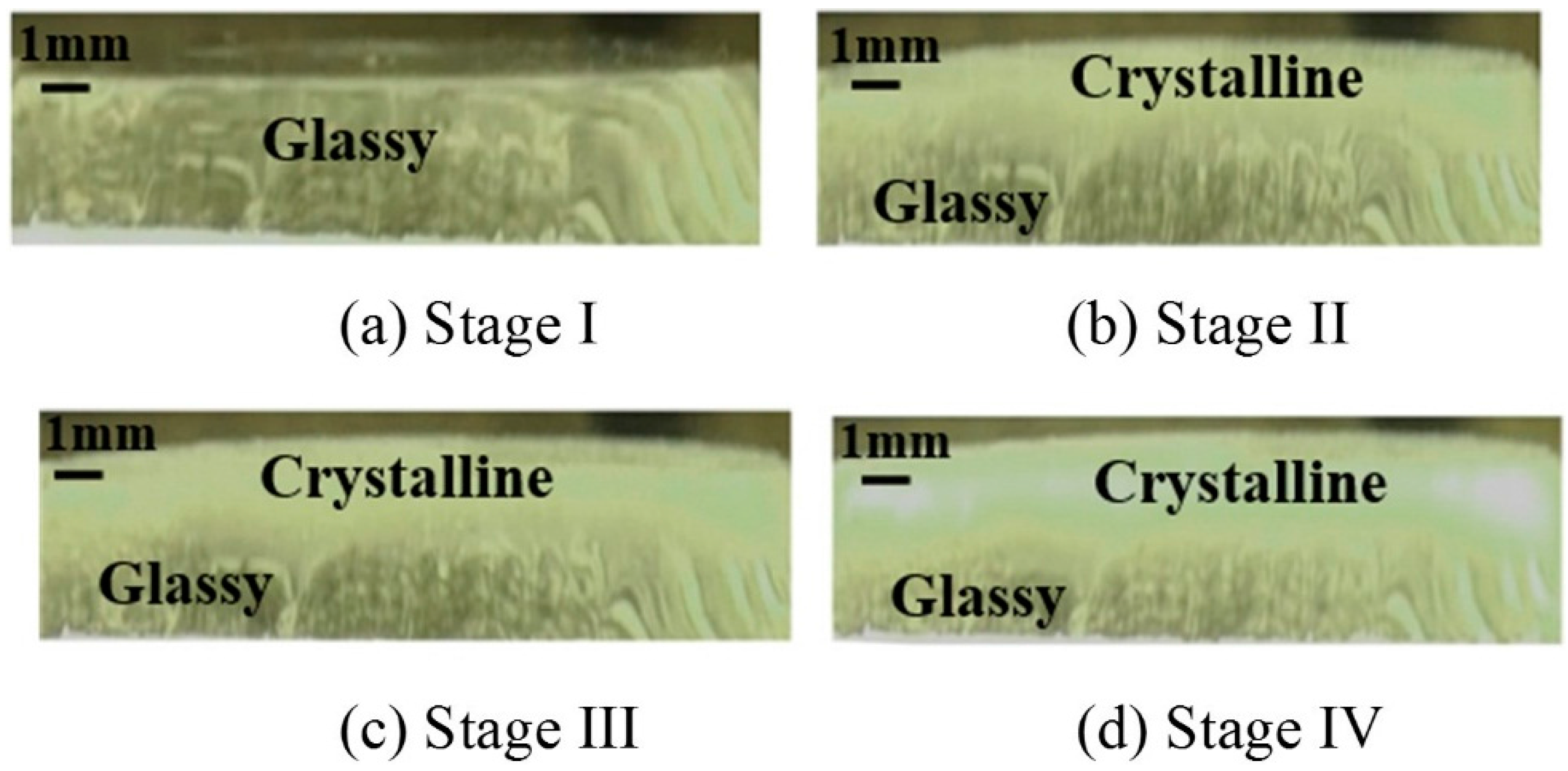
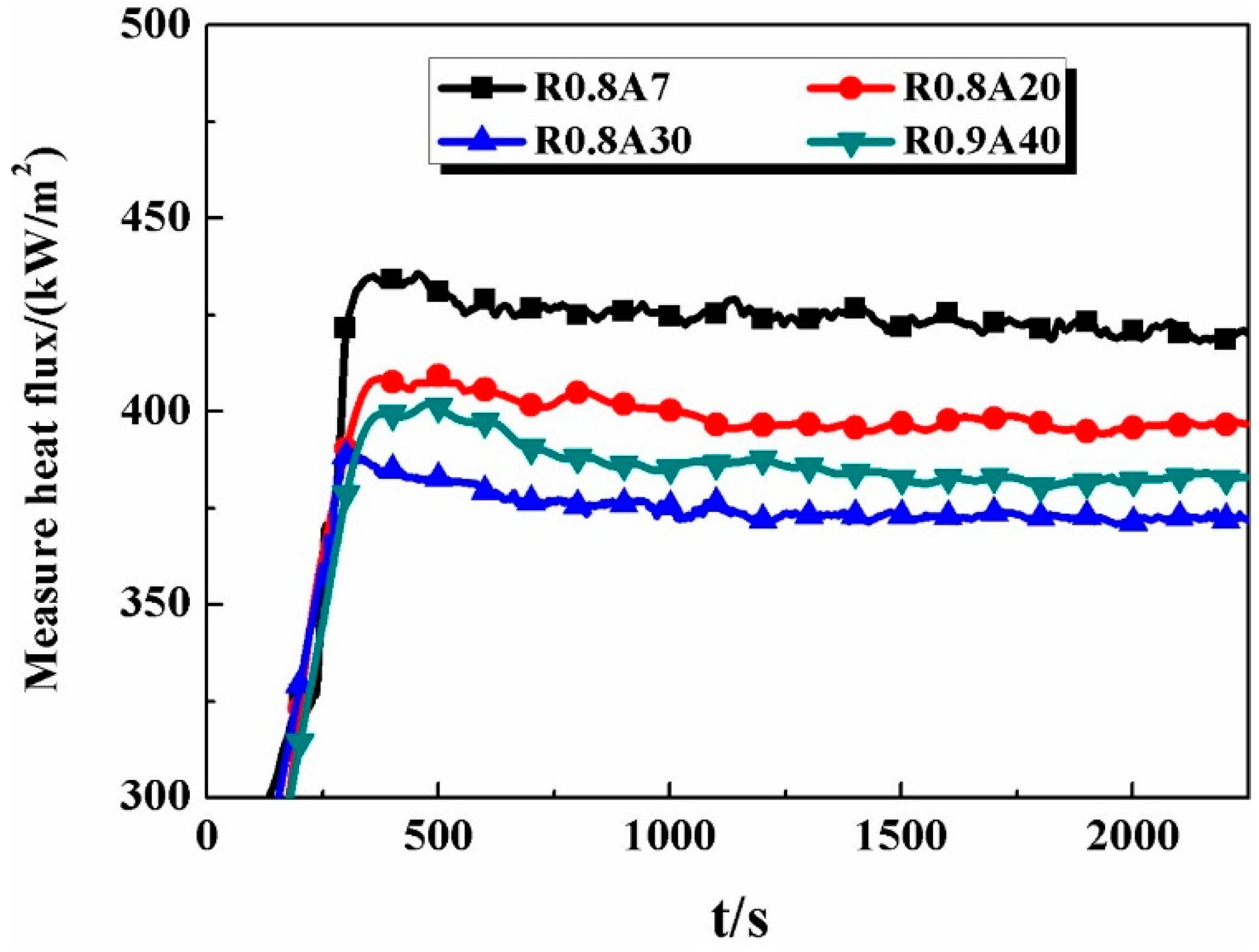
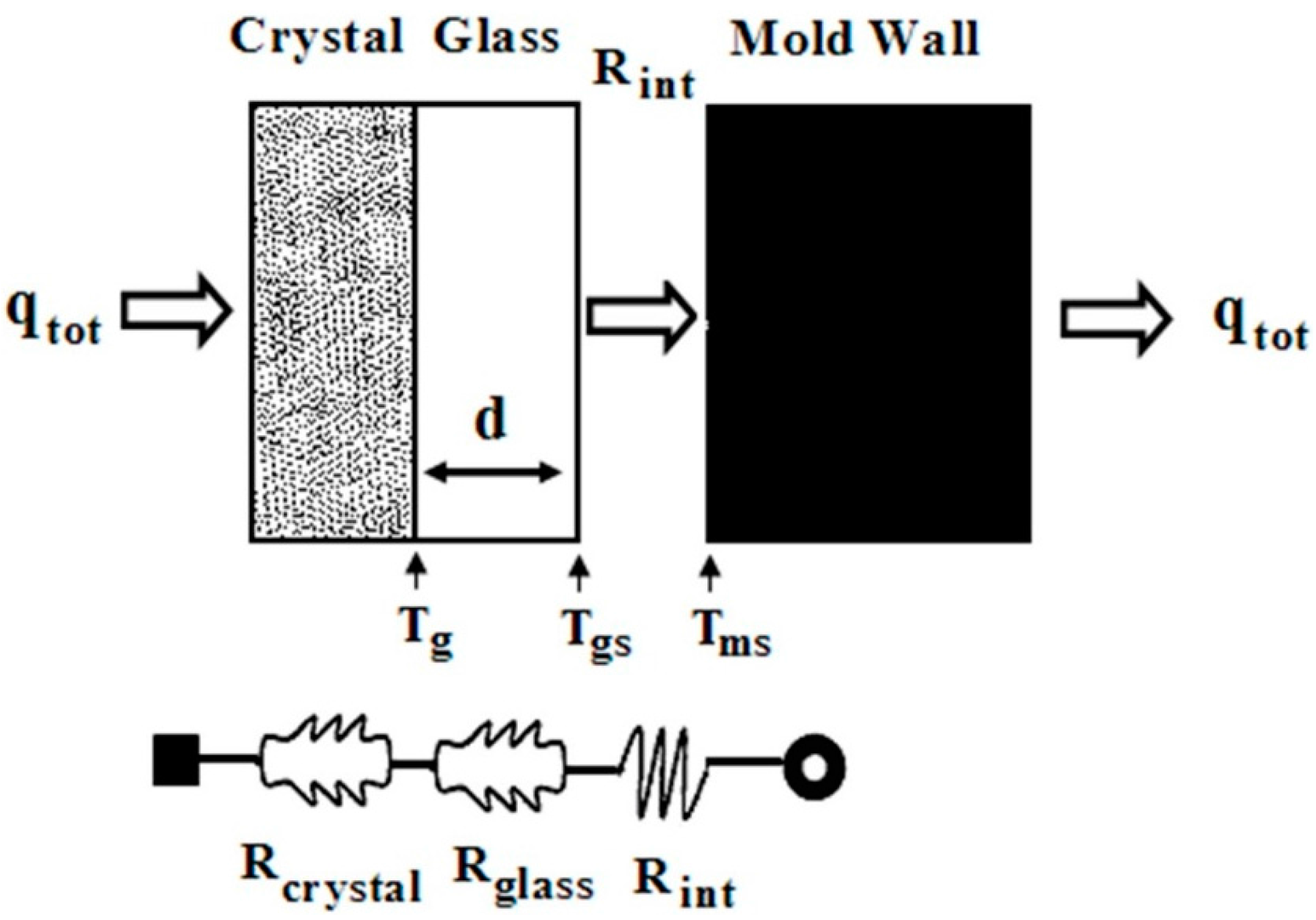
3.1. The Heat Transfer Procedure
