Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
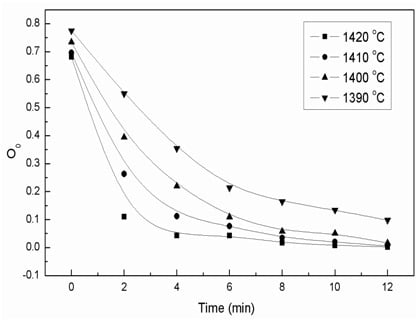
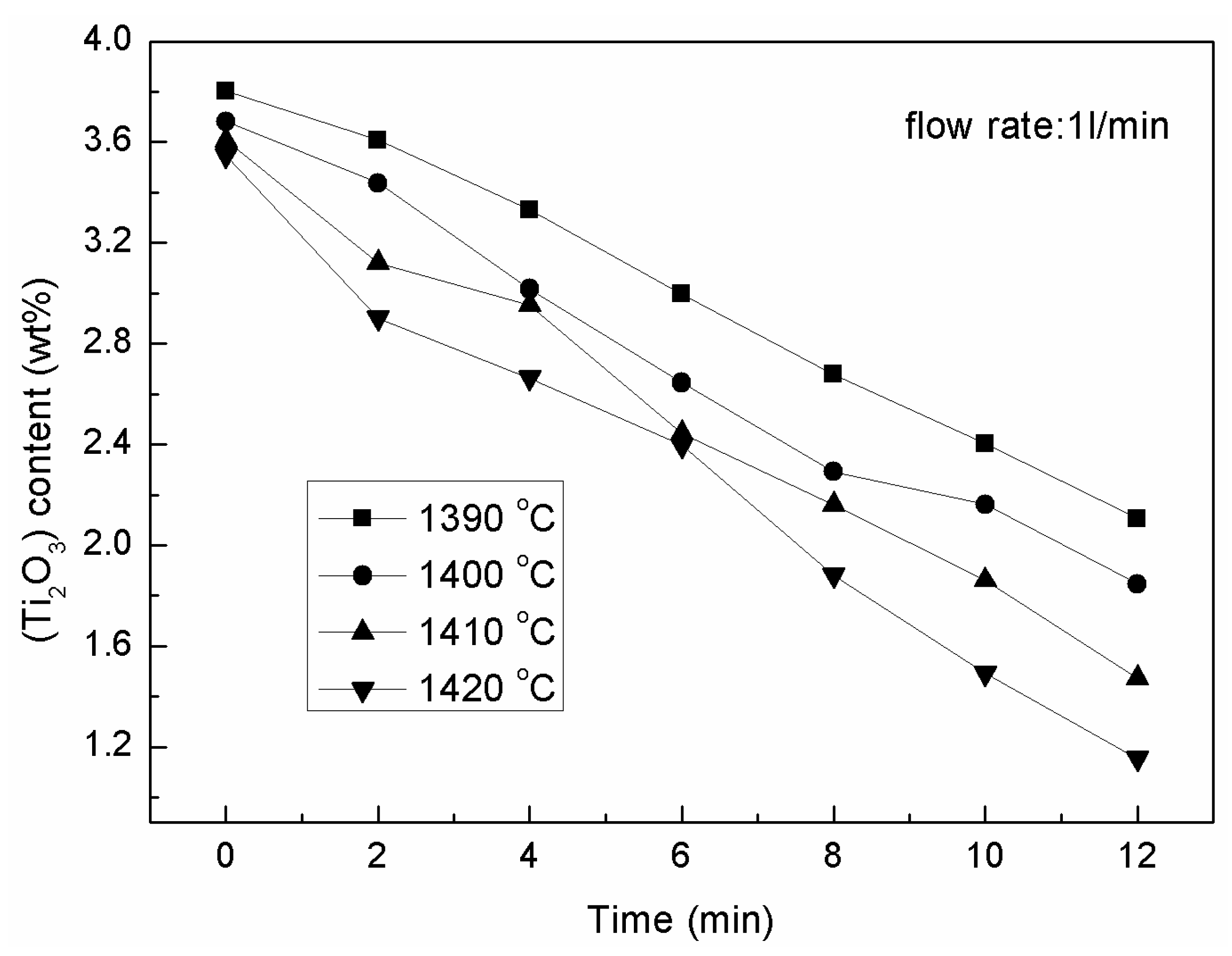
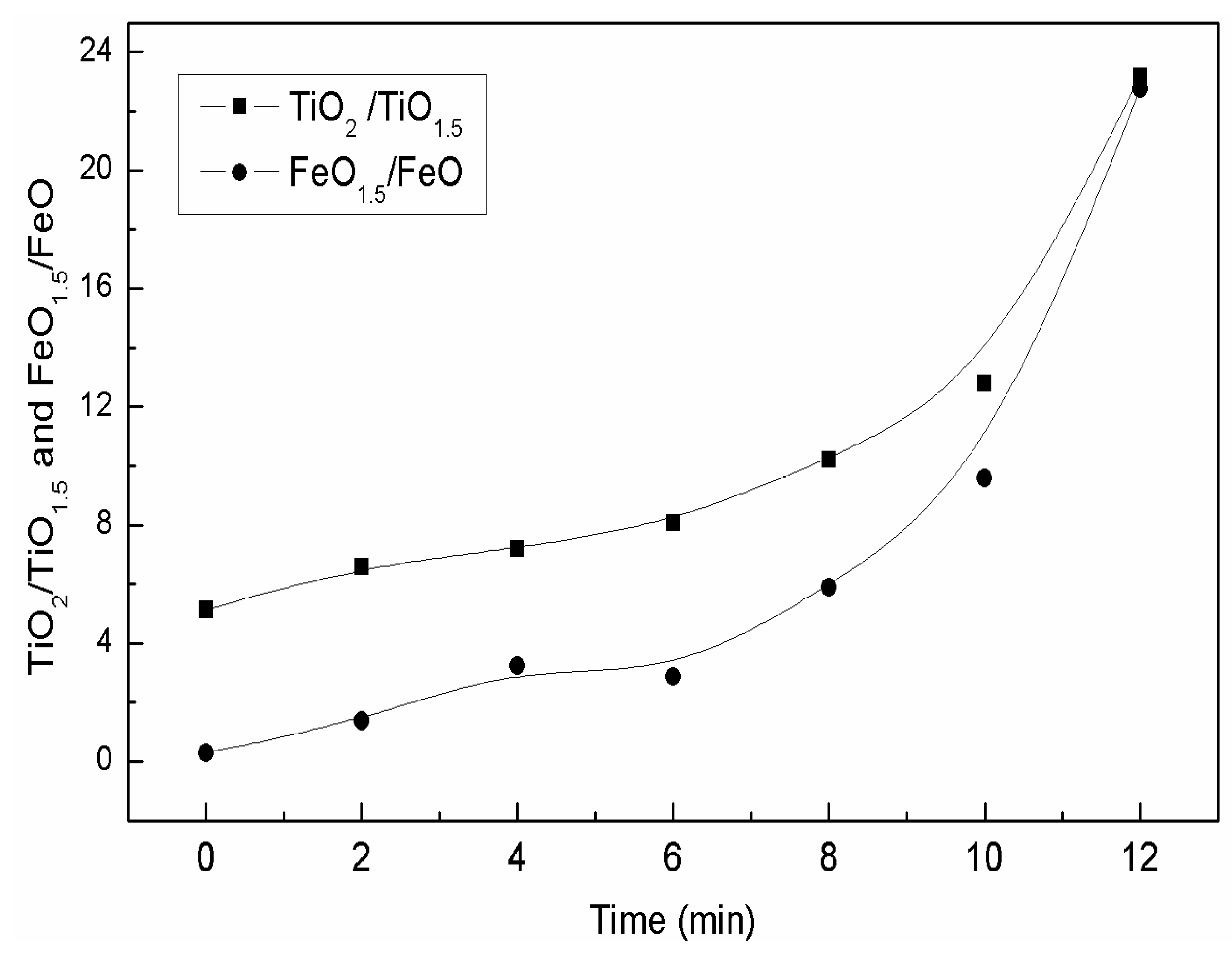
