Palladium(II) Recovery from Hydrochloric Acid Solutions by N,N′-Dimethyl-N,N′-Dibutylthiodiglycolamide
Abstract
:1. Introduction
2. Experimental Section
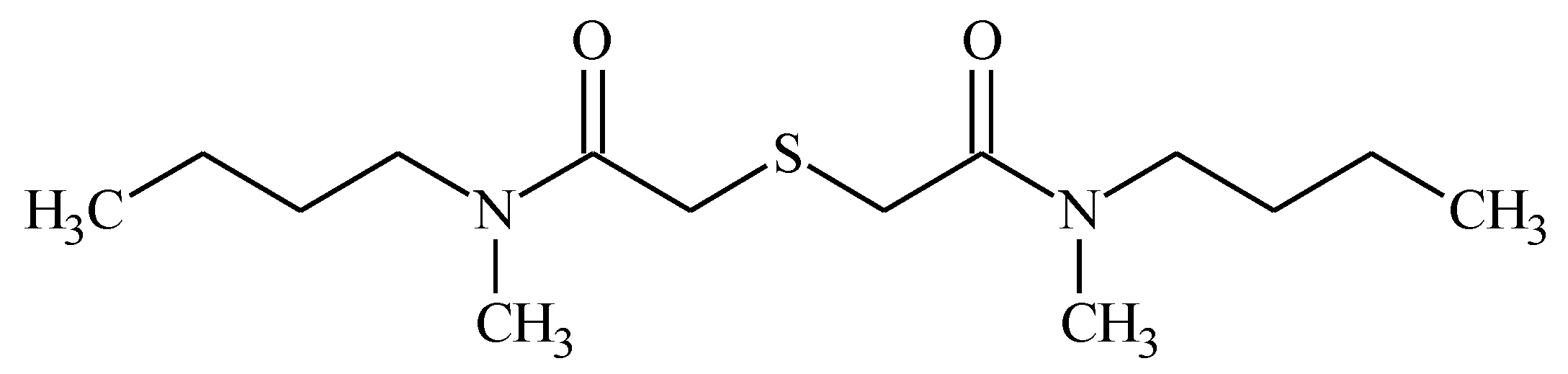
2.1. Synthesis and Characterization
2.2. Solvent Extraction Experiments
3. Results and Discussion
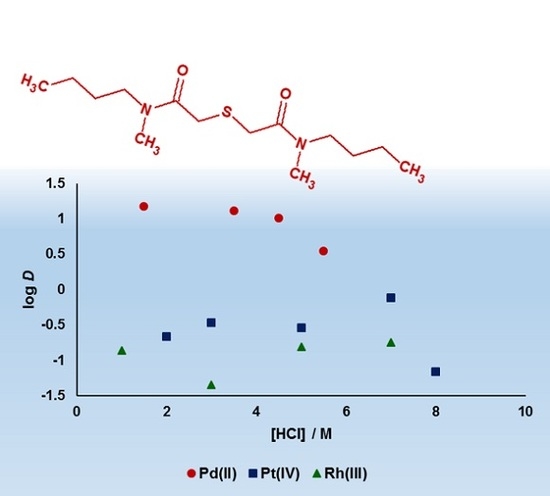
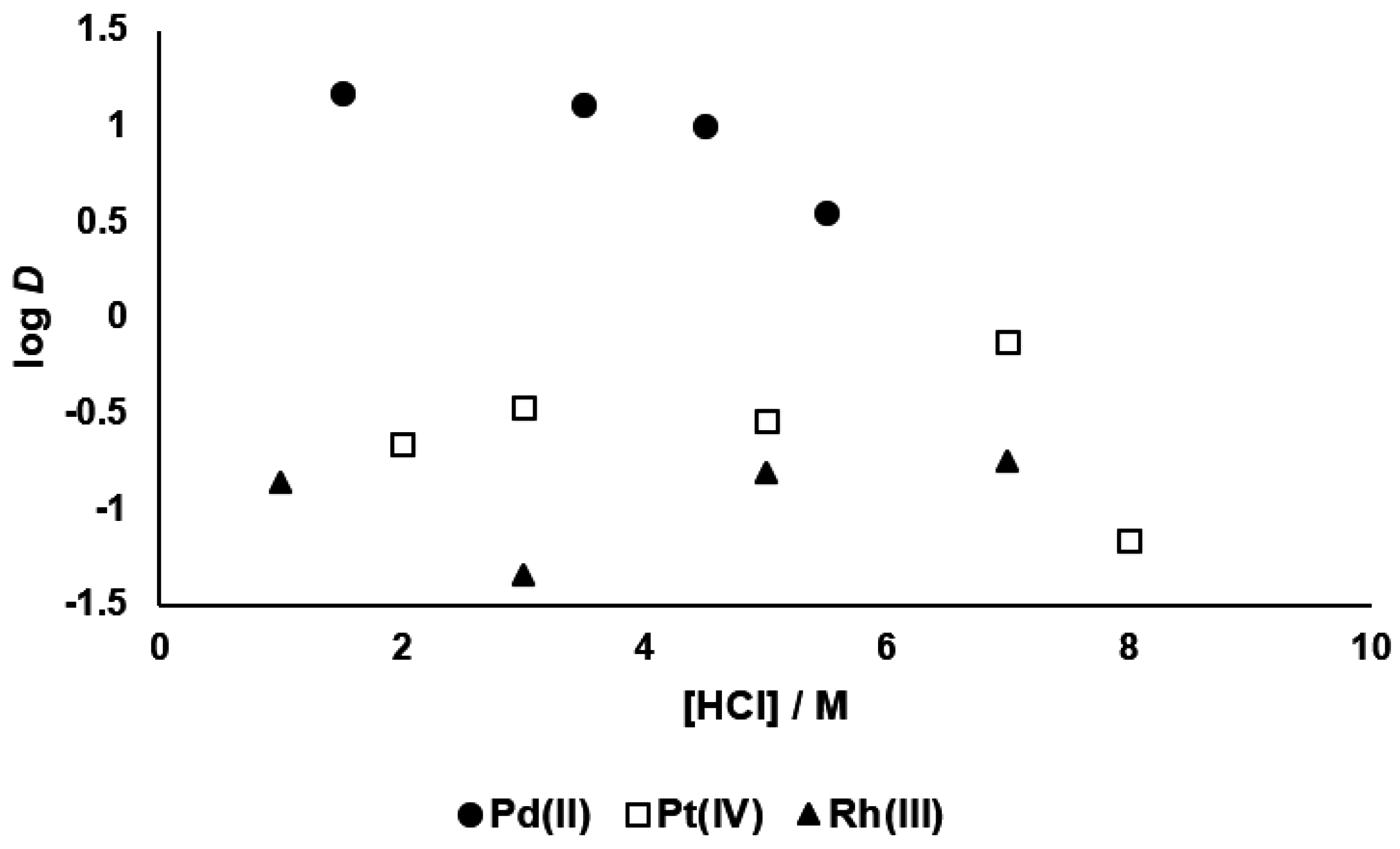
3.1. Palladium(II), Platinum(IV) and Rhodium(III) Extraction, and Palladium(II) Stripping
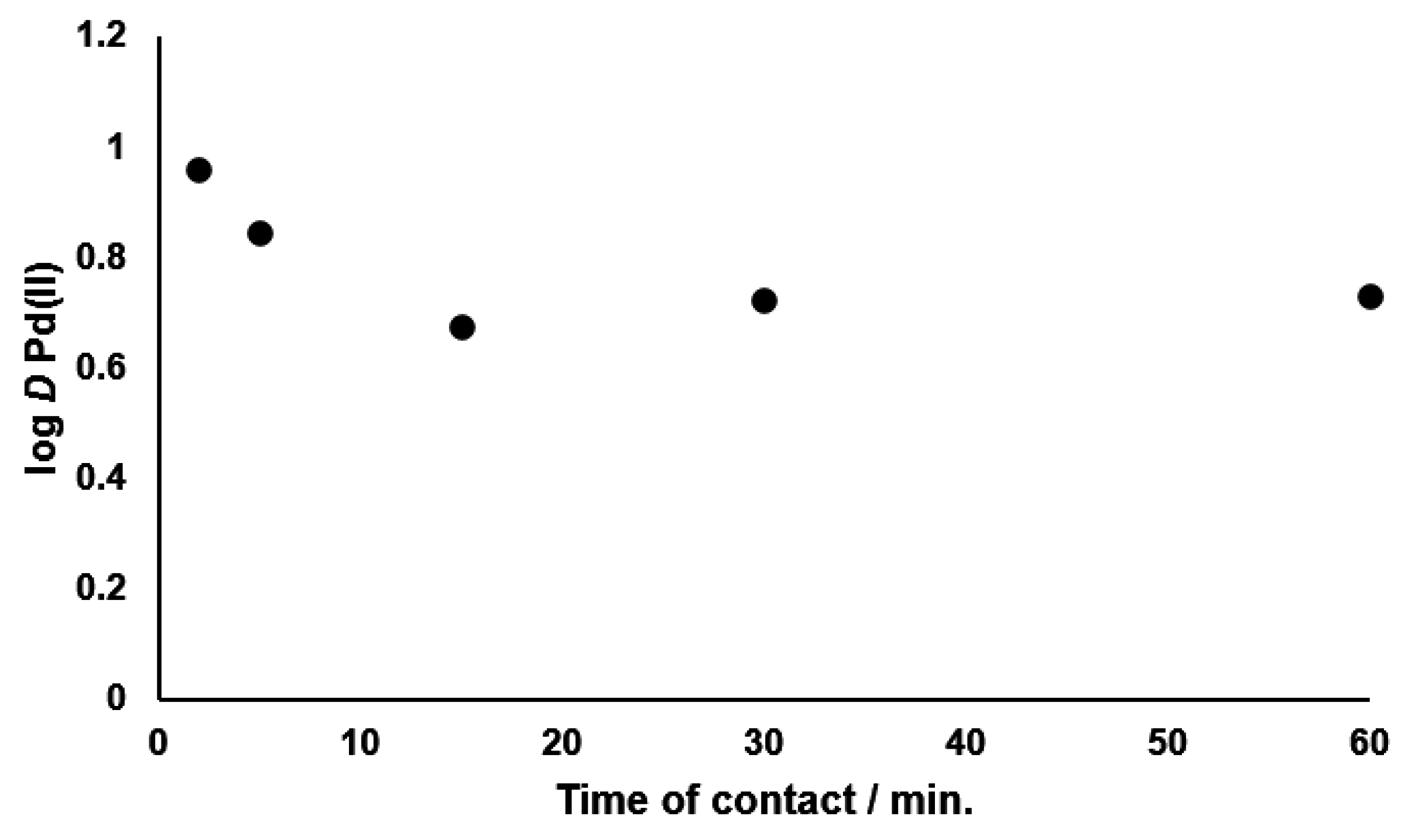
3.2. Kinetics of Palladium(II) Extraction
3.3. Effect of DMDBTDGA Concentration on Palladium(II) Extraction

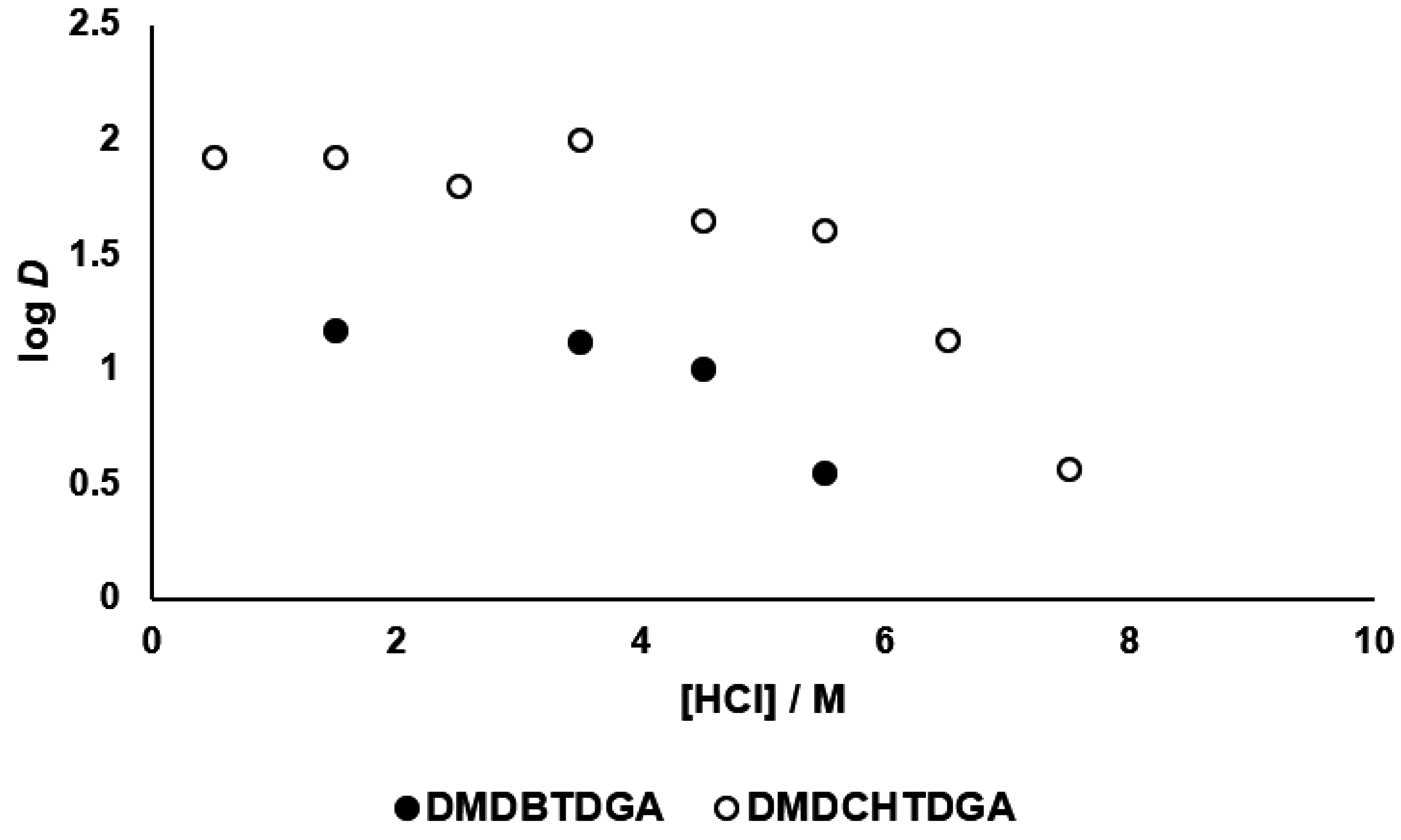
3.4. Selectivity for Palladium(II) Extraction
| [HCl] = 4 M | Solution 1 | Solution 2 | |
|---|---|---|---|
| Pt(IV) | Pt(IV) | Rh(III) | |
| DMDBTDGA | 261 | 356 | ~2880 |
| DMDCHTDGA * | 354 | 289 | ~2000 |
| [HCl] = 4 M | Solution 3 | Solution 4 | Solution 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt(IV) | Rh(III) | Al(III) | Pt(IV) | Rh(III) | Fe(III) | Pt(IV) | Rh(III) | Al(III) | Fe(III) | |
| DMDBTDGA | 271 | 759 | 673 | 5 | 66 | 26 | 5 | 126 | 169 | 26 |
| DMDCHTDGA * | 314 | ~2180 | ~7300 | 20 | 996 | 30 | 32 | ~1650 | ~2260 | 38 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Report on Critical Raw Materials for the EU—European Commission, May 2014. Available online: http://www.google.pt/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0CB4QFjAAahUKEwj0q5HDpdHIAhVCURoKHZwgCBQ&url=http%3A%2F%2Fec.europa.eu%2FDocsRoom%2Fdocuments%2F10010%2Fattachments%2F1%2Ftranslations%2Fen%2Frenditions%2Fnative&usg=AFQjCNEF6SkHHErPeB2gXS_dxBBFtlw_7A&sig2=qkh5AeKsO4cyRpuIZtVrWQ (accessed on 20 October 2015).
