Effect of Laser Surface Treatment on the Corrosion Behavior of FeCrAl-Coated TZM Alloy
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
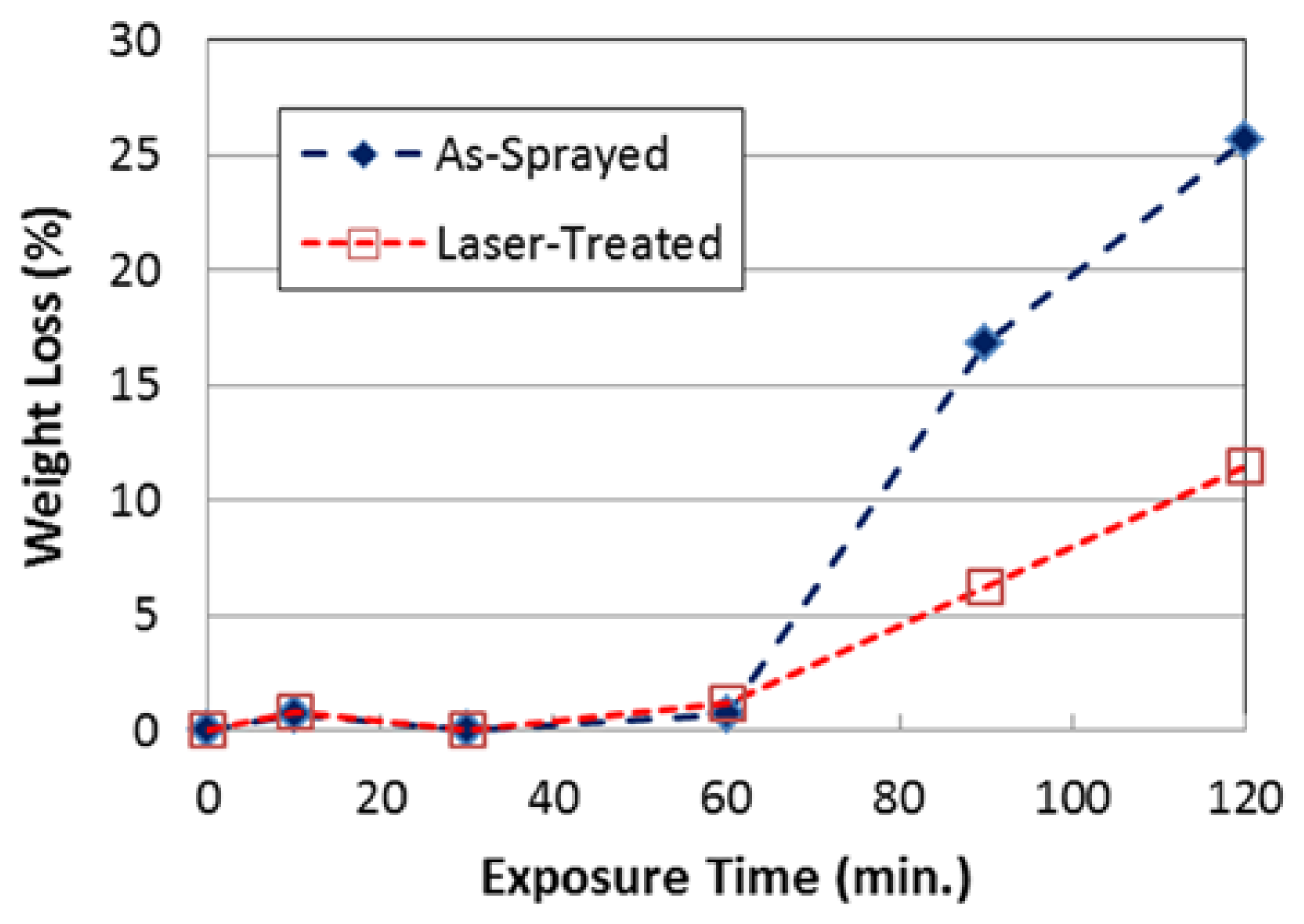
3.1. Oxidation Resistance of FeCrAl-Coated TZM Alloy at 1100 °C
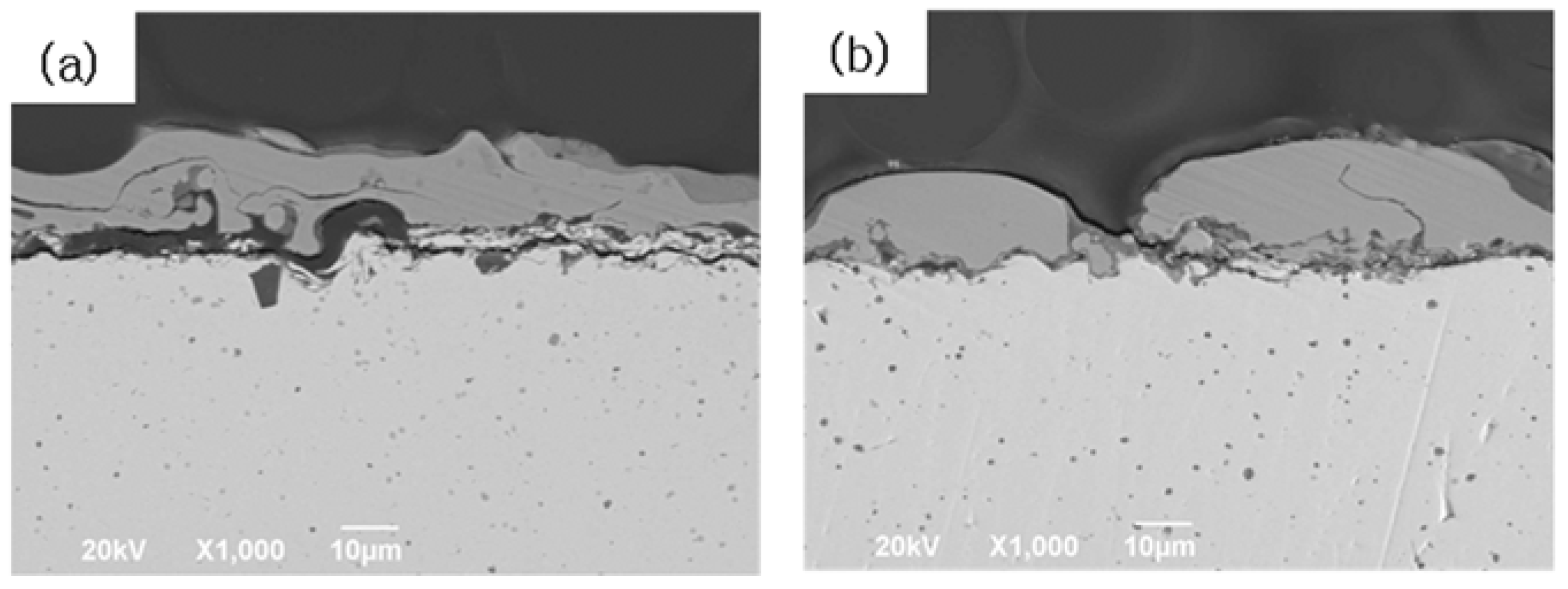


3.2. Microstructural Variations of FeCrAl-Coated TZM Alloy at 1100 °C


3.3. Corrosion Behavior of FeCrAl-Coated TZM Alloys in 3.5% NaCl

| Parameters | Rs(Ω·cm2) | CPE1(μF·cm−2) | R1(Ω·cm2) | CPE2(μF·cm−2) | R2(Ω·cm2) |
|---|---|---|---|---|---|
| Values | 2.56 | 0.038 | 6.86 | 2.52 | 6.38 × 109 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alur, A.P.; Chollacoop, N.; Kumar, K.S. High-temperature compression behavior of Mo-Si-B alloys. Acta Mater. 2004, 52, 5571–5587. [Google Scholar] [CrossRef]
- Smolik, G.R.; Petti, D.A.; Schuetz, S.T. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283, 1458–1462. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.M.; Cho, S.H.; Son, Y.I.; Kim, D. Oxidation of MoSi2-coated and uncoated TZM (Mo-0.5Ti-0.1Zr-0.02C) alloys under high temperature plasma flame. Mater. Trans. 2013, 54, 1517–1523. [Google Scholar] [CrossRef]
- Chakraborty, S.P. Studies on the development of TZM alloy by aluminothermic coreduction process and formation of protective coating over the alloy by plasma spray technique. Int. J. Refract. Met. Hard Mater. 2011, 29, 623–630. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Yan, J.; Sun, A. Preparation and characterization of molybdenum disilicide coating on molybdenum substrate by air plasma spraying. Appl. Surf. Sci. 2013, 284, 881–888. [Google Scholar] [CrossRef]
- Naumenko, D.; Le-Coze, J.; Wessel, E.; Fischer, W.; Quadakkers, W.J. Effect of trace amounts of carbon and nitrogen on the high temperature oxidation resistance of high purity FeCrAl alloys. Mater. Trans. 2002, 43, 168–172. [Google Scholar] [CrossRef]