3.1. The Effects of the Isothermal Oxidation on the (TiO) and (Ti2O3) Content in the Slag
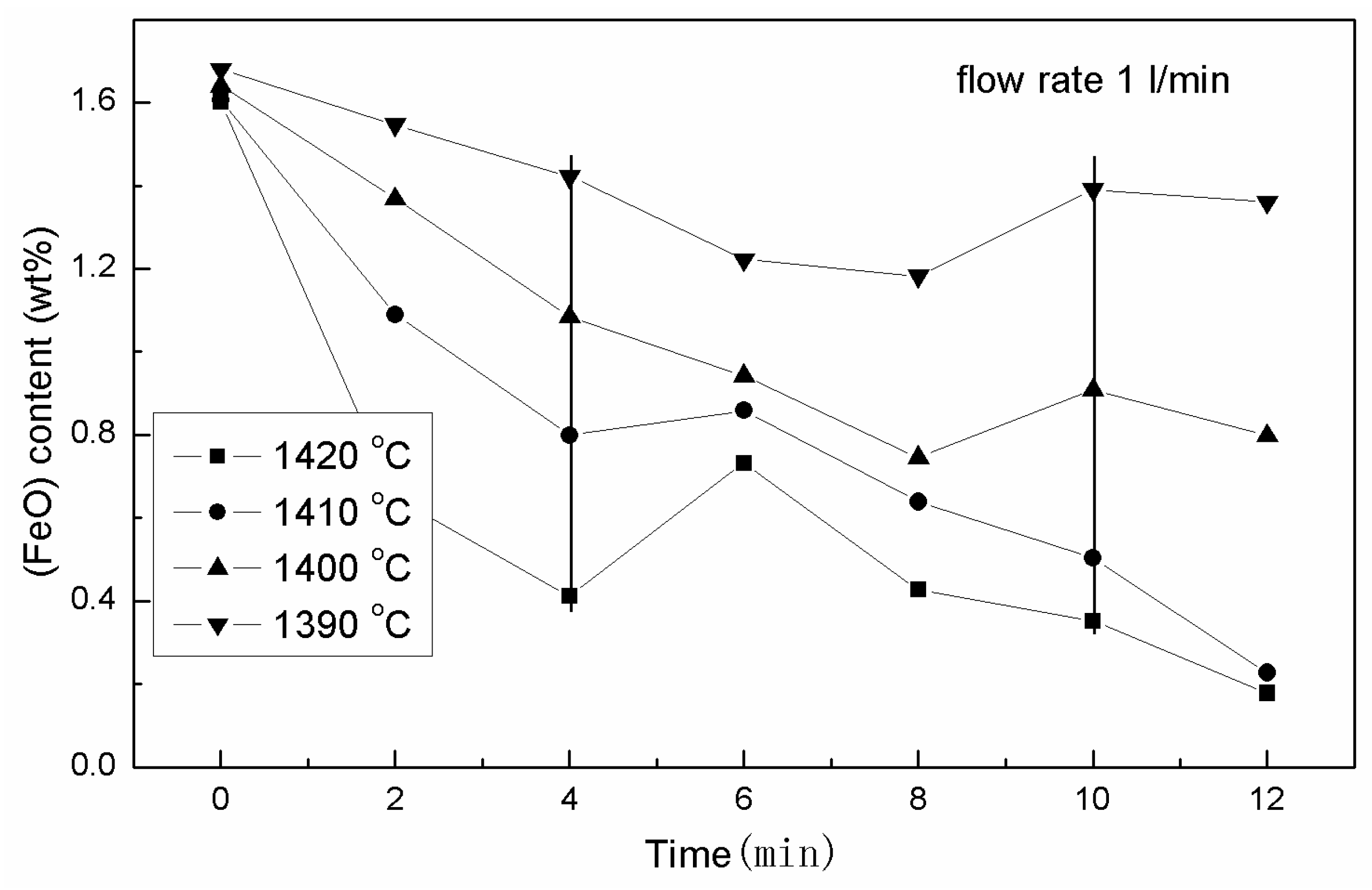