3.2. The Effect of Al2O3 Content on Heat Flux
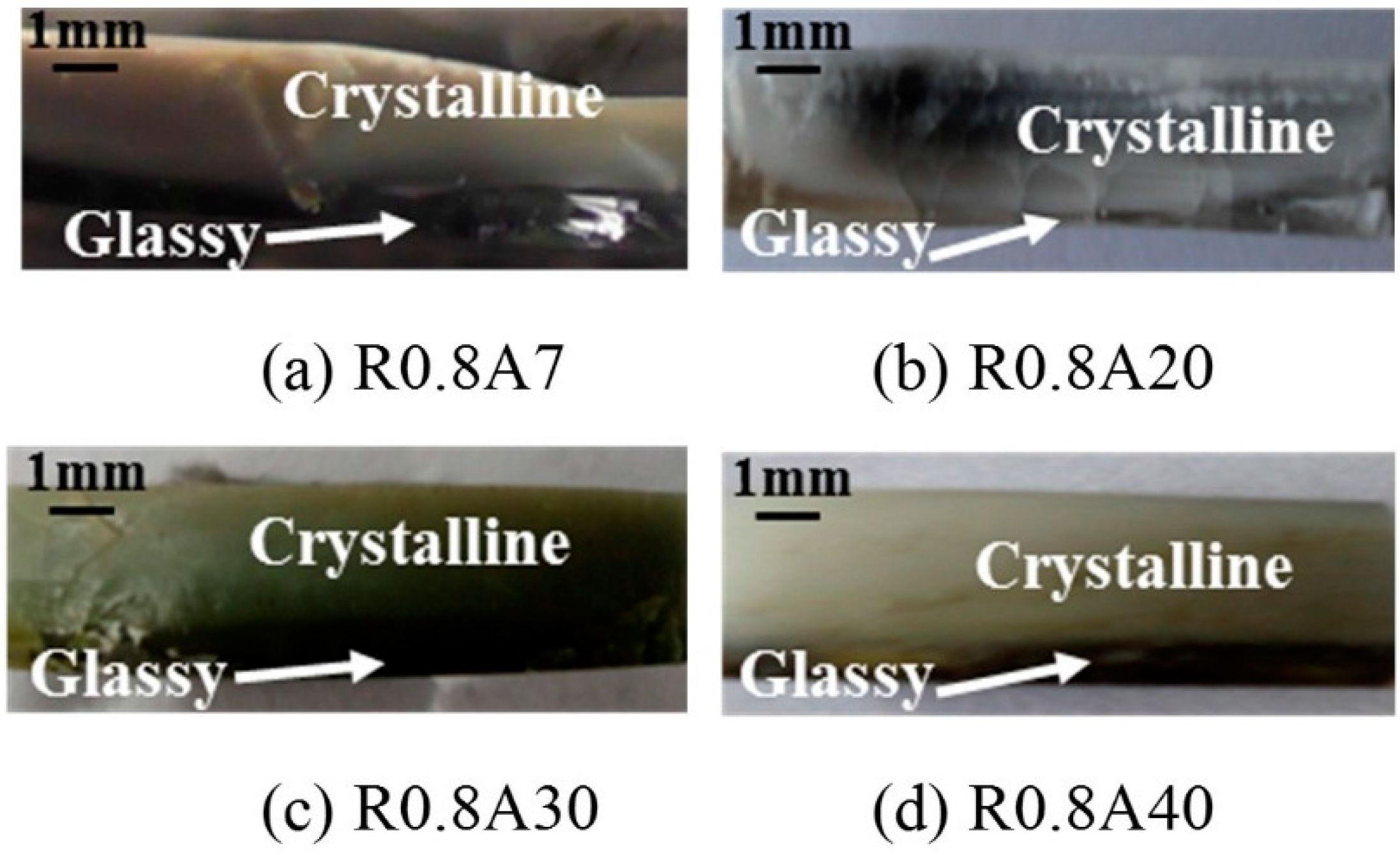
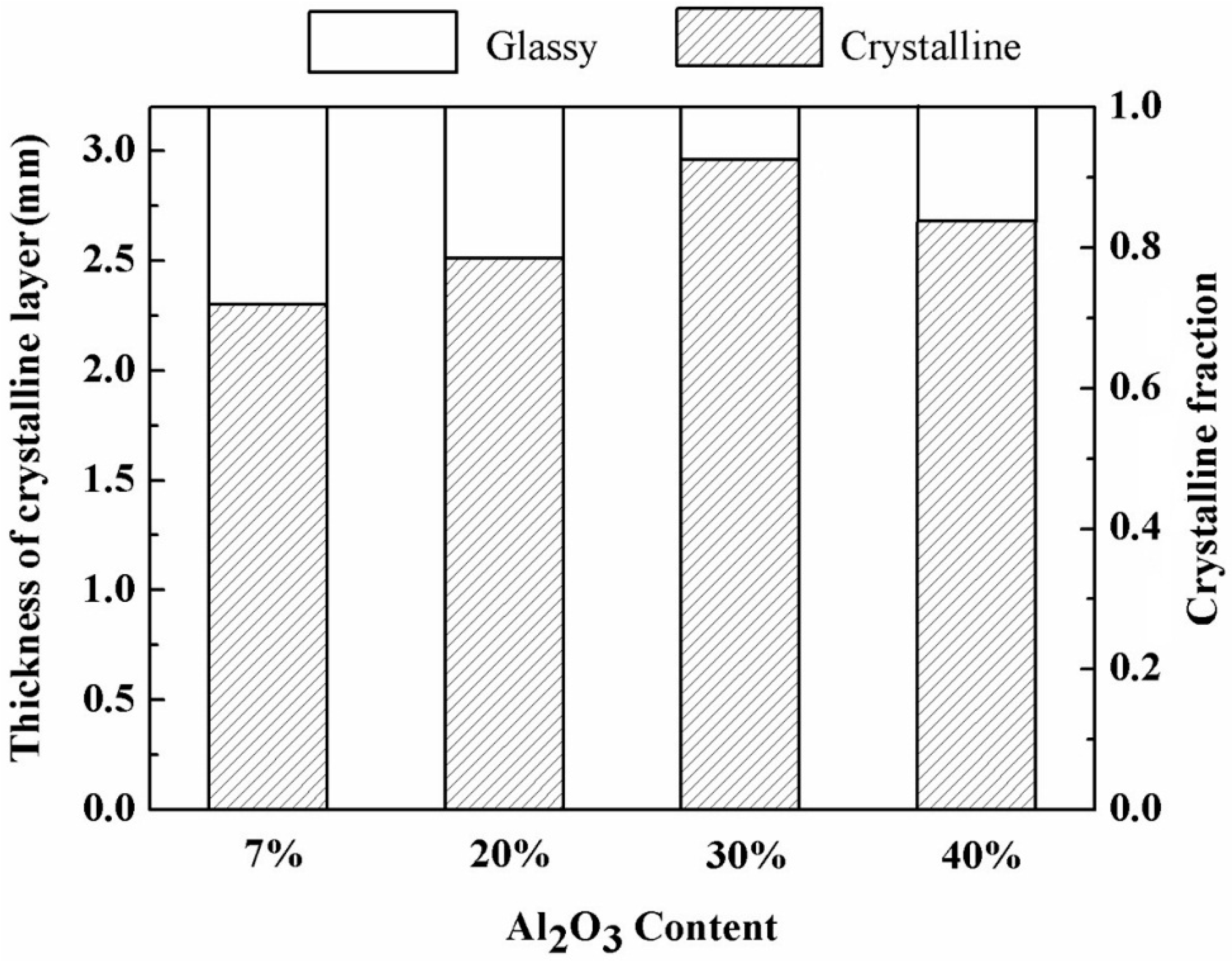