- Hao, S.; Zhao, L.; He, D. Surface microstructure and high temperature corrosion resistance of arc-sprayed FeCrAl coating irradiated by high current pulsed electron beam. Nucl. Instrum. Methods B Beam Interact. Mater. Atoms 2013, 312, 97–103. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Wang, S.; Guo, L. Laser surface remelting of plasma-sprayed nanostructured Al2O3-13 wt. % TiO2 coatings on magnesium alloy. J. Alloys Compd. 2010, 503, 127–132. [Google Scholar] [CrossRef]
- Qian, M.; Li, D.; Liu, S.B.; Gong, S.L. Corrosion performance of laser-remelted Al-Si coating on magnesium alloy AZ91D. Corros. Sci. 2010, 52, 3554–3560. [Google Scholar] [CrossRef]
- Sova, A.; Grigoriev, S.; Okunkova, A.; Smurov, I. Cold spray deposition of 316L stainless steel coatings on aluminium surface with following laser post-treatment. Surf. Coat. Technol. 2013, 235, 283–289. [Google Scholar] [CrossRef]
- Marrocco, T.; Hussain, T.; McCartney, D.G.; Shipway, P.H. Corrosion performance of laser posttreated cold sprayed titanium coatings. J. Therm. Spray Technol. 2011, 20, 909–917. [Google Scholar] [CrossRef]
- Ciubotariu, C.R.; Frunzaverde, D.; Marginean, G.; Serban, V.A.; Birdeanu, A.V. Optimization of the Laser Remelting Process for HVOF-Sprayed Stellite 6 Wear Resistant Coatings. Optics Laser Technol. 2016, 77, 98–103. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, J.; Gong, D.; Li, J.; Ding, M. Improvement of Solar Absorbing Property of Ni-Mo Based Thermal Spray Coatings by Laser Surface Treatment. Vacuum 2015, 121, 64–69. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. High-Temperature Oxidation Behavior of Cr-Coated Zirconium. In Proceedings of the LWR Fuel Performance Meeting, Charlotte, NC, USA, 15–19 September 2013; p. 840.
- Yang, F.; Wang, K.S.; Hu, P.; He, H.C.; Kang, X.Q.; Wang, H.; Liu, R.Z.; Volinsky, A.A. La doping effect on TZM alloy oxidation behavior. J. Alloys Compd. 2014, 593, 196–201. [Google Scholar] [CrossRef]