- Johnson Matthey, Precious Metals Management. Available online: http://www.platinum.matthey.com/about-pgm/applications (accessed on 20 October 2015).
- Hagelüken, C. Recycling the platinum group metals: A European perspective. Platinum Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 537–549. [Google Scholar]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; Costa, A.M.R. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cox, M. Solvent extraction in hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 455–505. [Google Scholar]
- Nikoloski, A.N.; Ang, K.-L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extr. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Reddy, B.R.; Raju, B.; Lee, J.Y.; Park, H.K. Process for the separation and recovery of palladium and platinum from spent automobile catalyst leach liquor using LIX 84I and Alamine 336. J. Hazard. Mater. 2010, 180, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Tanaka, M.; Morisaku, K.; Tamura, K. Extraction of platinum(IV) in hydrochloric acid solution using diglycolamide and thiodiglycolamide. Solvent Extr. Res. Dev. Jpn. 2006, 13, 101–106. [Google Scholar]
- Costa, M.C.; Assunção, A.; Costa, A.M.R.; Nogueira, C.; Paiva, A.P. Liquid-liquid extraction of platinum from chloride media by N,N′-dimethyl-N,N′-dicyclohexyltetradecylmalonamide. Solvent Extr. Ion Exch. 2013, 31, 12–23. [Google Scholar] [CrossRef]