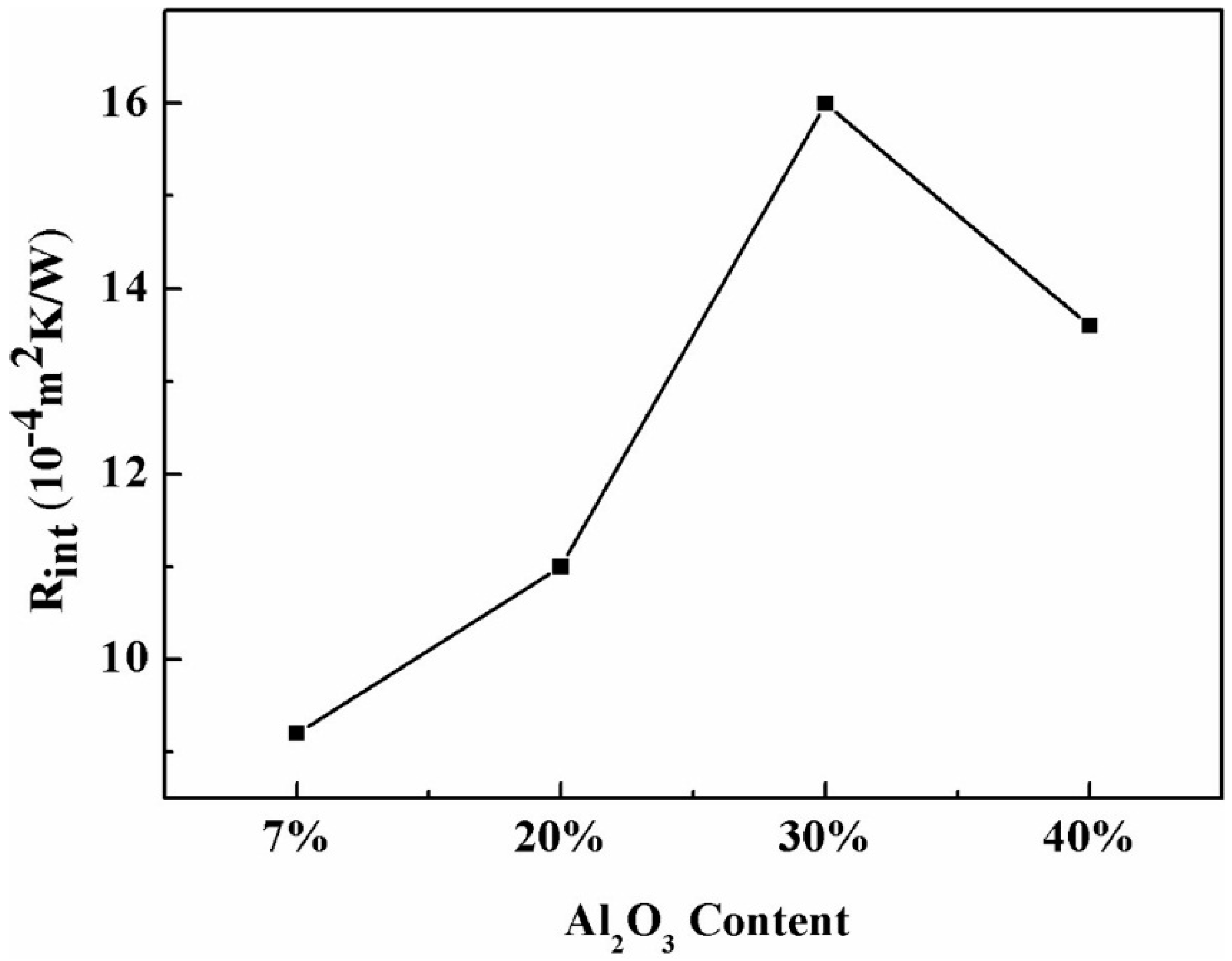
3.3. The Effect of Al2O3 Content on Interface Thermal Resistance
4. Conclusions
- The final steady-state heat flux of Sample R0.8A7 (Al2O3, 7%) was 423 kW·m−2, while it decreased to 419 kW·m−2 (Sample R0.8A20) and then to 372 kW·m−2 (Sample R0.8A30) when the Al2O3 content increased from 7% to 20% and then to 30%, respectively; however, it increased to 383 kW·m−2 as the Al2O3 content was continuously increased to 40%.