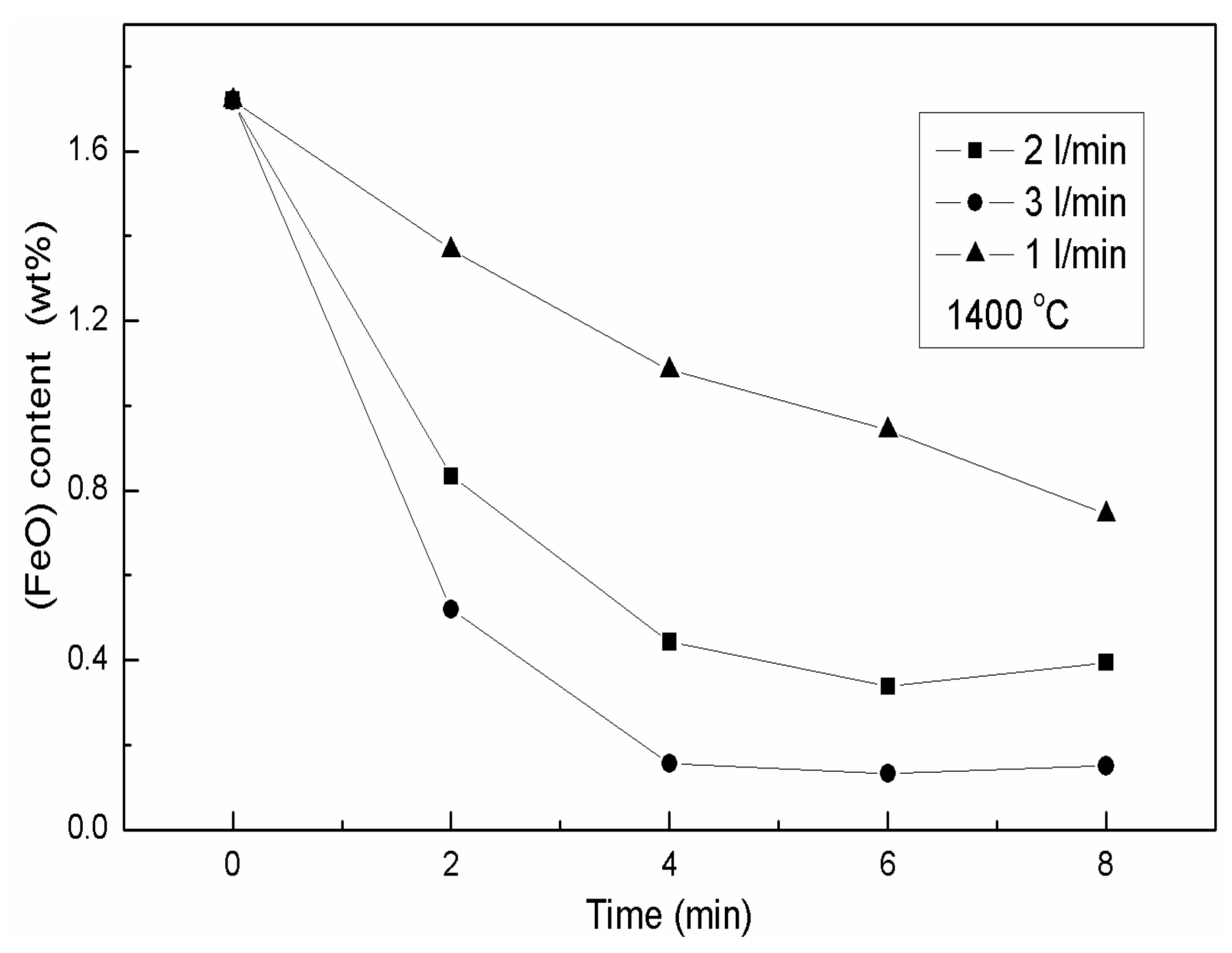
3.2. The Effects of the Isothermal Oxidation on the Ferrous Content in the Slag
3.2.1. Variation of the (FeO) Content
3.2.2. Variation of the FeM Content