- Malik, P.; Paiva, A.P. Solvent extraction studies for platinum recovery from chloride media by a N,N′- tetrasubstituted malonamide derivative. Solvent Extr. Ion Exch. 2009, 27, 36–49. [Google Scholar] [CrossRef]
- Paiva, A.P.; Carvalho, G.I.; Costa, M.C.; Costa, A.M.R.; Nogueira, C. Recovery of platinum and palladium from chloride solutions by a thiodiglycolamide derivative. Solvent Extr. Ion Exch. 2014, 32, 78–94. [Google Scholar] [CrossRef]
- Narita, H.; Morisaku, K.; Tamura, K.; Tanaka, M.; Shiwaku, H.; Okamoto, Y.; Suzuki, S.; Yaita, T. Extraction properties of palladium(II) in HCl solution with sulfide containing monoamide compounds. Ind. Eng. Chem. Res. 2014, 53, 3636–3640. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K. Palladium extraction with N,N,N′,N′-tetra-n-octyl-thiodiglycolamide. Miner. Eng. 2008, 21, 483–488. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Li, Y.; Wu, J.; Li, S.; Chen, S.; Zhu, L. Extraction of precious metals with a new amide extractant. Adv. Mater. Res. 2014, 878, 399–405. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover palladium(II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Das, A.; Ruhela, R.; Singh, A.K.; Hubli, R.C. Evaluation of novel ligand dithiodiglycolamide (DTDGA) for separation and recovery of palladium from simulated spent catalyst dissolver solution. Sep. Purif. Technol. 2014, 125, 151–155. [Google Scholar] [CrossRef]