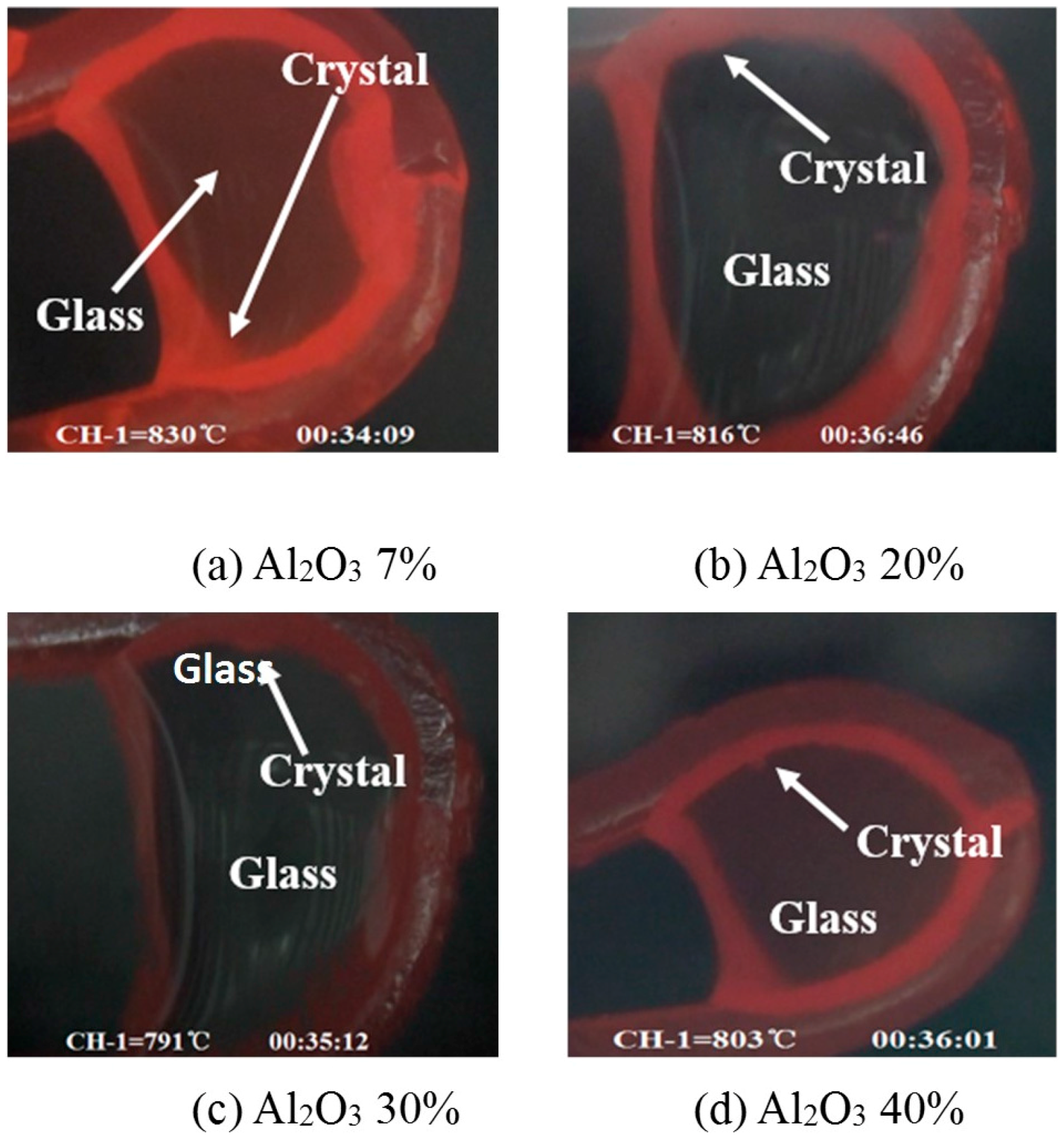
- Both crystalline layer thickness and fraction increased first, then decreased with the further addition of Al2O3. Additionally, it was the mold flux crystallization, together with the resulted interfacial thermal resistance, that dominated the heat transfer performance of the mold flux—not the specific precipitated crystal phase.