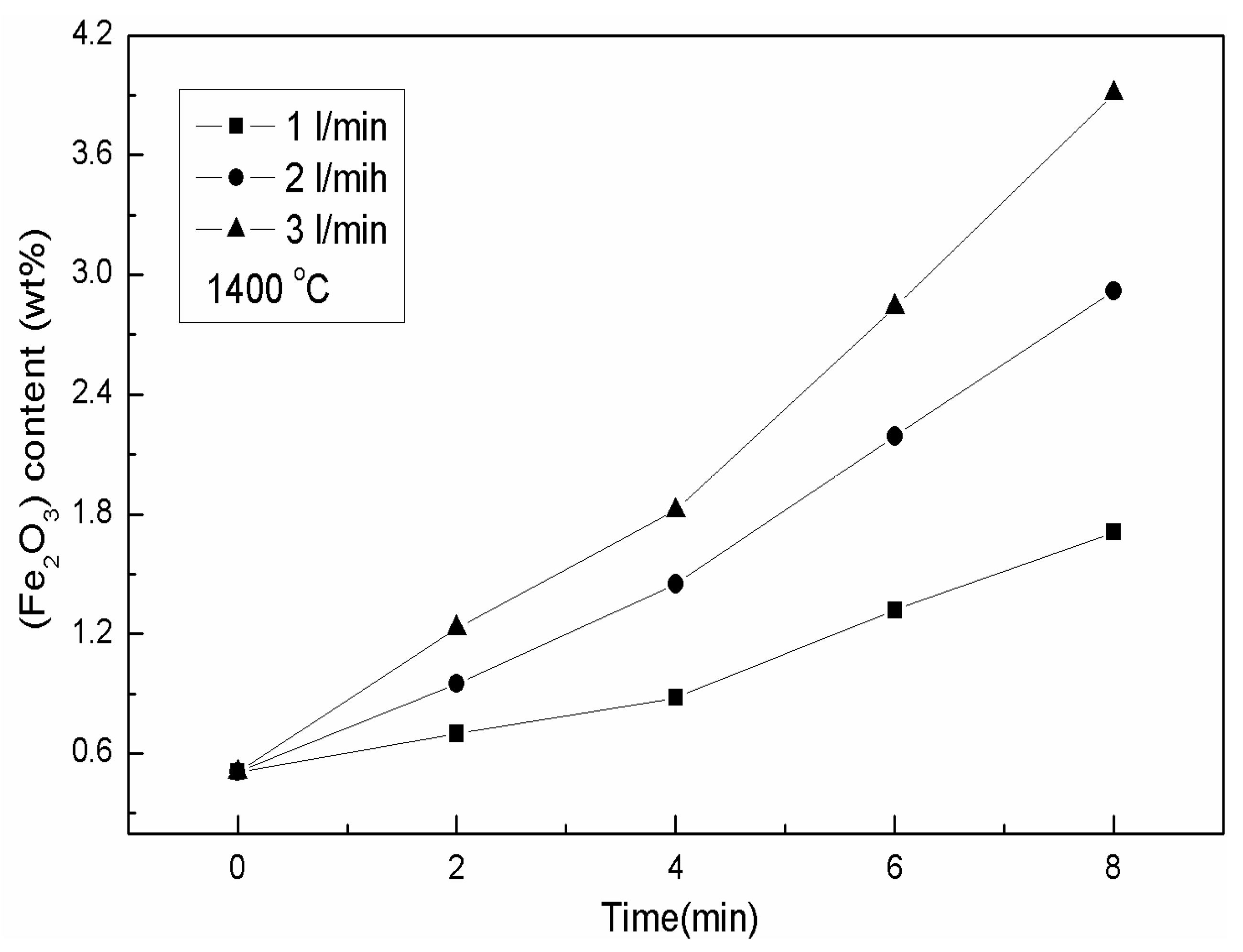
3.2.3. Variation of Fe2O3 Content
3.2.4. Variation of the Total Iron Content
3.3. Isothermal Oxidation Kinetics of Molten Slag