- Narita, H.; Morisaku, K.; Tanaka, M. Highly efficient extraction of rhodium(III) from hydrochloric acid solution with amide-containing tertiary amine compounds. Solvent Extr. Ion Exch. 2015, 33, 407–417. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Liquid-liquid extraction of palladium(II) from chloride media by N,N′-dimethyl-N,N′-dicyclohexylthiodiglycolamide. Sep. Purif. Technol. 2015, 156, 363–368. [Google Scholar] [CrossRef]
- Paiva, A.P.; Carvalho, G.I.; Costa, M.C.; Costa, A.M.R.; Nogueira, C.A. Recovery of Palladium(II) from a Spent Automobile Catalyst Leaching Solution. In Proceedings of the 3rd International Conference on Wastes: Solutions, Treatments and Opportunities, Viana do Castelo, Portugal, 14–16 September 2015; Vilarinho, C., Castro, F., Carvalho, J., Russo, M., Araújo, J., Eds.; CVR (Centro para a Valorização de Resíduos): Guimarães, Portugal, 2015; pp. 61–63. [Google Scholar]
- Narita, H.; Tanaka, M.; Morisaku, K. Extraction Properties of Platinum Group Metals with Diamide Compounds. In Proceedings of the 17th International Solvent Extraction Conference (ISEC 2005), Beijing, China, 19–23 September 2005; Conference Proceeding Editorial Department: Beijing, China, 2005; pp. 227–232. [Google Scholar]
- Ribeiro, L.C.; Santos, M.S.; Paiva, A.P. Apparent molar volumes of N,N-disubstituted monoamides: A convenient tool to interpret iron(III) extraction profiles from hydrochloric acid solutions. Solvent Extr. Ion Exch. 2013, 31, 281–296. [Google Scholar] [CrossRef]