- The Rint increased from 9.2 × 10−4 m2·kW−1 (Sample R0.8A7) to 11.0 × 10−4 m2·kW−1 (Sample R0.8A20) and then to 16.0 × 10−4 m2·kW−1 (Sample R0.8A30) when the addition of Al2O3 content increased from 7% to 20% and then to 30%, respectively; however, it decreased to 13.6 × 10−4 m2·kW−1 (Sample R0.8A40) when the Al2O3 content reached 40%.
- The variation in the interfacial thermal resistance Rint was proportional to crystalline fraction of the mold fluxes, as the interface thermal resistance was mainly caused by the deformation and shrinkage of the mold flux during its crystallization process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Thomas, B.G. Inclusions in continuous casting of steel. In Proceedings of the XXIV National Steelmaking Symposium, Morelia, Mich, Mexico, 26–28 November 2003; pp. 138–183.
- Atkinson, H.V.; Shi, G. Characterization of inclusions in clean steels: A review including the statistics of extremes methods. Prog. Mater. Sci. 2003, 48, 457–520. [Google Scholar] [CrossRef]
- Senk, D. Effects of Casting Flux Interaction with Steel Melt on Lubrication in CC Moulds. Mater. Sci. Forum 2010, 638, 3628–3633. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Cho, J.; Park, M.; Lee, H.; Kang, Y. A reaction between high Mn-High Al steel and CaO–SiO2-Type molten mold flux: Part I. Composition evolution in molten mold flux. Metall. Mater. Trans. B 2013, 44, 299–308. [Google Scholar] [CrossRef]
- Blazek, K.; Yin, H.; Skoczylas, G.; McClymonds, M.; Frazee, M. Development and evaluation of Lime-Alumina-Based mold powders for casting high-Aluminum TRIP steel grades. AIST Trans. 2011, 8, 232–240. [Google Scholar]
- Zhang, Z.; Wen, G.; Tang, P.; Sridhar, S. The influence of Al2O3/SiO2 ratio on the viscosity of mold fluxes. ISIJ Int. 2008, 48, 739–746. [Google Scholar] [CrossRef]
- Yu, X.; Wen, G.H.; Tang, P.; Wang, H. Investigation on viscosity of mould fluxes during continuous casting of aluminum containing TRIP steels. Ironmak. Steelmak. 2009, 36, 623–630. [Google Scholar] [CrossRef]
- Kim, G.; Sohn, I. Effect of Al2O3 on the viscosity and structure of calcium silicate-based melts containing Na2O and CaF2. J. Non-Crys. Sol. 2012, 358, 1503–1537. [Google Scholar] [CrossRef]
- Cho, J.; Blazek, K.; Frazee, M.; Yin, H.; Park, J.; Moon, S. Assessment of CaO–Al2O3 based mold flux system for high aluminum TRIP casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef]
- Xiong, Y.; Wen, G.; Tang, P.; Yang, B. Characteristics of heat flux through slag film of mold slag used for high Al-TRIP steel casting. J. Iron Steel Res. Int. 2010, 17, 11–16. [Google Scholar]
- Zhou, L.; Wang, W.; Ma, F.; Li, J.; Wei, J.; Matsuura, H.; Tsukihashi, F. A Kinetic Study of the Effect of Basicity on the Mold Fluxes Crystallization. Metall. Mater. Trans. B 2012, 43, 354–362. [Google Scholar] [CrossRef]
- Allibert, M.; Gaye, H.; Geiseler, J. Slag Atlas; Eisenhüttenleute, V.D., Ed.; Verlag Stahleisen GmbH Press: Dtisseldorf, Germany, 1995. [Google Scholar]
- Zhou, L.; Wang, W.; Wei, J.; Zhou, K. Melting and Heat Transfer Behavior of F-Free Mold Fluxes for Casting Medium Carbon Steels. ISIJ Int. 2015, 55, 821–829. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, L.; Gu, K. Effect of mold flux melting and crystal dissolution on the radiative heat transfer for continuous casting. Met. Mater. Int. 2010, 16, 913–920. [Google Scholar] [CrossRef]
- Beck, J.V. 1HCP1D: A Program for Calculating Surface Heat Fluxes from Transient Temperatures Inside Solids; Beck Engineering Consultants Company: Houston, TX, USA, 1997. [Google Scholar]