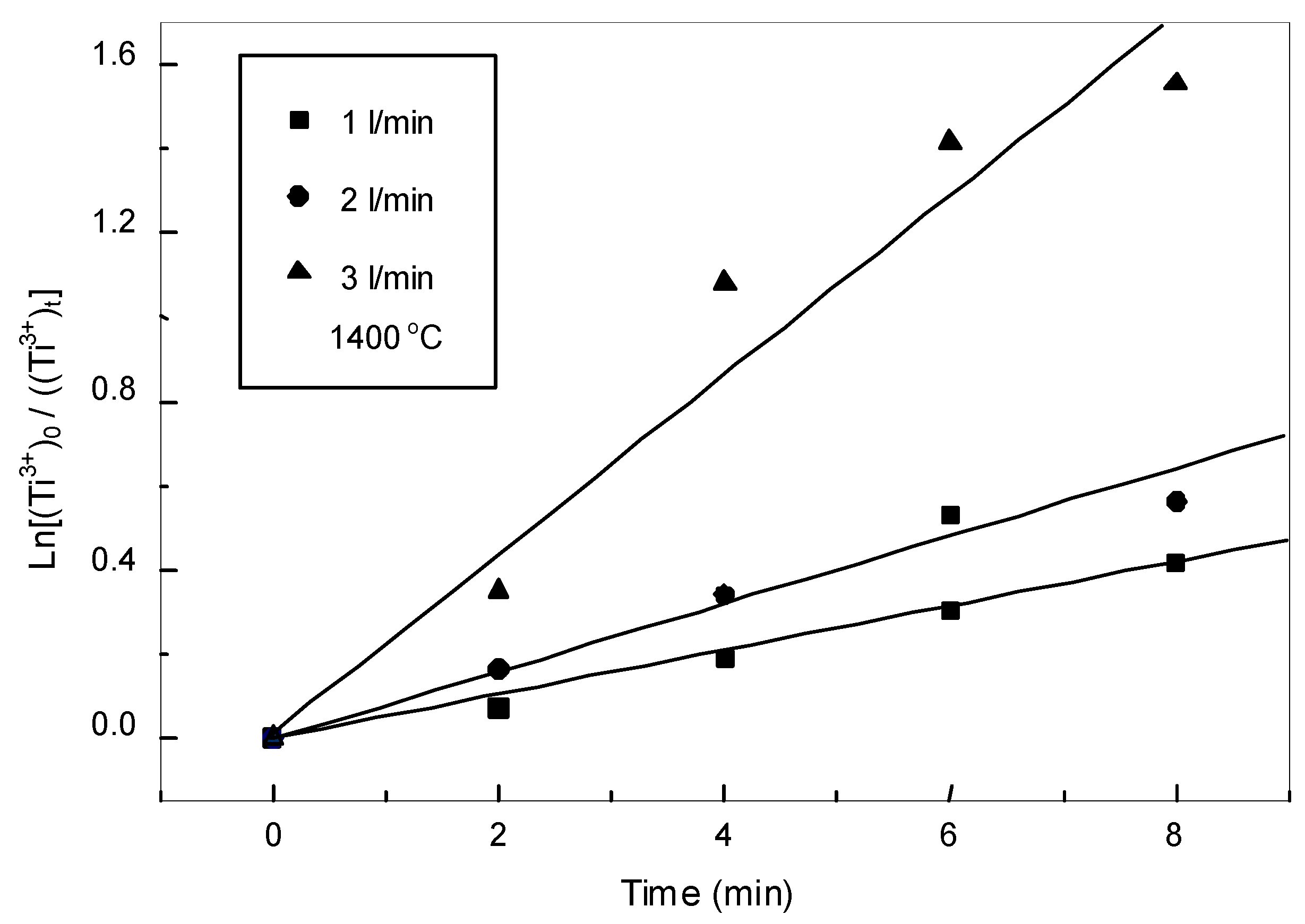
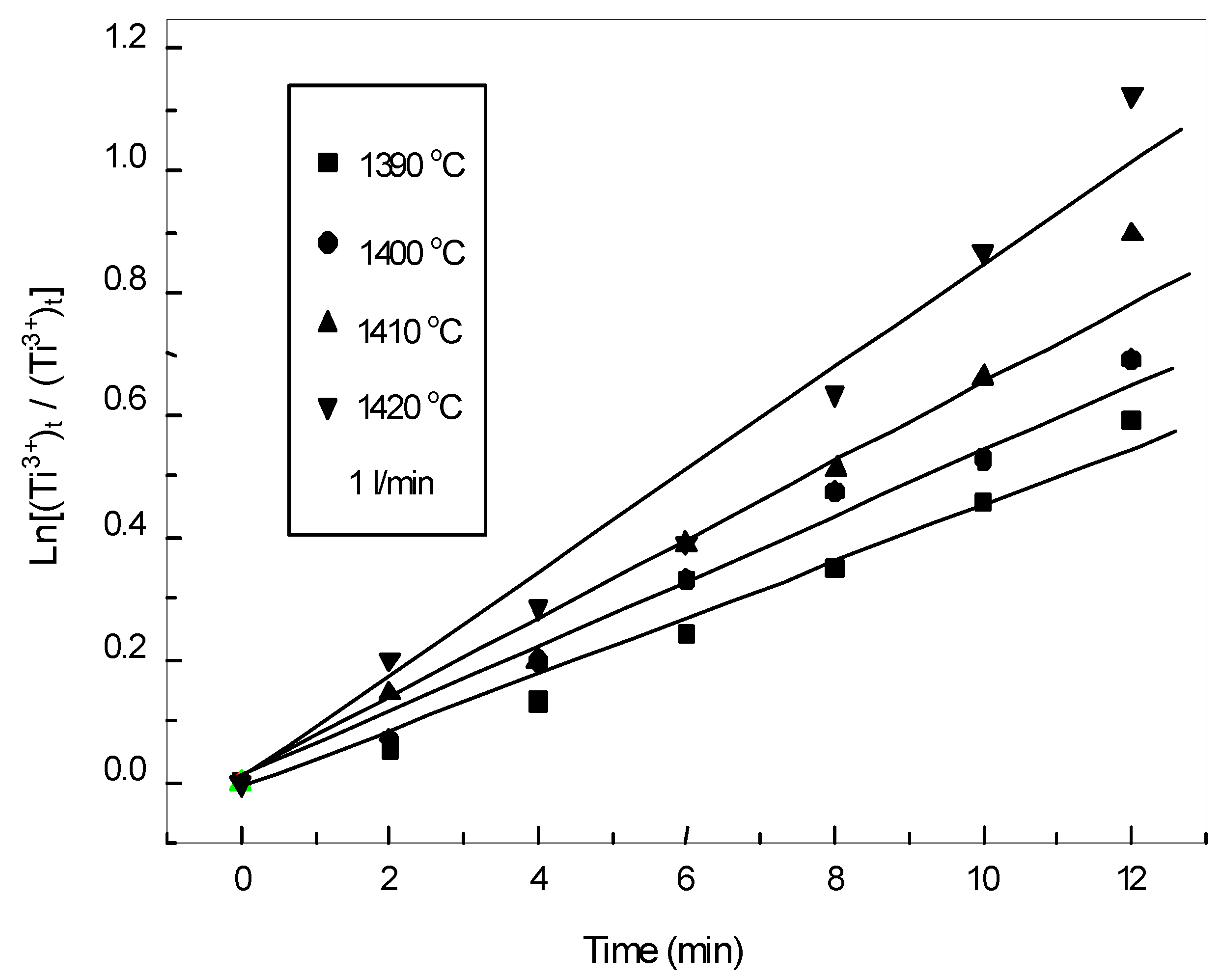
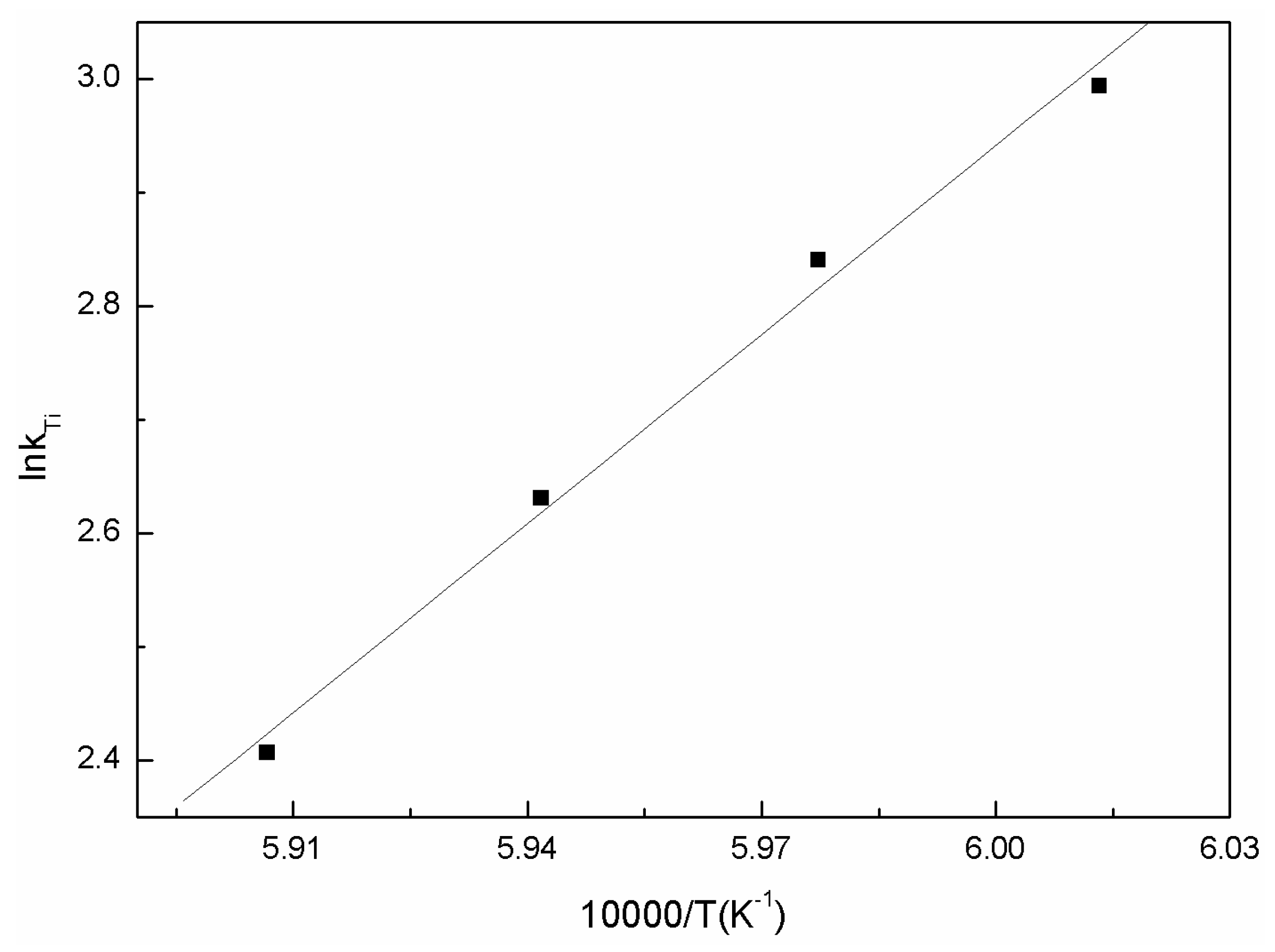
3.3.1. Oxidation Kinetics of Low Valence Titanium
3.3.2. Oxidation Mechanism of Ti3+
- (1)
- Oxygen gas arrived at the interface between gas phase (gas film) and the slag phase.