- Cleare, M.J.; Charlesworth, P.; Bryson, D.J. Solvent extraction in platinum group metal processing. J. Chem. Technol. Biotechnol. 1979, 29, 210–224. [Google Scholar] [CrossRef]
- Levitin, G.; Schmuckler, G. Solvent extraction of rhodium chloride from aqueous solutions and its separation from palladium and platinum. React. Funct. Polym. 2003, 54, 149–154. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Preston, J.S.; du Preez, A.C. Solvent extraction of platinum-group metals from hydrochloric acid solutions by dialkyl sulphoxides. Solvent Extr. Ion Exch. 2002, 20, 359–374. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, A.P.; Martins, M.E.; Ortet, O. Palladium(II) Recovery from Hydrochloric Acid Solutions by N,N′-Dimethyl-N,N′-Dibutylthiodiglycolamide. Metals 2015, 5, 2303-2315. https://doi.org/10.3390/met5042303
Paiva AP, Martins ME, Ortet O. Palladium(II) Recovery from Hydrochloric Acid Solutions by N,N′-Dimethyl-N,N′-Dibutylthiodiglycolamide. Metals. 2015; 5(4):2303-2315. https://doi.org/10.3390/met5042303
Chicago/Turabian StylePaiva, Ana Paula, Mário E. Martins, and Osvaldo Ortet. 2015. "Palladium(II) Recovery from Hydrochloric Acid Solutions by N,N′-Dimethyl-N,N′-Dibutylthiodiglycolamide" Metals 5, no. 4: 2303-2315. https://doi.org/10.3390/met5042303
APA StylePaiva, A. P., Martins, M. E., & Ortet, O. (2015). Palladium(II) Recovery from Hydrochloric Acid Solutions by N,N′-Dimethyl-N,N′-Dibutylthiodiglycolamide. Metals, 5(4), 2303-2315. https://doi.org/10.3390/met5042303