- Zhang, H.; Wang, W.; Zhou, D.; Ma, F.; Lu, B.; Zhou, L. A Study for Initial Solidification of Sn-Pb Alloy during Continuous Casting: Part I. The Development of the Technique. Metall. Mater. Trans. B 2014, 45, 1038–1047. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Cicutti, C.E.; Cramb, A.W.; Ishii, H. Development of double and single hot thermocouple technique for in situ observation and measurement of mold slag crystallization. ISIJ Int. 1998, 38, 348–356. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Zhou, K. Viscosity and crystallization behavior of F-free mold for casting medium carbon steels. ISIJ Int. 2015, 55, 1916–1924. [Google Scholar] [CrossRef]
- Mills, K.C.; Fox, A.B.; Li, Z.; Thackray, R.P. Performance and properties of mould fluxes. Ironmak. Steelmak. 2005, 32, 26–34. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Zhou, K. Effect of Al2O3 on the Crystallization of Mold Flux for Casting High Al Steel. Metall. Mater. Trans. E 2015, 2, 99–108. [Google Scholar] [CrossRef]
- Taylor, R.; Mills, K.C. Physical properties of casting powders III: Thermal conductivities of casting powders. Ironmak. Steelmak. 1988, 15, 187–194. [Google Scholar]
- Wang, W.; Gu, K.; Zhou, L.; Ma, F.; Sohn, I.; Min, D.; Matsuura, H.; Tsukihashi, F. Radiative heat transfer behavior of mold fluxes for casting low and medium carbon steels. ISIJ Int. 2011, 51, 1838–1845. [Google Scholar] [CrossRef]
- Ozawa, S.; Susa, M.; Goto, T.; Endo, R.; Mills, K.C. Lattice and radiation conductivities for mould fluxes from the perspective of degree of crystallinity. ISIJ Int. 2006, 46, 413–419. [Google Scholar] [CrossRef]
- Kyokai, N.T.; Shinkokai, N.G. Handbook of Physico-Chemical Properties at High Temperature; Shiraishi, Y., Kawai, Y., Eds.; Iron and Steel Institute of Japan: Tokyo, Japan, 1988. [Google Scholar]
- Gu, K.; Wang, W.; Wei, J.; Matsuura, H.; Tsukihashi, F.; Sohn, I.; Min, D.J. Heat-transfer phenomena across mold flux by using the inferred emitter technique. Metall. Mater. Trans. B 2012, 43, 1393–1404. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Nagasaka, T.; Hino, M. Surface roughness of solidified mold flux in continuous casting process. ISIJ Int. 1999, 39, 1150–1159. [Google Scholar] [CrossRef]
- Cho, J.W.; Emi, T.; Shibata, H.; Suzuki, M. Heat transfer across mold flux film in mold during initial solidification in continuous casting of steel. ISIJ Int. 1998, 38, 834–842. [Google Scholar] [CrossRef]



| Sample | CaO | SiO2 | Al2O3 | MgO | F− | Na2O | Li2O | R |
|---|---|---|---|---|---|---|---|---|
| R0.8A7 | 33.53 | 42.05 | 7.02 | 2.00 | 5.88 | 9.02 | 0.50 | 0.8 |
| R0.8A20 | 27.84 | 34.70 | 20.01 | 2.02 | 5.91 | 9.02 | 0.50 | 0.8 |
| R0.8A30 | 23.37 | 29.16 | 30.01 | 2.01 | 5.94 | 9.01 | 0.50 | 0.8 |
| R0.8A40 | 18.89 | 23.60 | 40.00 | 2.00 | 5.98 | 9.01 | 0.50 | 0.8 |
| Al2O3 Cont. | Tg/c (°C) | Tms (°C) | dcrystalline (mm) | dglassy (mm) |
|---|---|---|---|---|
| 7% | 830 | 103 | 2.30 | 1.00 |
| 20% | 816 | 100 | 2.51 | 0.79 |
| 30% | 791 | 96 | 2.96 | 0.34 |
| 40% | 803 | 97 | 2.68 | 0.62 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wang, W.; Zhou, K. A Study of the Heat Transfer Behavior of Mold Fluxes with Different Amounts of Al2O3. Metals 2016, 6, 139. https://doi.org/10.3390/met6060139
Zhou L, Wang W, Zhou K. A Study of the Heat Transfer Behavior of Mold Fluxes with Different Amounts of Al2O3. Metals. 2016; 6(6):139. https://doi.org/10.3390/met6060139
Chicago/Turabian StyleZhou, Lejun, Wanlin Wang, and Kechao Zhou. 2016. "A Study of the Heat Transfer Behavior of Mold Fluxes with Different Amounts of Al2O3" Metals 6, no. 6: 139. https://doi.org/10.3390/met6060139