- (2)
- (Ti3+) was oxidized on the interface between slag and gas, thus (Ti4+) was produced via the following reaction:
- (3)
- (Ti4+) ions diffused into the molten slag.
3.3.3. Oxidation Kinetics of Iron in the Slag
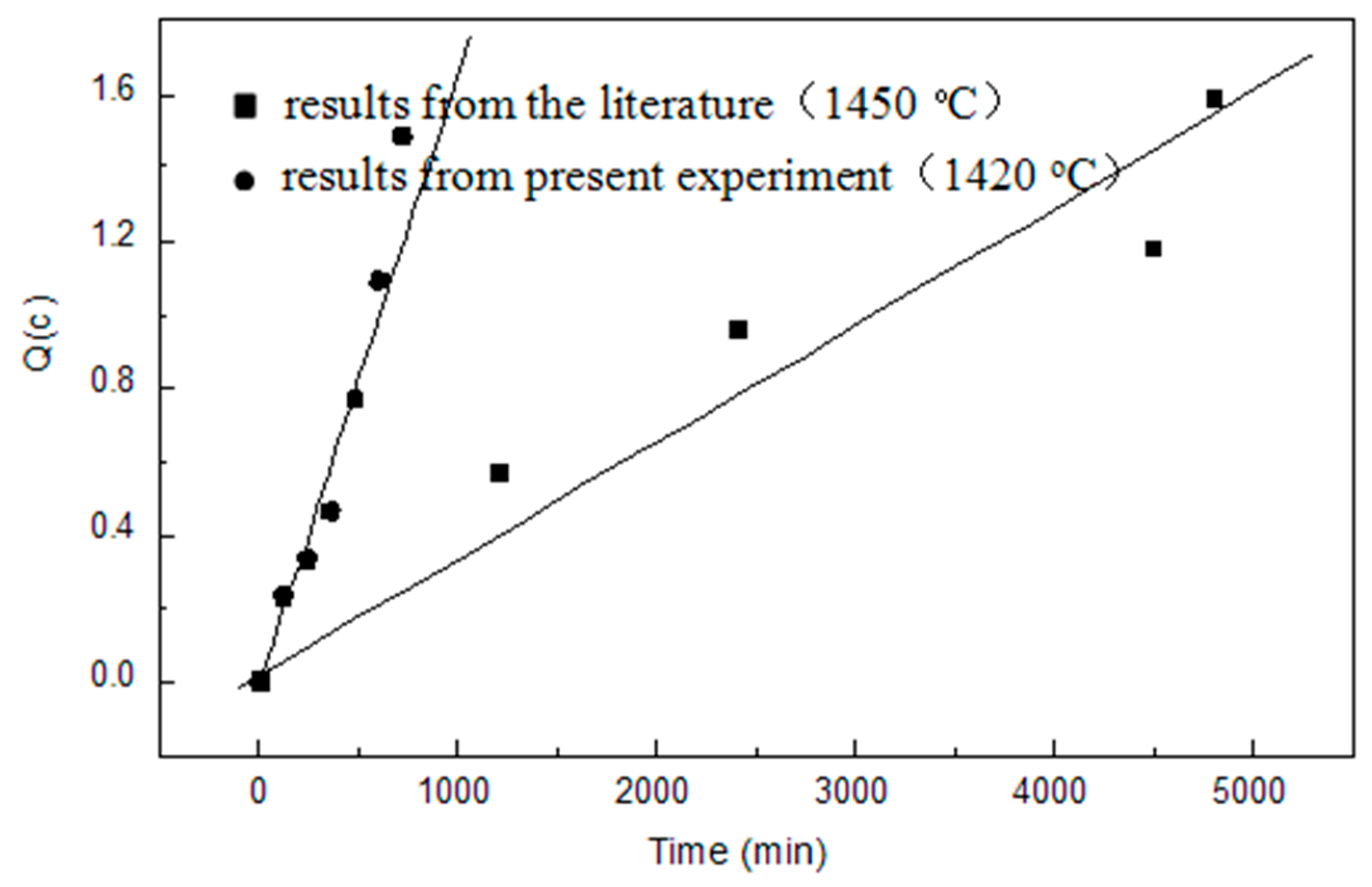
3.4. Oxygen Capacity of the Slag
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhang, L. Selective enrichment of TiO2 and precipitation behavior of perovskite phase. Trans. Nonferrous Met. Soc. 2006, 16, 421–425. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.D.; Zhang, Z.T. Crystallization behavior of rutile in the synthesized Ti-bearing blast burnace slag using single hot thermocouple technique. ISIJ Int. 2011, 51, 1396–1402. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.Q.; Lou, T.P.; Sui, Z.T. Selective enrichment and growth of Ti component in titaniferrous slag. Acta Metall. Sin. 2002, 38, 400–403. [Google Scholar]
- Sui, Z.T. Precipitation selectivity of boron components from slags. Acta Mater. 1999, 47, 1337–1344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.H.; Zhang, W.; Li, G.Q. Thermodynamic analysis of extraction of synthetic rutile from modified slag. Ind. Eng. Chem. Res. 2013, 52, 4924–4931. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y. Effect of perovskite phase precipitation on viscosity of Ti-bearing blast furnace slag under the dynamic oxidation condition. J. Non Cryst. Solids 2006, 352, 123–129. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Sui, Z.T. Dynamic oxidation of the Ti-bearing blast furnace slag. ISIJ Int. 2006, 46, 458–465. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y. Precipitation selectivity of perovskite phase from Ti-bearing blast furnace slag under the dynamic oxidation condition. J. Non-Cryst. Solids 2007, 353, 2214–2290. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhang, J.H.; Feng, N.X. Crystallization and coarsening kinetics of rutile phase in modified Ti-bearing blast furnace slag. Ind. Eng. Chem. Res. 2012, 51, 12294–12298. [Google Scholar]
- Zhang, W.; Zhang, L.; Zhang, J.H.; Feng, N.X. Effect of oxidation on phase transformation in Ti-bearing blast furnace slag. Adv. Mater. Res. 2013, 641, 363–366. [Google Scholar] [CrossRef]
- Wang, X.D.; Mao, Y.W.; Liu, X.Y.; Zhu, Y.K. Study on crystallization behavior of blast furnace slag containing TiO2. J. Iron Steel Res. 1990, 3, 1–6. [Google Scholar]
- Li, Y.H.; Lou, T.P.; Sui, Z.T. Kinetics of non-isothermal precipitate process of perovskite phase in CaO-TiO2-SiO2-Al2O3-MgO system. J. Mater. Sci. 2000, 35, 5635–5637. [Google Scholar] [CrossRef]
- Pistourious, P.C.; Coetzee, C. Physicochemical aspects of titanium slag production and solidification. Metall. Mater. Trans. B 2003, 34, 581–586. [Google Scholar] [CrossRef]