- Leyens, C. Oxidation and Protection of Titanium Alloys and Titanium Aluminides. In Titanium and Titanium Alloys, 1st ed.; Leyens, C., Peters, M., Eds.; Wiley-VCH: Weinheim, Germany, 2003; pp. 187–230. [Google Scholar]
- Rajkumar, V.B.; Kumar, K.C.H. Thermodynamic modeling of the Fe-Mo system coupled with experiments and ab initio calculations. J. Alloys Compd. 2014, 611, 303–312. [Google Scholar] [CrossRef]
- Verdian, M.M.; Raeissi, K.; Slehi, M. Corrosion performance of HVOF and APS thermally sprayed NiTi intermetallic coatings in 3.5% NaCl solution. Corros. Sci. 2010, 52, 1052–1059. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behavior of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Kim, W.J.; Ahn, S.H.; Kim, H.G.; Kim, J.G.; Ozdemir, I.; Tsunekawa, Y. Corrosion performance of plasma-sprayed cast iron coatings on aluminum alloy for automotive components. Surf. Coat. Technol. 2005, 200, 1162–1167. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-M.; Ha, T.-H.; Park, J.-S.; Kim, H.-G. Effect of Laser Surface Treatment on the Corrosion Behavior of FeCrAl-Coated TZM Alloy. Metals 2016, 6, 29. https://doi.org/10.3390/met6020029
Kim J-M, Ha T-H, Park J-S, Kim H-G. Effect of Laser Surface Treatment on the Corrosion Behavior of FeCrAl-Coated TZM Alloy. Metals. 2016; 6(2):29. https://doi.org/10.3390/met6020029
Chicago/Turabian StyleKim, Jeong-Min, Tae-Hyung Ha, Joon-Sik Park, and Hyun-Gil Kim. 2016. "Effect of Laser Surface Treatment on the Corrosion Behavior of FeCrAl-Coated TZM Alloy" Metals 6, no. 2: 29. https://doi.org/10.3390/met6020029
APA StyleKim, J.-M., Ha, T.-H., Park, J.-S., & Kim, H.-G. (2016). Effect of Laser Surface Treatment on the Corrosion Behavior of FeCrAl-Coated TZM Alloy. Metals, 6(2), 29. https://doi.org/10.3390/met6020029