- Lou, Y.P.; Li, Y.H.; Sui, Z.T. Study of precipitation of perovskite phase from the oxide slag. Acta Metall. Sin. 2000, 36, 140–144. [Google Scholar]
- Xue, X.X.; Duan, P.N.; Zhou, M. Review of thermodynamic studies on titanium oxides in metallurgical slags. J. Baotou Univ. Iron Steel Technol. 1999, 18, 357–362. [Google Scholar]
- Li, J.; Zhang, Z.T.; Zhang, M.; Guo, M.; Wang, X.D. The influence of SiO2 on the extraction of Ti element from Ti-bearing blast furnace slag. Steel Res. Int. 2011, 82, 613–614. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.F.; Lou, T.P.; Sui, Z.T. Precipitation kinetics of calcium cerite phase in slag bearing rare earths. J. Iron Steel Res. 2004, 16, 64–67. [Google Scholar]
- Liu, X.H.; Sui, Z.T. Leaching of Ti-bearing blast furnace slag by pressuring. Trans. Nonferrous Met. Soc. 2002, 12, 1281–1284. [Google Scholar]
- Li, L.S.; Sui, Z.T. Physical chemistry behavior of enrichment the selectivity of TiO2 in perovskite. Acta Phys. Chem. Sin. 2001, 17, 845–849. [Google Scholar]
- Xia, Y.H.; Sui, Z.T. Computer simulation of phase separation in CaO-MgO-Fe2O3-Al2O3-SiO2 glass. J. Northeast. Univ. 1999, 5, 511–514. [Google Scholar]





| Chemical Composition (wt. %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | TiO2 | Ti2O3 | FeO | Al2O3 | MgO | MnO | FeM * | S | Others |
| 26.54 | 24.37 | 16.90 | 4.06 | 1.72 | 13.76 | 8.48 | 0.53 | 2.50 | 0.67 | 0.47 |
| Temperature (°C) | Viscosity (Pa·s) |
|---|---|
| 1383 | 0.331 |
| 1373 | 0.339 |
| 1363 | 0.357 |
| 1353 | 0.379 |
| 1343 | 0.408 |
| T/K | kTi | −lnkTi |
|---|---|---|
| 1663 | 0.0501 | 2.993734 |
| 1673 | 0.05838 | 2.840782 |
| 1683 | 0.07203 | 2.630673 |
| 1693 | 0.09013 | 2.406502 |
| Air Flow Rate (L/min) | kTi | −lnkTi |
|---|---|---|
| 1 | 0.05838 | 2.840182 |
| 2 | 0.07578 | 2.579921 |
| 3 | 0.18741 | 1.674437 |
| T/K | kFe | −lnkFe |
|---|---|---|
| 1663 | 0.10507 | 2.296901 |
| 1673 | 0.12945 | 2.044461 |
| 1683 | 0.15741 | 1.848901 |
| 1693 | 0.17544 | 1.740458 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, W.; Zhang, J.; Li, G. Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions. Metals 2016, 6, 105. https://doi.org/10.3390/met6050105
Zhang L, Zhang W, Zhang J, Li G. Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions. Metals. 2016; 6(5):105. https://doi.org/10.3390/met6050105
Chicago/Turabian StyleZhang, Li, Wu Zhang, Juhua Zhang, and Guangqiang Li. 2016. "Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions" Metals 6, no. 5: 105. https://doi.org/10.3390/met6050105
APA StyleZhang, L., Zhang, W., Zhang, J., & Li, G. (2016). Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions. Metals, 6(5), 105. https://doi.org/10.3390/met6050105